Abstract
Background Familial platelet disorder with propensity to myeloid malignancy (FPD/AML) is an autosomal dominant disease characterised by platelet abnormalities and risk of MDS/AML. FPD/AML results from germline mutations in RUNX1. The phenotype is heterogeneous with ∼35% of affected individuals developing MDS/AML. This clinical variability may obscure the diagnosis and prevent appropriate screening. We describe 2 families with novel C-terminal RUNX1 mutations and FPD/AML phenotypes, in which potentially affected siblings were used as haematopoietic stem cell transplant (HSCT) donors prior to identification of the inherited mutation.
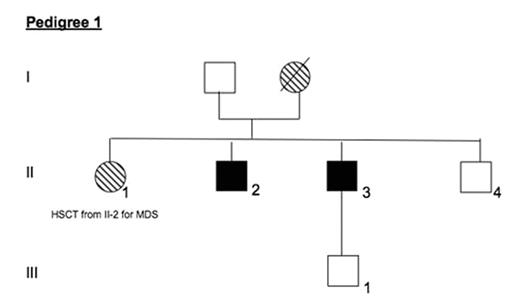
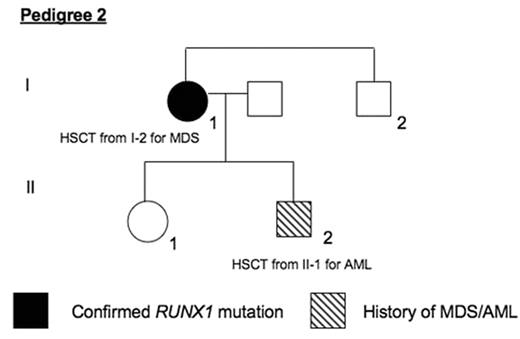
Methods DNA was collected from members of two families with histories of thrombocytopenia and MDS/AML. Direct PCR sequencing was performed on exons 1 and 3–8 of the RUNX1 gene. Results Pedigree 1 has 4 affected members from 2 generations. The proband (I-1) had a history of chronic thrombocytopenia and developed MDS at age 63 and died of AML at age 64. Her daughter (II-1) presented with MDS at age 37 and underwent 2 HSCTs from II-2, relapsing 8 months after the first and remaining well 6 years after the second HSCT. Individuals II-2 and II-3 were recently noted to have mild thrombocytopenia with normal bone marrow morphologies. DNA from blood and bone marrow of II-2 and II-3 revealed an out-of-frame 7 base pair (bp) deletion in exon 8 (1007_1013del) of RUNX1 at amino acid 336 (Gly336fsTer564). Pedigree 2 has 2 affected individuals from 2 generations. The proband (I-1) had a history of chronic thrombocytopenia since childhood. She developed MDS at age 52 and received an HSCT from her brother (I-2) in 2007 with normal engraftment and no early transplant complications. Her son (II-2) had presented with AML at age 8 and received an HSCT from his sister (II-1) at that time. He remains well 26 years later. DNA from blood and saliva of I-1 revealed a substitution of C for T in exon 7 (877C>T) of [italic[RUNX1, resulting in a stop codon and an early-truncated protein at position 292 (Arg292Ter).
Conclusions Two novel, C-terminal RUNX1 mutations are described in families with FPD/AML phenotypes. Both families were investigated after affected individuals received HSCT from siblings (who were also at risk of having inherited the mutation). The sibling donors exhibited normal or near-normal platelet counts prior to HSCT, obscuring a FPD/AML diagnosis. Therefore, all patients with MDS/AML should be screened for a family history of thrombocytopenia and/or MDS/AML and considered for investigation of RUNX1 mutations prior to HSCT.
Author notes
Disclosure: No relevant conflicts of interest to declare.