This study challenges a recent proposal that disulfide bond isomerization switches cell-surface tissue factor between encrypted (non-coagulant) and decrypted (procoagulant) states.
Blood clotting is initiated when factor VIIa binds to the integral membrane protein, tissue factor. Curiously, most tissue factor on the surface of resting cells exists in an “encrypted” state with very little clotting activity.1 Damaging the plasma membrane, treating cells with calcium ionophore, or inducing apoptosis all dramatically increase tissue factor activity (“decryption”). Most clotting enzymes are active only when assembled on membranes containing anionic phospholipids (especially phosphatidylserine). Cells actively sequester phosphatidylserine to the inner leaflet of the plasma membrane, but this membrane asymmetry is abolished by agents known to induce tissue factor decryption. The most straightforward explanation for tissue factor decryption is therefore phosphatidylserine exposure, although decryption may also involve tissue factor dimerization and/or localization to membrane rafts.1
A new hypothesis for tissue factor encryption was recently proposed in which a critical disulfide bond (Cys186-Cys209) is disrupted in encrypted tissue factor.2,3 Protein disulfide isomerase (PDI), a protein normally resident in the endoplasmic reticulum, was proposed to control the formation of this critical disulfide on cell-surface tissue factor in a mechanism that neatly explains many facets of tissue factor biology. In addition to triggering clotting, the factor VIIa-tissue factor complex can also initiate signaling via limited proteolysis of protease-activated receptor-2 (PAR-2). Ahamed et al reported that tissue factor with the disrupted disulfide bond is still competent to engage in signaling, proposing that disulfide-mediated encryption/decryption of tissue factor is a molecular switch between procoagulant and signaling modes.2 They also provided evidence that breaking the Cys186-Cys209 disulfide of tissue factor is under control of nitric oxide pathways, bringing another vascular regulatory process into the mix.
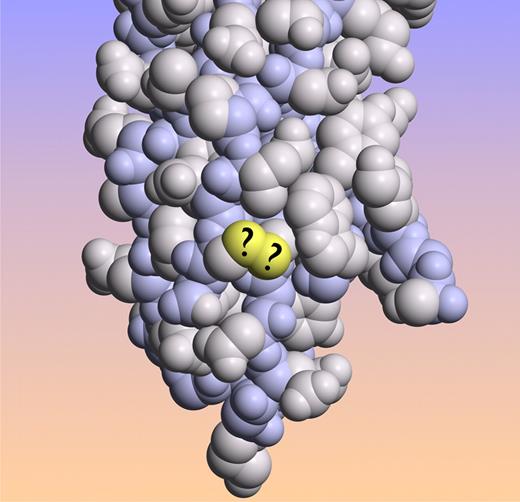
Tissue factor crystal structure with the sulfur atoms of the proposed labile Cys186-Cys209 disulfide bond in yellow. (Image created from coordinates file 1DAN from the Protein Data Bank.)
Tissue factor crystal structure with the sulfur atoms of the proposed labile Cys186-Cys209 disulfide bond in yellow. (Image created from coordinates file 1DAN from the Protein Data Bank.)
In this issue of Blood, Pendurthi and colleagues report negative results from multiple tests of the hypothesis that disulfide formation/disruption controls tissue factor coagulant and signaling activities. These include demonstrating that knocking down PDI expression using siRNA had no effect on cell-surface tissue factor decryption or signaling, nor did adding exogenous PDI or blocking anti-PDI antibodies. Studies supporting the disulfide formation/breakage hypothesis have largely been conducted with living cells, as it is apparently very difficult to demonstrate disulfide-mediated encryption/decryption—or effects of PDI—with purified full-length tissue factor incorporated into phospholipid vesicles.2-4 Those studies have typically demonstrated that treating intact cells with oxidizing agents like HgCl2 causes rapid tissue factor decryption, although such treatments are known to dramatically increase phosphatidylserine exposure.5 Pendurthi and colleagues argue that changes in phospholipid asymmetry, rather than tissue factor disulfide bond rearrangement, most likely explain the procoagulant effects of HgCl2 treatment. Based on multiple lines of reasoning, they call into question the validity of the proposal that tissue factor encryption/decryption involves PDI-dependent disulfide isomerization.
It is always difficult to know where the truth lies when 2 groups publish such diametrically opposed con-clusions, and doubtless this new report will spur further research into the puzzling mechanism(s) of tissue factor encryption. Nature does not decrypt her secrets willingly.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■