Abstract
Standard therapy for chemosensitive relapsed diffuse large B-cell lymphoma (DLBCL) is high-dose chemotherapy followed by autologous stem cell transplantation (ASCT) based on the results of the Parma study (
NEJM 1995;333:1540–5
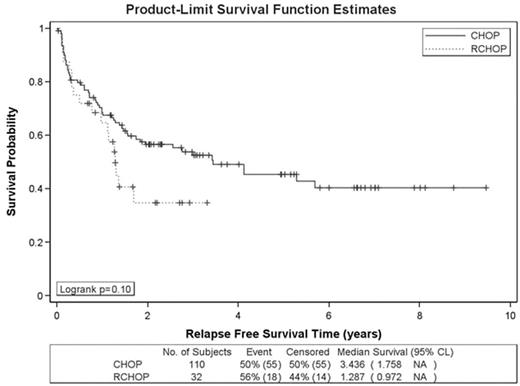
). However, the addition of rituximab to initial chemotherapy has improved progression-free and overall survival compared with CHOP alone. Patients who relapse after R-CHOP may have disease which is less sensitive to second line therapy or, alternatively, the benefit of ASCT may be independent of the impact of rituxuimab, and, therefore, the true benefit of ASCT in current patients with relapsed DLBCL is unclear. We performed a retrospective cohort analysis of patients who received ASCT for DLBCL during the period of 1997–2006. Our goal was to evaluate the benefit of ASCT for patients with relapsed DLBCL who received R-CHOP as initial chemotherapy compared to those who were treated with CHOP alone. A total of 145 patients at first relapse with chemotherapy sensitive disease who underwent ASCT were identified. 111 received a CHOP-like regimen, and 34 received R-CHOP as their initial chemotherapy regimen. All patients received salvage chemotherapy prior to ASCT. 86.5% of CHOP patients and 88.2% of R-CHOP patients achieved a CR/CRu with their initial chemotherapy (p=0.99). At initial diagnosis, Ann Arbor stage and ECOG performance status (PS) between the two groups were also similar. The two groups of patients had significant differences in the following characteristics: median age at ASCT (51 for CHOP vs 57 for R-CHOP, p=0.002), Ann Arbor stage of disease at relapse (22.5% localized disease for CHOP vs 55.9% localized disease for R-CHOP, p=0.001), and percentage of patients who underwent nuclear imaging to evaluate response to salvage chemotherapy (37.8% in CHOP vs 79.4% in R-CHOP, p<0.0001). The majority of patients in both groups received either CBV or Bu/Cy conditioning chemotherapy at ASCT. 2-year relapse-free survival (RFS) was 57% in the CHOP group versus 35% in the R-CHOP/ASCT group (p=0.10). 2-year OS was 75% (CHOP) and 67% (R-CHOP/ASCT), respectively (p=0.09). Longer follow-up is essential and further analysis examining the potential impact of rituximab containing salvage therapy in rituximab naïve patients is needed. In conclusion, although this is a retrospective comparison, the trend toward inferior RFS and OS in the patients who initially received R-CHOP suggests that the benefit of ASCT in patients who subsequently relapse after R-CHOP may be inferior compared to patients who received CHOP alone.Author notes
Disclosure:Membership Information: Ephraim P. Hochberg, MD; Advisory Board: Genentech, Inc; Speaker’s Bureau: Genentech, Inc, Biogen Idec, Inc, Amgen, Inc.; David C. Fisher, MD; Speaker’s Bureau: GENIE, BEN.
2007, The American Society of Hematology
2007