Abstract
Xenoantibody production directed at a wide variety of T lymphocyte–dependent and T lymphocyte–independent xenoantigens remains the major immunologic obstacle for successful xenotransplantation. The B lymphocyte subpopulations and their helper factors, involved in T-cell–independent xenoantibody production are only partially understood, and their identification will contribute to the clinical applicability of xenotransplantation. Here we show, using models involving T-cell–deficient athymic recipient mice, that rapidly induced, T-cell–independent xenoantibody production is mediated by marginal zone B lymphocytes and requires help from natural killer (NK) cells. This collaboration neither required NK-cell–mediated IFN-γ production, nor NK-cell–mediated cytolytic killing of xenogeneic target cells. The T-cell–independent IgM xenoantibody response could be partially suppressed by CD40L blockade.
Introduction
Successful xenotransplantation may alleviate the ever-increasing need for donor organs, but severe immunologic barriers, of which xenoreactive antibodies represent the most important 1, still hamper (pre)clinical application. Pigs are now generally considered the most suitable organ donors for clinical xenotransplantation,1 but transplantation of pig organs into nonhuman primates, currently constituting the best validated preclinical model, leads to xenograft rejection within a few hours. This hyperacute rejection is mediated by pre-existing, so-called “natural antibodies,” which are directed at a specific Galα1,3Galβ1,4GlcNAc (Gal) oligosaccharide, that is present on (especially endothelial) proteins of most lower animal species, but not of nonhuman primates or man.2-4 Anti-Gal natural antibodies can rapidly activate the complement system of the recipient, leading to hyperacute rejection.5 Several procedures have been explored in recent years to solve the problem of hyperacute rejection.6,7 The most appealing ones were the development of genetically modified donor pig strains that either expressed transgenes of human complement regulatory proteins, able to interfere with complement activation,8 or that lacked the α1,3-galactosyltransferase gene to synthesise αGal.9,10 The latter pig strain seemed particularly attractive, as Gal antigens had been found to be also strongly involved in acute vascular xenograft rejection, a second type of xenograft rejection that develops within a few days in situations where hyperacute rejection is prevented.7,11 It was hoped, therefore, that elimination of Gal epitopes would prevent both hyperacute rejection and acute vascular rejection
A number of recent reports on the transplantation of α1,3-galactosyltransferase-ko pig kidney or heart grafts in nonhuman primates have shown that loss of Gal expression in donor pig organs could indeed prevent hyperacute rejection, but that T-cell–dependent IgG xenoantibodies were induced against non-Gal xenoantigens, unless a treatment regimen was used that resulted in specific T-cell xenotolerance.12-14 In the latter case, long-term functional renal xenograft survival was obtained, but after a few months, mild focal thrombotic microangiopathy was observed, suggesting that additional modifications to the treatment regimen are needed to permit the development of the kind of long-term survival required for clinical applications.13 Although nonimmune factors such as incompatibilities between pig endothelial cells and the primate coagulation system may play a role in the occurrence of microangiopathy and/or loss of α1,3-galactosyltransferase-ko organs, involvement of T-cell–independent anti–non-Gal immune xenoreactivity cannot be excluded. Anti-Gal xenoantibody-producing B cells were recently described as Mac1− B1b-like splenic B cells.15 In contrast, T-cell–independent anti–non-Gal xenoantibody formation, which may contribute to residual xenoreactivity in these mentioned studies,12-14 is as yet poorly understood. Here, we challenged T-cell–deficient athymic mice with hamster heart xenografts or xenoantigens, a combination that does not involve Gal expression incompatibility, and report that rapidly induced anti–non-Gal xenoantibody production was mediated by marginal zone B (MZB) cells and required help from natural killer (NK) cells.
Materials and methods
Animals
T-cell–deficient nude C57BL/6 H-2b and BALB/c H-2d male mice, 8 to 10 weeks old, purchased from M&B (DK-8680; Ry, Denmark) were used as recipients. The functional T-cell deficiency of these mice was confirmed at regular intervals by verifying their inability to reject allogeneic heart or skin grafts. Inbred golden hamsters (AU/Hö Han Rj), purchased from the Center d'Elevage R. Janvier (Le Genest-St-Isle, France), were used as donors. IFN-γ−/− and TCRβ−/− C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). CB17SCID mice were kindly provided by D. Schols (Rega Institute, University of Leuven, Leuven, Belgium). Nude mice were kept in a specific pathogen–free facility. All experiments were approved by the Ethics Committee on Research Animal Care of the University of Leuven.
Surgical procedures
Heterotopic xenoheart transplantation was performed as previously described.16 Grafts were implanted in the recipient neck and graft beating was checked daily, either by inspection or palpation. Cessation of beating indicated xenograft rejection, which was confirmed by histologic examination.
For splenic tissue transplantations, 1-mm3 fragments of spleens from either naive or lethally irradiated donor mice were placed under the kidney capsules of recipient mice via a small subcostal incision. Tissue from 1.5 donor spleens was used for 1 recipient. Alternatively, spleen tissue fragments were stirred in collagenase (1 mg/mL) at 37°C for 1 hour. After filtration, cells were washed twice and counted. Cellular viability of these grafts was more than 95%.
Immunization
Mice were immunized using 100 μg TNP-Ficoll (Biosearch Technologies, Novato, CA) through intravenous injection, or 200 μL hamster red blood cells (RBCs) through intravenous injection.
Measurement of serum antibodies
Mouse anti–TNP-Ficoll serum IgM and IgG2a were determined by flow cytometry, using TNP-Ficoll microbeads (Microbeads Bioscience, Arlington, MA) and FITC-labeled goat-anti-mouse IgM and hamster anti-mouse IgG2a antibodies (Serotec, Kidlington, United Kingdom). Serum mouse anti-hamster IgM and IgG were determined as previously described.16
In vivo depletion of NKT/NK cells and in vivo blockade of Ly49D and CD40L
As previously reported,17,18 NK/NKT cells were depleted by intraperitoneal injection of TM-β1 (kindly provided by T. Tanaka, Osaka University, Japan). In vivo blockade of Ly49D receptor was performed by intraperitoneal administration of blocking anti-Ly49D mAb (4E5; Pharmingen, Erembodegem, Belgium) or control rat Ig2a (Serotec), at a dose of 100 μg, 1 day before hamster RBC immunization or hamster heart transplantation. CD40L blockade was performed by intraperitoneal injection of 300 μg of MR1 (anti-CD40L; kindly provided by L.B. and A.K.), or control hamster IgG (Biotrend, Keulen, Germany) on days −1, 0, 1, 3, and 5 relative to the day of hamster RBC immunization.
Monoclonal antibodies and flow cytometry
Flow cytometric analysis of peripheral blood mononuclear cells and splenocytes was performed as described previously.16 Antibodies used were as follows: FITC-, PE-, or PerCP-conjugated anti-mouse H2Kb, CD3e, F4/80, CD11b, CD21/CD35, CD5, NK 1.1, DX5, CD45R/B220, Ly-G6, Ly49D, and CD23, all purchased from BD Biosciences Pharmingen (Erembodegem, Belgium); PE-conjugated goat anti-mouse IgM (Cedarlane, Burlington, ON) and FITC-conjugated rat anti-mouse IgD (Southern Biotechnology, Birmingham, AL).
Cell isolation procedures
Magnetic-activated cell sorting (MACS) was used to isolate NK1.1+, CD19+, and DX5+ cells from spleens. In brief, a single-cell suspension depleted from RBCs and debris was incubated either with purified anti-mouse NK1.1 (IgG2a; BD Pharmingen) followed by anti-mouse IgG2a + b microbeads (Miltenyi Biotec, Munchen Gladbach, Germany) each for 15 minutes at 4°C, or with anti-mouse DX5 or anti-mouse CD19 microbeads (Miltenyi Biotec) for 20 minutes at 4°C. Magnetic separation was subsequently performed using the LS column (Miltenyi Biotec). The purity of selected cell population was more than 95%.
For isolation of MZB and B1b cells, CD19+ MACS-isolated cells were stained with anti-CD21/CD35(CR2/CR1)–FITC and anti-CD23(FceRII)–PE (eBioscience, Halle, Belgium) and resuspended in PBS at a concentration of 3 × 106 cells/mL. On a FACSVantage (Becton Dickinson, San Jose, CA), CD21negCD23neg B1b and CD21highCD23low MZB cells were sorted,15,19 collected, and resuspended in PBS for intravenous injection. Purity of selected cell populations was more than 90%.
NK cytotoxicity assay
NK cytotoxicity was tested using either purified NK1.1+ cells or DX5+ cells or nylon wool–enriched NK cells using the standard 51Cr-release assay, as described previously.20
Light microscopy, immunohistochemistry, and electron microscopy
For routine microscopic examination, spleens or grafted hearts were formalin fixed and stained with hematoxylin and eosin (HE). For immunohistochemistry, tissues were snap-frozen in liquid nitrogen and kept at −70°C until use. Cryostat sections of 4 μm were air-dried overnight at room temperature and fixed in acetone. Next, endogenous peroxidase activity was blocked by adding 0.3% H2O2, and nonspecific staining was blocked by incubation with 5% normal rabbit or goat serum. Sections were incubated with the following mAbs: rat anti–mouse CD21/CD35, FDC-M1, CD3e, DX5, C3, and P-selectin; hamster anti–mouse CD120a; biotinylated rat anti–mouse CD45R/B220, syndecan 1 (CD138), or CD5 (all from BD Pharmingen); rat anti–mouse CD169 (sialoadhesin; ImmunoKontact, Abingdon, United Kingdom); goat anti–mouse IgM (Serotec) and IgD (Southern Biotechnology); biotinylated MOMA-1 (ImmunoKontact); and biotinylated F4/80 (Serotec). Where unconjugated mAbs were used, sections were subsequently incubated with biotinlyated rabbit anti–rat or anti–goat Ig mAb (DAKO, Merelbeke, Belgium). Finally, sections were incubated in horseradish peroxidase (HRP)–conjugated avidin-biotin complex (ABC), positive staining was visualized using DBA and/or substrate-chromogen solution (DAKO, Carpinteria, CA), and sections were counterstained with Mayer hematoxylin. Microscopic analysis was performed with a Zeiss Axioplan2 microscope (Zeiss, Gottingen, Germany) and Carl Zeiss Vision (KS 400V 3.00; Zeiss, Hallbergmoos, Germany). Original magnification 20×/0.50.
For electron microscopy, small spleen fragments were immediately fixed in 2.5% glutaraldehyde and 0.1 M phosphate buffer at 4°C. Postfixation was performed in 1% osmium tetroxide and 0.1 M phosphate buffer for 1 hour. Next, samples were dehydrated in a graded series of alcohol and embedded in epoxy resin. Ultrathin sections were cut, stained with uranyl acetate and lead citrate, and examined at 50 kV using a Zeiss EM 900 electron microscope (Zeiss, Oberkochen, Germany). Images were recorded digitally with a Jenoptik Progress C14 camera system (Jena, Germany), operated using Image-Pro Express software version 4.0 (Media Cybernetics, Silver Spring, MD).
Statistical analysis
The Mann-Whitney U test was used to estimate the level of significance of differences between groups of data. A P value less than .05 was considered evidence for a statistically significant difference.
Results
T-cell–independent xenoantibodies leading to hamster xenograft rejection in C57BL/6 nude mice are produced by MZB cells
C57BL/6 nude mice rejected hamster xenoheart grafts at day 5.1 plus or minus 0.5 (Table 1; group A), with high serum IgM xenoantibody levels (mean fluorescence intensity [MFI] 36.1 ± 8.3 [n = 6]; Figure 1A,B) and immunohistochemical signs typical of acute vascular rejection16 (IgM and complement deposition and endothelial P-selectin expression; Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Following splenectomy (on days −90, −14, 0, 2, or 3 relative to xenografting), xenografts survived indefinitely (Table 1; group B), and IgM xenoantibodies were absent (not shown). Splenectomized recipients given whole splenocytes from either syngeneic naive (Table 1; group C) or from xenograft-rejecting syngeneic eusplenic animals (Table 1; group D) failed to reject xenohearts. In contrast, transfer of serum, taken from eusplenic C57BL/6 nude mice on day 7 after xenoheart transplantation, to splenectomized recipients bearing a beating hamster heart graft, induced hyperacute rejection (Table 1; group E). These data indicate that xenograft rejection is dependent both on splenocytes and splenic architecture in the induction phase, whereas the effector phase of xenograft rejection can be spleen independent.
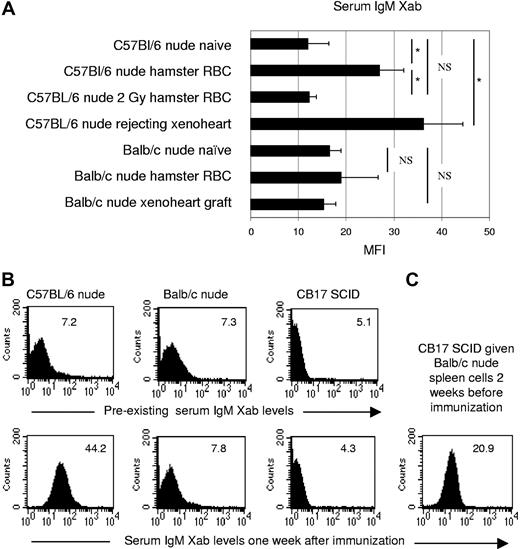
IgM xenoantibody response in C57BL/6 nude, BALB/c nude, and CB17SCID mice given BALB/c nude splenocytes. (A) Naive nude animals were either immunized with hamster RBCs (C57BL/6, n = 7; BALB/c, n = 3) or given a hamster xenoheart (C57BL/6, n = 7; BALB/c, n = 6). Serum hamster-specific IgM xenoantibodies were assayed either on day 7 (hamster RBC), or between days 2 and 6 (C57BL/6, at the time of xenograft rejection), or between days 3 and 12 (BALB/c, no rejection of xenografts). One C57BL/6 nude group was given 2 Gy TBI 1 week before hamster RBC immunization (n = 6). Naive BALB/c nude (n = 12) and C57BL/6 nude (n = 9) animals were used as controls. Bars represent the mean (± SE) MFI values. *P < .05; NS indicates not significant for comparison between groups. (B) Histograms illustrating IgM xenoantibody serum levels of naive (top panels) or hamster RBC–immunized (bottom panels) nude C57BL/6, nude BALB/c, or CB17 SCID mice (MFI is indicated in the top right corner). (C) Histogram illustrating IgM xenoantibody serum levels of CB17 SCID mice after transfer of 120 × 106 BALB/c nude splenocytes on day −14. Serum IgM xenoantibodies were determined on day 7 relative to hamster RBC immunization (1 of 2 identical experiments is shown).
IgM xenoantibody response in C57BL/6 nude, BALB/c nude, and CB17SCID mice given BALB/c nude splenocytes. (A) Naive nude animals were either immunized with hamster RBCs (C57BL/6, n = 7; BALB/c, n = 3) or given a hamster xenoheart (C57BL/6, n = 7; BALB/c, n = 6). Serum hamster-specific IgM xenoantibodies were assayed either on day 7 (hamster RBC), or between days 2 and 6 (C57BL/6, at the time of xenograft rejection), or between days 3 and 12 (BALB/c, no rejection of xenografts). One C57BL/6 nude group was given 2 Gy TBI 1 week before hamster RBC immunization (n = 6). Naive BALB/c nude (n = 12) and C57BL/6 nude (n = 9) animals were used as controls. Bars represent the mean (± SE) MFI values. *P < .05; NS indicates not significant for comparison between groups. (B) Histograms illustrating IgM xenoantibody serum levels of naive (top panels) or hamster RBC–immunized (bottom panels) nude C57BL/6, nude BALB/c, or CB17 SCID mice (MFI is indicated in the top right corner). (C) Histogram illustrating IgM xenoantibody serum levels of CB17 SCID mice after transfer of 120 × 106 BALB/c nude splenocytes on day −14. Serum IgM xenoantibodies were determined on day 7 relative to hamster RBC immunization (1 of 2 identical experiments is shown).
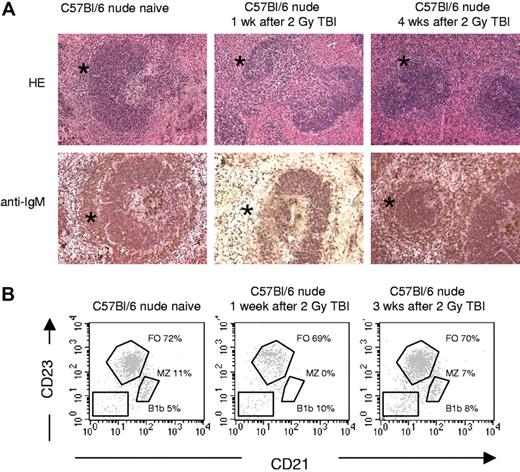
Many xenoantigens are T-cell–independent type II antigens.16,21 The B-cell populations contributing to T-cell–independent type II antibody production have been reported to consist of both MZB cells in the spleen and B1b lymphocytes.22 In view of the effect of splenectomy in our model, we proposed a role for MZB cells, and in order to investigate this, recipient mice were given 2 Gy total body irradiation (TBI), resulting—as previously reported23,24 —in a selective and almost complete, but temporary depletion of MZB cells (confirmed by HE and immunohistochemical stainings, and by flow cytometry; Figure 2). Of note, low-dose TBI had neither an effect on pre-existing natural antibody serum levels nor on the frequency of splenic NK1.1+ cells (not shown). Immunization with hamster RBCs 1 week after TBI in C57BL/6 nude mice failed to result in significant serum IgM xenoantibody levels (MFI 12.2 ± 1.5 [n = 6] versus 27 ± 5 in nonirradiated immunized [n = 7] and 12 ± 4.4 in naive [n = 9]; Figure 1A). Accordingly, xenografts transplanted on day 2 or 14 were accepted indefinitely (Table 1; groups F,G) without IgM xenoantibody production (not shown). In contrast, xenografting at week 4 after TBI, when MZB cells had recovered, again resulted in acute vascular rejection (Table 1; group H) accompanied with high IgM xenoantibody levels (not shown).
Effect of 2 Gy on splenic white pulp and number of splenic MZB cells in C57BL/6 nude mice. (A) Overview of HE and IgM stainings, performed on frozen sections (all pictures taken at the same magnification): spleens from C57BL/6 nude mice before and 1 week and 4 weeks after 2 Gy TBI irradiation. The marginal zones are indicated by asterisks, showing that IgM+ MZB cells disappeared by 1 week after TBI and exhibited partial repopulation by 4 weeks after TBI. See “Light microscopy, immunohhistochemistry, and electron microscopy” for complete image acquisition information. (B) Flow cytometric analysis of splenocytes from C57BL/6 mice before and 1 week or 3 weeks after 2 Gy TBI irradiation. Results shown were obtained in the B220+ lymphocyte gate, MZB cells were identified as CD21hiCD23lo, follicular B (FO) cells as CD21loCD23hi, and B1b cells as CD21negCD23neg.
Effect of 2 Gy on splenic white pulp and number of splenic MZB cells in C57BL/6 nude mice. (A) Overview of HE and IgM stainings, performed on frozen sections (all pictures taken at the same magnification): spleens from C57BL/6 nude mice before and 1 week and 4 weeks after 2 Gy TBI irradiation. The marginal zones are indicated by asterisks, showing that IgM+ MZB cells disappeared by 1 week after TBI and exhibited partial repopulation by 4 weeks after TBI. See “Light microscopy, immunohhistochemistry, and electron microscopy” for complete image acquisition information. (B) Flow cytometric analysis of splenocytes from C57BL/6 mice before and 1 week or 3 weeks after 2 Gy TBI irradiation. Results shown were obtained in the B220+ lymphocyte gate, MZB cells were identified as CD21hiCD23lo, follicular B (FO) cells as CD21loCD23hi, and B1b cells as CD21negCD23neg.
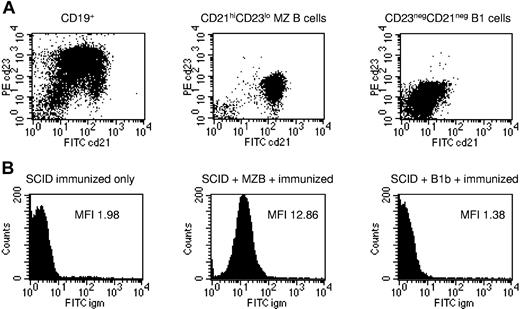
In order to confirm that MZB cells and not B1b lymphocytes were responsible for rapidly induced IgM xenoantibody production, MACS-enriched CD19+ B cells from BALB/c nude spleens were purified using fluorescence-activated cell sorter (FACS) sorting into CD21highCD23low MZB cells and CD21negCD23neg B1b cells15,19 (Figure 3A). CB17 severe combined immunodeficiency (SCID) mice were given 2 to 5 × 106 subset B cells intravenously on day 0, and 200 μL whole hamster blood on day 7. Serum IgM xenoantibodies were measured on day 12 (Figure 3B): as expected, unreconstituted SCID mice did not produce IgM xenoantibodies (mean MFI 1.5 ± 0.1 SE [individual values 1.4, 1.3, 1.3, 1.8; n = 4]). CB17SCID mice given CD19+CD21negCD23neg B1b cells were equally unable to produce a significant IgM xenoantibody response (mean MFI 1.7 ± 0.2 SE [individual values 1.9, 1.8, 1.4; n = 3]), whereas in contrast, those given CD19posCD21highCD23low MZB cells produced significantly higher IgM xenoantibody levels (mean MFI 10 ± 1.4 SE [individual values 8.4, 8.8, 12.8; n = 3]; P < .05 for difference with B1b-transferred mice, Mann-Whitney U; Figure 3B).
MZB cells, not B1b cells, are responsible for rapidly induced IgM xenoantibody formation. (A) MACS-enriched CD19pos B cells from BALB/c nude spleens (left panel) were purified using FACS sorting into CD21highCD23low MZB cells (middle panel) and CD21negCD23neg B1b cells (right panel). Representative plots are shown from 3 identical experiments. (B) CB17 SCID mice were given 2 to 5 × 106 subset B cells intravenously on day 0, and 200 μL whole hamster blood on day 7. Serum IgM xenoantibody titers in an unreconstituted SCID mouse (left panel), a SCID mouse given CD19posCD21highCD23low MZB cells (middle panel), and a SCID mouse given CD19posCD21negCD23neg B1b cells (right panel). Representative plots are shown from 1 of 3 to 4 mice from 3 identical experiments.
MZB cells, not B1b cells, are responsible for rapidly induced IgM xenoantibody formation. (A) MACS-enriched CD19pos B cells from BALB/c nude spleens (left panel) were purified using FACS sorting into CD21highCD23low MZB cells (middle panel) and CD21negCD23neg B1b cells (right panel). Representative plots are shown from 3 identical experiments. (B) CB17 SCID mice were given 2 to 5 × 106 subset B cells intravenously on day 0, and 200 μL whole hamster blood on day 7. Serum IgM xenoantibody titers in an unreconstituted SCID mouse (left panel), a SCID mouse given CD19posCD21highCD23low MZB cells (middle panel), and a SCID mouse given CD19posCD21negCD23neg B1b cells (right panel). Representative plots are shown from 1 of 3 to 4 mice from 3 identical experiments.
Despite functional MZB cells, BALB/c nude mice fail to produce IgM xenoantibodies and to reject xenografts
In contrast to C57BL/6 nude mice, BALB/c nude mice permanently accepted hamster heart xenografts (Table 2; group A), showing neither IgM nor complement deposition (Figure S1), and failed to produce significant levels of IgM xenoantibodies following either xenografting (MFI 15.3 ± 2.4; n = 7) or hamster RBC immunization (MFI 18.9 ± 7.7 [n = 3] versus 16.5 ± 2.5 [n = 12] in naive; Figure 1A,B). Transfer of serum from xenoheart-rejecting C57BL/6 nude recipients to xenoheart graft–bearing BALB/c nude recipients resulted in hyperacute rejection (Table 2; group B), indicating that the failure of BALB/c nude mice to reject heart xenografts was due to their inability to produce sufficient quantities of IgM xenoantibodies. Of note, complement activity (CH50 assay) in C57BL/6 and BALB/c nude mice was found to be identical (not shown).
The marginal zone and B-cell follicles in BALB/c nude mice were similar to those of C57BL/6 nude mice, as evidenced by immunohistochemistry (Figure S2) and flow cytometry for MZB-characterizing IgDlo, CD23lo, IgMhi and CD21hi on splenocytes and peripheral blood lymphocytes (not shown). Moreover, following transfer of BALB/c nude splenocytes into CB17 SCID mice, which did not have pre-existing IgM xenoantibodies (MFI 5.1, 5.9 [n = 2]; Figure 1C) and which were unable to generate an IgM xenoantibody response by themselves (MFI 2.9, 4.3; n = 2), hamster RBC immunization gave rise to high IgM xenoantibody levels (MFI 33.2, 20.9; n = 2), suggesting that BALB/c nude mice have a functioning MZB-cell compartment, but lack “help” signals required for IgM xenoantibody production. In order to identify this helper factor, 7 days prior to xenografting, BALB/c nude recipients were given under the kidney capsule either spleen tissue from untreated C57BL/6 nude mice, or spleen tissue from 8-Gy TBI–treated C57BL/6 nude mice (leading to depletion of splenic B cells, as confirmed by immunohistochemistry; not shown), or were given radioresistant C57BL/6 nude splenocytes obtained by collagenase digestion of 8-Gy TBI–irradiated C57BL/6 spleens. In most cases, in each group (5 of 8, 9 of 11, and 4 of 6, respectively; Table 2; groups C-F), acute vascular rejection developed with increased IgM xenoantibody levels (not shown). Appropriate control experiments confirmed that, under these experimental conditions, the B cells responsible for IgM xenoantibody production and acute vascular rejection were exclusively contained within the BALB/c nude recipient MZB-cell compartment (Table S1). Also, splenic tissue grafts from 8-Gy TBI–treated BALB/c nude mice did not restore the ability for IgM xenoantibody production and xenograft rejection (Table 2; group E), ruling out a strain-independent, irradiation-induced effect. These data indicated that functionally intact MZB cells in BALB/c nude mice lacked “help” that could be delivered by CB17 SCID mice or by radioresistant C57BL/6 nude splenocytes.
NK1.1+-cell help is required for T-independent IgM xenoantibody production and is defective in BALB/c nude mice
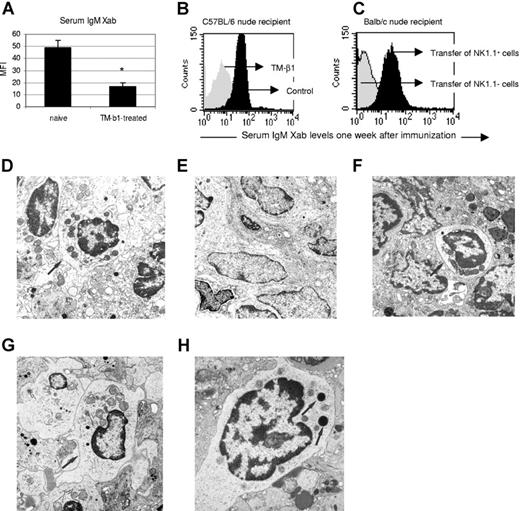
Phenotyping of collagenase-treated, lethally irradiated C57BL/6 nude spleen cells, as used in the aforementioned transfer experiments, showed that 50% of cells expressed NK1.1 (not shown). In addition, in vivo treatment of C57BL/6 nude mice with anti-IL2Rβ mAb, previously reported to efficiently deplete NK1.1+ cells in vivo for at least 5 weeks,17,18 abrogated IgM xenoantibody production following hamster RBC immunization (MFI 16.9 ± 3.1 [n = 9] versus 48.9 ± 6.4 [n = 3]; Figure 4A,B). Furthermore, transfer to BALB/c nude recipients of purified C57BL/6 NK1.1+ but not NK1.1− splenocytes 1 week before xenografting resulted in acute vascular rejection in 4 out of 6 recipients (Table 2; groups G,H), severe inflammatory lesions in the remaining 2, and markedly increased IgM xenoantibody serum levels in all (Figure 4C). Taken together, these data indicate that in order to produce IgM xenoantibody, MZB cells require critical help from NK1.1+ cells, and that this help signal is lacking in BALB/c nude mice.
NK1.1+-cell help is required for IgM xenoantibody production by MZB cells, and is deficient in BALB/c nude mice. (A-C) Hamster RBC immunization was performed in naive or TM-β1–treated C57BL/6 nude mice. Serum IgM xenoantibody levels were measured on day 7 after immunization. (A) Bars represent means (± SE) MFI of 3 naive and 9 TM-β1–treated mice (*P < .05 for comparison between groups). (B) Histogram illustrates the MFI of 1 representative animal of both groups. (C) At 1 week before hamster heart xenografting, 6 × 106 NK1.1+ or NK1.1− cells from C57BL/6 nude naive mice were transferred to BALB/c nude naive recipients (Table 2; groups G,H). Serum IgM xenoantibody levels were measured at the time of graft rejection, or at day 7. Histograms illustrate 1 representative sample of both groups. (D-H) Electron microscopy of spleen tissue from (D) a lethally irradiated C57BL/6 nude mouse, (E) a lethally irradiated BALB/c nude mouse, (F) a BALB/c nude mouse first given transplants of C57BL/6 nude splenic tissue under the kidney capsule, subsequently given a hamster heart xenograft, and—when in the process of rejecting the xenograft—exposed to a lethal irradiation dose, and (G) a BALB/c nude mouse that first received NK1.1+ cells from C57BL/6 nude naive mice, subsequently was given a hamster heart xenograft and finally given lethal irradiation. All pictures were taken at the same original magnification (×11 000). Arrows indicate radioresistant large granular lymphocytes in the white pulp in panels D, F, and G, and these cells are absent in panel E. (H) Detail of a large granular lymphocyte from the same spleen shown in panel G and reveals the dense cytoplasmic granules (←; original magnification, ×19 000). Spleen samples used for electron microscopic examination were taken at 5 days after lethal irradiation. See “Light microscopy, immunohistochemistry, and electron microscopy” for complete image acquisition information.
NK1.1+-cell help is required for IgM xenoantibody production by MZB cells, and is deficient in BALB/c nude mice. (A-C) Hamster RBC immunization was performed in naive or TM-β1–treated C57BL/6 nude mice. Serum IgM xenoantibody levels were measured on day 7 after immunization. (A) Bars represent means (± SE) MFI of 3 naive and 9 TM-β1–treated mice (*P < .05 for comparison between groups). (B) Histogram illustrates the MFI of 1 representative animal of both groups. (C) At 1 week before hamster heart xenografting, 6 × 106 NK1.1+ or NK1.1− cells from C57BL/6 nude naive mice were transferred to BALB/c nude naive recipients (Table 2; groups G,H). Serum IgM xenoantibody levels were measured at the time of graft rejection, or at day 7. Histograms illustrate 1 representative sample of both groups. (D-H) Electron microscopy of spleen tissue from (D) a lethally irradiated C57BL/6 nude mouse, (E) a lethally irradiated BALB/c nude mouse, (F) a BALB/c nude mouse first given transplants of C57BL/6 nude splenic tissue under the kidney capsule, subsequently given a hamster heart xenograft, and—when in the process of rejecting the xenograft—exposed to a lethal irradiation dose, and (G) a BALB/c nude mouse that first received NK1.1+ cells from C57BL/6 nude naive mice, subsequently was given a hamster heart xenograft and finally given lethal irradiation. All pictures were taken at the same original magnification (×11 000). Arrows indicate radioresistant large granular lymphocytes in the white pulp in panels D, F, and G, and these cells are absent in panel E. (H) Detail of a large granular lymphocyte from the same spleen shown in panel G and reveals the dense cytoplasmic granules (←; original magnification, ×19 000). Spleen samples used for electron microscopic examination were taken at 5 days after lethal irradiation. See “Light microscopy, immunohistochemistry, and electron microscopy” for complete image acquisition information.
Electron microscopic examination showed the presence of numerous large granular lymphocytes (LGLs) in the splenic white pulp of lethally irradiated C57BL/6 but not BALB/c nude mice (Figure 4D,E), and also in spleens of lethally irradiated BALB/c nude mice that rejected xenografts after having received either a C57BL/6 nude splenic tissue transplant (Figure 4F) or MACS-purified C57BL/6 nude NK 1.1+ cells (Figure 4G). These data support the hypothesis that the identity of the radioresistant helper cell, which is able to restore the capacity of xenograft rejection and T-cell–independent IgM xenoantibody production in BALB/c nude mice in the previously mentioned splenic tissue transplantation and cell-transfer experiments (Table 2), is an NK cell.
The sole involvement of NKT cells was subsequently ruled out on the basis of 2 observations. First, 8 of 10 C57BL/6 TCRβ−/− recipients given a hamster heart graft rejected their xenograft after 5 to 7 days (Table 2; group J), with significantly higher IgM xenoantibody (Xab) levels on the day of rejection (mean MFI ± SE 24.8 ± 3.5 versus 3.1 ± 1.7 in naive) and histopathologic signs typical of acute vascular rejection (not shown). Second, 3 of 4 BALB/c nude mice, given C57BL/6 TCRβ−/− spleen cells prior to hamster heart grafting, acutely rejected the xenografts on day 6 or 7 (Table 2; group K).
In the following control experiments, we demonstrated that following adoptive transfer in BALB/c nude mice, at least a fraction of C57BL/6 NK cells migrated to the spleen and survived locally until the day of xenografting. This was further supported by the in vitro observations that BALB/c nude NK cells fail to kill C57BL/6 target cells, and that the rapidly induced IgM xenoantibodies do not cross-react with allogeneic C57BL/6 target cells.
Flow cytometric analysis of spleens of BALB/c nude hosts that rejected xenoheart grafts after adoptive transfer of C57BL/6 TCRβ−/− spleen cells (Table 2; group K) revealed, at the time of rejection, a clearly definable H2Kb+DX5+ cell population (establishing 3.3%, 1.5%, 2.8%, and 1.7% of total spleen cells; not shown). This was confirmed in a second experiment: B-cell–depleted spleen cells (obtained by CD19− MACS) from C57BL/6 TCRβ−/− mice (containing 20% DX5+ cells) were transferred to BALB/c nude mice (n = 2) at a dose of either 32 × 106 or 5 × 106 CD19− TCRβ−/− spleen cells. As can be seen in Figure S3, on day 7 after adoptive transfer, the C57BL/6 NK cells, identifiable as the NK1.1+DX5+ or H2Kb+DX5+ cell population, could be clearly identified in both reconstituted BALB/c nude mice, as well as in the TCRβ−/− naive control, but not in the naive BALB/c nude control.
In addition, we performed NK cytotoxicity assays using BALB/c nude DX5+ NK cells as effector cells and C57BL/6 blast cells as target cells and demonstrated that BALB/c nude NK cells fail to elicit anti-C57BL/6 alloreactive cytotoxicity. A total of 2 independent experiments were performed, with identical results (Table S2).
Finally, we showed, using a sensitive flow cytometric assay, that circulating IgM xenoantibodies (obtained from BALB/c nude mice that rejected a hamster heart following adoptive transfer of C57BL/6 NK cells) do not cross-react with C57BL/6 cells (results not shown).
NK1.1+-cell help requires neither NK1.1+-cell mediated IFN-γ production nor NK-cell–mediated killing of xenotargets; the IgM xenoantibody response involves CD40-CD40L interaction
Hamster heart transplantation in BALB/c nude recipients given C57BL/6 IFN-γ−/− NK1.1+ cells still led to acute vascular rejection in 3 of 4 mice (Table 2; group I), and severe inflammatory lesions in the remaining mouse.
Lack of Ly49D expression in BALB/c mice has previously been reported to account for their inability to lyse xenogeneic Chinese hamster ovary cells.25 After confirming that BALB/c nude NK cells do not express Ly49D (not shown) and exhibit defective lysis of golden hamster target cells, we used blocking anti-Ly49D antibodies to show that lysis of golden hamster target cells, and not of Yac-1 tumor cells, by C57BL/6 nude NK cells is critically dependent on activation of Ly49D (Figure S4). However, in vivo treatment with anti-Ly49D antibodies, despite producing full in vivo blockade of splenic NK Ly49D receptors and despite abrogating the ability to lyse golden hamster target cells ex vivo, failed to interfere with the IgM xenoantibody response upon immunization with hamster RBCs (n = 2; Figure S4), or xenografting (n = 1; acute vascular rejection on day 7). Hence, NK-mediated lysis of T-cell–independent xenoantigen-expressing target cells is not involved in the NK1.1+-MZB cell interaction during the T-cell–independent IgM xenoantibody response.
C57BL/6 nude mice were treated with MR1 (n = 6) or control Ig (n = 6) from day −1 to day 5 relative to the day of hamster RBC immunization, and the level of serum IgM xenoantibody were determined on day 7. In vivo CD40L blockade was associated with significantly lower serum IgM xenoantibody levels (MFI 17.6 ± 3.2 SE [n = 6] in MR1-treated versus 28.1 ± 4.2 SE in coIg-treated [n = 6]; P < .05; not shown), indicating that the MZB-cell–mediated IgM xenoantibody response is—at least in part—dependent on CD40-CD40L interaction.
MZB cells and NK1.1+-cell help are only partially responsible for the TNP-Ficoll IgM response
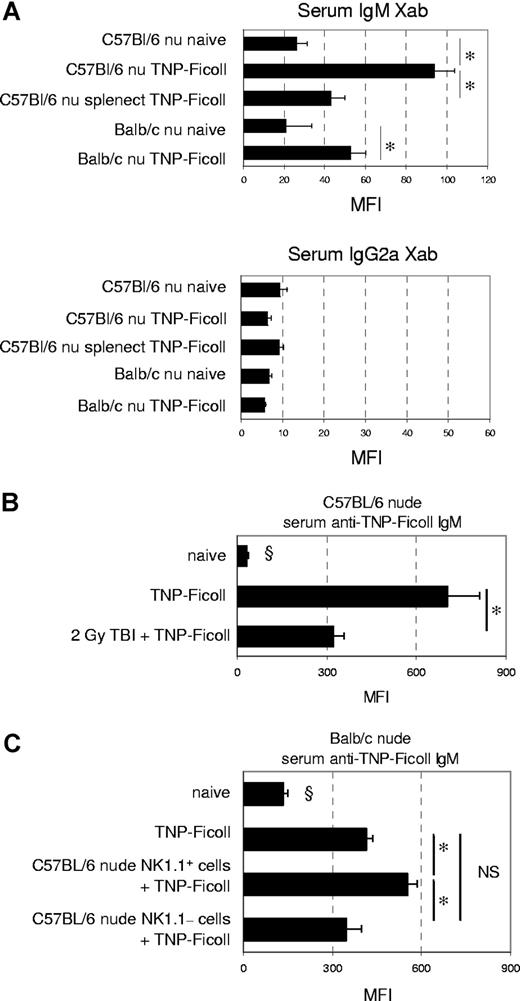
The difference in IgM xenoantibody response between C57BL/6 and BALB/c nude mice was compared with the IgM response to TNP-Ficoll, a prototypical T-cell–independent type II antigen (Figures 5A, S5). In contrast to C57BL/6 nude mice, which produced high anti–TNP-Ficoll IgM levels (MFI 93.8 ± 9.9 [n = 6] versus 26 ± 5.2 in naive [n = 6]), BALB/c nude mice exhibited a less pronounced, but significant, response (MFI 52.5 ± 7.6 [n = 6] versus 20.8 ± 12.8 in naive [n = 6]), which was similar to that of splenectomized C57BL/6 nude mice (MFI 42.9 ± 6.7; n = 6). As expected, IgG2a formation was essentially absent in all mice, confirming the T-cell–deficient setting of the experiments. MZB-cell–depleted C57BL/6 nude mice, given 2 Gy TBI on day −14, exhibited significantly lower anti–TNP-Ficoll IgM serum levels than nonirradiated immunized controls (MFI 321 ± 38 versus 704 ± 108, respectively; Figure 5B). Transfer of C57BL/6 nude NK1.1+ cells enabled BALB/c nude mice to produce significantly higher TNP-Ficoll IgM levels (MFI 550 ± 34) than NK1.1−-cell–transferred (MFI 347 ± 50) and nontransferred controls (413 ± 24; Figure 5C). These data indicate that an important part of the TNP-Ficoll IgM response is mediated by MZB cells, and that this part is also dependent on NK1.1+ cells.
MZB cells and NK1.1+-cell help are only partially responsible for the TNP-Ficoll IgM response (A) On day 7 after TNP-Ficoll immunization of naive or splenectomized mice, serum was obtained for flow cytometric analysis of TNP-Ficoll–specific IgM and IgG2a levels. Bars represent the mean (± SE) MFI values of groups of 6 animals each. *P < .05 for comparison between groups. (B) TNP-Ficoll immunization was performed in naive or 2-Gy–irradiated C57BL/6 nude mice. Serum TNP-Ficoll IgM levels were measured on day 7 after immunization. Bars represent means (± SE) MFI of 4 naive nonimmunized, 5 naive immunized, and 6 irradiated immunized mice. *P < .05 for comparison between groups; §P < .05 for comparison with all other groups. (C) At 1 week before TNP-Ficoll immunization, 6 × 106 NK1.1+ or NK1.1− cells (n = 4 per group) from C57BL/6 nude naive mice were transferred to BALB/c nude naive recipients. Serum TNP-Ficoll IgM levels were measured on day 7 after immunization and compared with those of immunized and control BALB/c nude mice (n = 3 per group). Bars represent means (± SE) MFI of each group. *P < .05 for comparison between groups; §P < .05 for comparison with all other groups.
MZB cells and NK1.1+-cell help are only partially responsible for the TNP-Ficoll IgM response (A) On day 7 after TNP-Ficoll immunization of naive or splenectomized mice, serum was obtained for flow cytometric analysis of TNP-Ficoll–specific IgM and IgG2a levels. Bars represent the mean (± SE) MFI values of groups of 6 animals each. *P < .05 for comparison between groups. (B) TNP-Ficoll immunization was performed in naive or 2-Gy–irradiated C57BL/6 nude mice. Serum TNP-Ficoll IgM levels were measured on day 7 after immunization. Bars represent means (± SE) MFI of 4 naive nonimmunized, 5 naive immunized, and 6 irradiated immunized mice. *P < .05 for comparison between groups; §P < .05 for comparison with all other groups. (C) At 1 week before TNP-Ficoll immunization, 6 × 106 NK1.1+ or NK1.1− cells (n = 4 per group) from C57BL/6 nude naive mice were transferred to BALB/c nude naive recipients. Serum TNP-Ficoll IgM levels were measured on day 7 after immunization and compared with those of immunized and control BALB/c nude mice (n = 3 per group). Bars represent means (± SE) MFI of each group. *P < .05 for comparison between groups; §P < .05 for comparison with all other groups.
Discussion
Our study shows that T-cell–independent xenoantibody production to non-Gal xenoantigens in a concordant hamster-mouse combination exclusively depends on radiosensitive MZB cells, and, hence, that it differs from the T-cell–independent xenoantibody response to the Gal antigen, which was recently shown by Ohdan et al to be mediated by 3-Gy TBI–resistant Mac1− B1b-like splenic B lymphocytes.15,26,27
Our findings may be relevant to the recently reported successful results in a preclinical xenograft model by Yamada et al13 In this study, in addition to a T-cell xenotolerance-inducing regimen—consisting of host thymectomy and xenothymus grafting—recipient baboons underwent splenectomy and were given treatment with CD40L–blocking antibodies. Based on our present study and our previous work, indicating that xenothymus grafting is insufficient to induce T-cell–independent xenotolerance,28 we hypothesize that the success of the treatment regimen by Yamada et al may be attributed in part also to effects on T-cell–independent xenoantibody production. This is further supported by the observation that in recipient baboons showing long-term graft survival, flow cytometric studies of peripheral blood lymphocytes and immunohistochemistry of tissues failed to document the presence of IgM xenoantibody.13
Our findings also reveal a novel role of NK cells for in vivo IgM xenoantibody formation. An exclusive role for NKT cells was definitively ruled out. Although NK cells have previously been shown to play a key role in in vitro T-cell–independent type II antibody production,29,30 contradicting evidence exists on their in vivo role.31-35 As NK1.1+ cells were critical for the entire IgM xenoantibody response, but only in part for the anti–TNP-Ficoll antibody response, it seems that the reported discrepancies on the role of NK cells may be due to the type of T-cell–independent antigen studied.
The precise mechanisms involved in NK-cell–mediated help for MZB cells are incompletely understood. NK-mediated IFN-γ production, previously described as playing a role in T-cell–independent type II Ig responses,29,30,35 was not involved in our model, nor was NK-cell killing of xenoantigen-expressing targets.25 However, the MZB-cell–mediated IgM xenoantibody response was—at least in part—dependent on CD40-CD40L signaling. Human and rat NK cells can express CD40L, and it has been shown that NK-cell CD40L cross-linking leads to their activation.36,37 In addition, Blanca et al have demonstrated that CD40-CD40L interaction regulates activation of human B cells by autologous NK cells.38 Although in vitro studies with murine cells have demonstrated that murine macrophages can activate NK cells through a CD40-CD40L–dependent mechanism,39,40 direct evidence of expression of CD40L by murine NK cells is—to our knowledge—as yet unavailable. Similar to what has previously been reported by Martin-Fontecha et al,41 we failed to detect CD40L expression in NK cells of C57BL/6 mice: neither by flow cytometry nor by reverse transcription–polymerase chain reaction (RT-PCR) could we document CD40L expression in resting NK cells or NK cells stimulated by IL-2, hamster blasts, or Yac-1 tumor cells (results not shown). Possibly, CD40L expression by murine NK cells is difficult to induce in vitro. In addition, flow cytometric detection of expression of CD40L protein, such as on T cells, is known to be technically challenging.42,43 Nevertheless, our observations are in concert with those by Wu et al, who showed that the in vivo T-cell–independent Ig response to phosphorylcholine in C57BL/6 mice also involves a T-cell–independent CD40L signal.44 The source of the CD40L signal and the nature of the NK-cell help signal in our model are the subject of ongoing studies. In this respect, Turner et al have shown in mice that CD40-dependent stimulation of antigen-presenting cells (APCs) can give rise to IL-12 production and subsequent NK cell activation,45 suggesting the existence of a NK-cell–independent CD40-CD40L pathway that can lead to NK cell activation. Likewise, Wu et al suggested the involvement of mast cells and auto- or paracrine B-cell signals as a source of CD40L help in the phosphorylcholine Ig response.44
In preliminary experiments, we showed that plasma cells (identified by expression of syndecan 1), CD21/CD35+ cells (Figure S6), and IgM+ cells (not shown) accumulated in the splenic white pulp of C57BL/6 nude mice at the time of xenograft rejection. In addition, DX5+ cells, which in naive C57BL/6 and BALB/c nude mice is located in the red pulp, could be found in the splenic white pulp of only C57BL/6 and not BALB/c nude mice after xenoheart transplantation (Figure S6). These findings suggest that MZB-cell and NK-cell interaction in the splenic white pulp is crucial for T-cell–independent IgM xenoantibody production, and that a defect in the migratory capacity of BALB/c nude NK cells may underlie their inability to produce T-cell–independent IgM xenoantibodies. In this respect, it has indeed been shown that the splenic periarteriolar lymphoid sheath is the principal site where T-cell–dependent and T-cell–independent humoral immune responses take effect,46-48 and also that NK cells can migrate from the red to the white pulp.49
The mechanism underlying long-term tolerance to xenografts in 2-Gy–irradiated mice is the subject of ongoing studies. Preliminary observations in such animals, several weeks after the recovery of MZB cells, show that low levels of circulating IgM xenoantibodies can be detected, and that long-term surviving xenografts exhibit weak immunoreactivity for IgM with an absence of signs of acute vascular rejection. These data suggest that, following recovery of MZB cells, IgM xenoantibody production occurs to some extent, and that continued graft survival at this point can be attributed to graft accommodation.
If MZB cells and NK cells are so important for T-cell–independent xenoantibody production, would depletion of MZB cells (eg, by splenectomy) or precluding NK1.1+-cell– and/or CD40L-dependent help entirely solve the T-cell–independent xenoantibody problem? Our comparative studies in C57BL/6 and BALB/c nude mice of the Ig response against the prototypical T-cell–independent type II antigen TNP-Ficoll suggest that this will probably not be the case. Indeed, only a part of the anti–TNP-Ficoll antibody response exhibited the characteristics of the antihamster xenoantibody response. In addition, it was neither completely absent in BALB/c nude mice, nor could it be completely abrogated in splenectomized or 2-Gy TBI–treated C57BL/6 nude mice, confirming earlier observations in Pyk-2–deficient mice, which have deficient MZB cells but still showed a significant—albeit strongly reduced—TNP-Ficoll IgM response.50 Although it is certain that multiple T-cell–independent non-Gal xenoantigens exist, their nature and contribution to xenoreactivity in different xenogeneic species combinations have as yet not been elucidated.14,21,51 Therefore, a fraction of the T-cell–independent xenoantibody response in the pig-to-primate model may resemble the murine TNP-Ficoll Ig response, and hence would be only partially controlled by splenectomy or CD40L blockade. It will be interesting to investigate whether this is the case, for example, for some endothelial xenoantigens in porcine small arteries, and whether xenoantibodies directed against these xenoantigens are involved in the process of thrombotic microangiopathy that was seen in most of the xenografts in the study by Yamada et al.13
Full immune xenotolerance involving both the T-cell–dependent and T-cell–independent compartments will probably be a requirement for xenotransplantation to be safely introduced into the clinic. Induction of mixed xenogeneic bone marrow chimerism has been shown to induce robust T-cell–dependent and T-cell–independent xenotolerance in several small animal models,27,52-55 but in large animals still faces problems such as growth factor incompatibility.56,57 Xenogeneic thymus transplantation has been shown to effectively and safely induce T-cell xenotolerance.13,58-60 Ideally, this approach should be combined with a procedure leading also to T-cell–independent xenotolerance. It is hoped that our present study, elucidating some of the cell populations and mechanisms involved in T-cell–independent xenoantibody formation, may contribute to this aim and as such also to the clinical applicability of xenotransplantation.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a grant from the Fund for Scientific Research (FWO) Flanders. A.D.B. is a postdoctoral fellow of the FWO Flanders.
We thankfully acknowledge the assistance of Willy Landuyt (Laboratory of Oncology and Experimental Radiotherapy) in irradiation experiments, and of Vic Van Duppen (Stem Cell Institute Leuven) in cell sorting procedures. We thank Patrick Matthys (Laboratory of Immunobiology, Rega-institute for Medical Research), and Xavier Bossuyt (Laboratory of Experimental Laboratory Medicine, Department of Molecular Cell Biology) for scientific discussions.
Authorship
Contribution: S.L. and Y.Y. designed and performed all experiments, assisted in some cases by Y.L. or D.M.B. O.R. and J.G. provided technical assistance. C.S. was responsible for animal care. L.B. and A.K. provided antibodies and contributed to experimental design. C.D.-P. supervised pathologic and immunohistochemical studies. R.D.V. performed electron microscopy studies. All authors contributed to data interpretation. A.D.B. and M.W. designed the study and wrote the manuscript.
S.L. and Y.Y. contributed equally to this study; M.W. and A.D.B. contributed equally to this study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mark Waer, Laboratory of Experimental Transplantation, University of Leuven, Campus Gasthuisberg, O&N 1 Box 811, Herestraat 49, B-3000 Leuven, Belgium; e-mail: mark.waer@gbiomed.kuleuven.be.