Abstract
Kupffer cells form a large intravascular macrophage bed in the liver sinusoids. The differentiation history and diversity of Kupffer cells is disputed; some studies argue that they are derived from blood monocytes, whereas others support a local origin from intrahepatic precursor cells. In the present study, we used both flow cytometry and immunohistochemistry to distinguish 2 subsets of Kupffer cells that were revealed in the context both of bone marrow transplantation and of orthotopic liver transplantation. One subset was radiosensitive and rapidly replaced from hematogenous precursors, whereas the other was relatively radioresistant and long-lived. Both were phagocytic but only the former population was recruited into inflammatory foci in response to CD8+ T-cell activation. We propose the name “sessile” for the radioresistant Kupffer cells that do not participate in immunoinflammatory reactions. However, we found no evidence that these sessile Kupffer cells arise from immature intrahepatic precursors. Our conclusions resolve a long-standing controversy and explain how different experimental approaches may reveal one or both of these subsets.
Introduction
Macrophages are a key cellular component of the innate immune system. They eliminate pathogens including bacteria and parasites and may directly kill tumor cells by receptor-mediated phagocytosis or release of tumor necrosis factor-α, oxygen metabolites, or proteinases.1 As professional antigen-presenting cells (APCs) they can also promote adaptive immune responses by processing and presenting antigen via major histocompatibility complex (MHC) class I and II.2,3 In cases of pathogenic infection or tissue damage, the release of cytokines and chemokines from activated macrophages recruits other cells of the immune system to sites of inflammation and tissue repair.
Kupffer cells (KCs) in the liver are the largest population of resident macrophages. They resemble tissue macrophages in most other nonlymphoid organs; however, their location within the hepatic sinusoids enables intimate contact with circulating blood, and this facilitates their role in clearance of bacterial products and interaction with blood-derived leukocytes. Kupffer cells have been implicated in both immunogenic and tolerogenic immune reactions.4-6
There is controversy about the origin of KCs and how homeostasis of the population is maintained over time. The traditional view is that tissue macrophages are not self-renewing and are replenished from bone marrow–derived monocytes.7,8 In contrast to this, it has been argued that KCs are a self-renewing population and divide as mature cells or originate from local intrahepatic progenitors.9-11 This issue assumes practical importance in clinical situations such as myeloid diseases treated with bone marrow allografts and also during liver transplantation. In both situations, large self-renewing APC populations that are not readily replaced from bone marrow sources are likely to affect the specificity of immune responses. Additionally, there are implications for experimental strategies in basic immunology that rely on the exchange of APCs by bone marrow transplantation in chimera experiments.
To evaluate the stability of intrahepatic macrophage populations and the extent of their replacement by bone marrow–derived and local progenitor cells, we have used several bone marrow and tissue transplantation models. Surprisingly, we identified 2 distinct KC populations within the liver, depending on the method of evaluation. Multiparameter flow cytometry of isolated hepatic leukocytes, a method frequently used to analyze the frequency of both intrahepatic and extrahepatic leukocyte populations, indicated a clear predominance of bone marrow–derived KCs that were rapidly replaced after irradiation reconstitution. However, in situ analysis by immunohistologic staining revealed a large population of residual non–bone marrow–derived KCs that was completely lost in various cell isolation protocols. These latter cells we here term sessile KCs. While both the bone marrow–derived and the sessile KCs shared the same morphology and phagocytic capabilities, only the bone marrow–derived KCs engaged in inflammatory responses. Thus the term “sessile” alludes both to their lack of rapid turnover and to their lack of capacity for local recruitment.
The different biologic functions of these 2 KC populations provide a possible explanation for the dichotomy of immunogenic and tolerogenic immune reactions within the liver. Furthermore, our results emphasize the necessity to reevaluate the representation of cell populations in tissue cell isolates by parallel in situ methods. This point was recently established for T lymphocytes as well.12
Materials and methods
Animals
Mice of the C57BL/6 strain (CD45.2) and the CD45.1 congenic strain B6.SJL-Ptprc<a>Pep3/BoyJ (termed B6.CD45.1) were purchased from the Jackson Labs (Bar Harbor, ME). The mice were housed under specific pathogen-free (SPF) conditions and were cared for by the Division of Laboratory Animal Care of the University of Rochester. All of the experiments were approved by the University Committee on Animal Resources.
Bone marrow chimeras
Six- to 8-week-old recipient mice were irradiated with various radiation dosages using an RS2000 x-ray irradiator (Rad Source Technologies, Coral Springs, FL) at 20 mA, 160 kV, releasing a radiation dose of 1.8 Gy (180 rad) per minute. The femora and tibiae of bone marrow donors were removed aseptically and dissected from the surrounding tissue. Marrow cavities were flushed with Hanks balanced salt solution (HBSS; Gibco-BRL/Invitrogen, Gaithersburg, MD) containing 5% FBS and 50 U/mL heparin with a syringe and a 27-gauge needle. The cells were washed in HBSS/FBS at 400g for 10 minutes, resuspended, and passed through a nylon mesh (BD Falcon, Bedford, MA) to remove debris. Depletion of mature T cells was achieved by incubation with anti-CD4 monoclonal antibody (mAb; clone RL172.4) and anti-CD8 mAb (clone 3-16.8) for 30 minutes on ice and subsequent lysis using guinea pig complement (Gibco-BRL/Invitrogen) at a concentration of 1:20 at 37°C, 5% CO2 for 30 minutes. Cells were then washed twice with PBS and resuspended at a cell concentration of 5 × 107 cells/mL. Recipient mice were injected with 107 bone marrow cells via tail vein injection using 1-mL syringes with 27-gauge needles.
Intrahepatic inflammatory models
TCR-transgenic CD8+ OT-I T cells (reactive to the SIINFEKL-peptide presented by MHCI Kb) from spleens and lymph nodes of B6.CD45.1+ mice were enriched by magnetic depletion of B cells, CD4+ T cells, dendritic cells (DCs), and natural killer (NK) cells using primary antibody (clone 212.Al anti-MHC class II, α-GK1.5 α-CD4, 2.4.G2 α-FcR, HB.191 α-NK1.1) plus Ig-coated beads (Qiagen, Valencia, CA). CD8+ T cells (5 × 106; > 93% purity) were injected intravenously into bone marrow transplant recipients and activated by daily intraperitoneal injections of 25 nmol SIINFEKL peptide (New England Peptide, Gardner, MA) for 3 days starting 24 hours after OT-I cell transfer. For influenza-induced intrahepatic foci, sedated C57BL/6 mice were infected intranasally with 103 plaque-forming units (PFUs) of H1N1 recombinant influenza strain A/WSN-OVAI.13 Livers were harvested on day 5 (peptide activation) or day 9 (influenza infection) and frozen in OCT freezing medium for sectioning and histologic analysis.
Mouse liver transplantation
Orthotopic mouse liver transplantation, initially reported by Qian et al,14 was performed according to a technique described by Steger et al.15 In brief, the donor liver was dissected from the surrounding hepatic ligaments; the right adrenal vein, pyloric vein, and proper hepatic artery were ligated and divided. For continuous bile flow, the gallbladder was removed after ligation of the cystic duct. A polyethylene stent tube (SIMS Portex, Kent, United Kingdom) was inserted into the lumen of the common bile duct and secured with 8-0 silk (Pearsalls, Taunton, United Kingdom). The infrahepatic inferior vena cava (IVC) and portal vein were clamped and the organ was perfused with 5 mL of 4°C normal saline through the portal vein. The liver was removed to a 4°C saline bath, and a 20-gauge polyurethane cuff was placed at the portal vein stump and secured with 8-0 silk. The liver graft was retained at 4°C until transplantation. The transplantation procedure was performed under inhalation anesthesia with isoflurane. After clamping of the infrahepatic and suprahepatic IVC and the portal vein, the recipient's liver was completely removed and the donor organ was placed orthotopically into the abdominal cavity. The suprahepatic and infrahepatic IVC were anastomosed with continuous running sutures using 10-0 nylon (Ethicon, Sommerville, NJ), and the portal vein was reconnected by cuff anastomosis. Reconstruction of the bile flow was achieved by inserting the graft's stent tube into the recipient's bile duct and securing it with 3 single 10-0 nylon sutures.
Isolation of liver nonparenchymal cells
Following bone marrow transplantation or liver transplantation, the recipient mice were killed with CO2 and the lymphocytes from the liver were isolated. The liver was perfused immediately with HBSS. After perfusion, the liver was dissected out of the abdominal cavity and homogenized through a cell strainer. The homogenized liver was incubated in 10 mL digestion buffer (RPMI 1640 containing 0.05% collagenase IV, 0.002% DNase I; Sigma-Aldrich, St Louis, MO) at 37°C for 40 minutes on a “belly dancer” gyratory shaker. After removing hepatocytes and cell clumps by centrifugation at 30g, the supernatant was then centrifuged at 400g and the pellet collected. The pellet was resuspended in Opti-prep (Axis-Shield, Oslo, Norway), giving a final solution of 22%, and centrifuged for 25 minutes at 1500g. The cells at the interface were collected, washed, and analyzed.
Staining reagents and flow-cytometric analysis
The antibodies used for staining were anti-TCRβ (H57–597), anti-CD8α (53–6.7), anti-CD4 (RM4-5), anti-CD45.1 (A20), anti-CD45.2 (104), anti-CD11b (M1/70), anti-CD45R/B220 (RA3–6B2), and anti-IAb (AF6-120.1), all from BD Biosciences PharMingen (San Diego, CA). Additional anti-F4/80 (CI:A3-1; Caltag/Invitrogen, Carlsbad, CA) was used for KC staining. The cells were stained for 20 minutes on ice, washed with PBS, and then fixed with 2% paraformaldehyde. The samples were analyzed using a FACSCalibur flow cytometer (BD Bioscience, San Jose, CA) and CellQuest software version 2.1 (BD Bioscience). Each data set was first gated based on forward scatter and side scatter on leukocytes and then further analyzed for fluorescence.
Latex bead injection and immunohistology
For phagocytosis studies, mice were tail vein injected with 0.2 mL of 1-μm carboxylate-modified fluorescent microspheres (Molecular Probes/Invitrogen, Carlsbad, CA). Depending on the experiment, animals were killed 2 hours to 5 days after injection and liver sections were prepared for confocal laser scanning microscopy.
For immunohistologic studies, 5-μm cryosections of OCT-embedded liver tissue fragments were cut, fixed in ice-cold methanol-acetone (1:1 mixture) for 5 minutes, air-dried for 15 minutes, then placed in PBS-T. Sections were then stained with the macrophage marker F4/80 (Caltag/Invitrogen) at a 1:50 dilution. Alexa 546 antirat secondary antibody (Molecular Probes/Invitrogen) was used at a 1:200 dilution to visualize F4/80 staining. Sections were then stained with FITC-conjugated CD45.2 (1:25 dilution; BD Bioscience PharMingen) and Cy5-conjugated CD45.1 (1:50 dilution; BD Bioscience PharMingen). The Cy5 fluorochrome with a fluorescence emission in the near-infrared spectrum (675 nm) was represented in blue false color throughout the experiments to yield a purple color in Cy5 + PE or Cy5 + Alexa546 double-positive donor-derived Kupffer cells. For analysis of costimulatory and activation markers and lineage, FITC- and PE-conjugated antibodies for the following surface markers were used: IAb, GR1, CD115, CXCR4, CD40, FcγR, TLR4R, CD14, CD80, and CD86 (all Bioscience PharMingen; dilution 1:50). Confocal fluorescent imaging was done using a Leica TCS SP1 (Leica Microsystems, Bannockburn, IL; University of Rochester Core Imaging Facility) equipped with oil immersion lenses: HCX PL APO 40×/1.25 numerical aperture (NA); HCX PL APO 100×/1.40 NA; HCX PL APO 63×/1.32 NA (magnifications of × 200 to × 400 were used). Images were acquired with Leica confocal software (version 2.5) and results were analyzed with Adobe Photoshop 6.0.1.
Statistical analysis
The statistical significance of the differences between groups of mice was tested using the Student t test. A P value ofless than .05 was considered significant. Means and SEM are given in text and tables.
Results
Kupffer cell turnover following bone marrow transplantation: analysis by flow cytometry
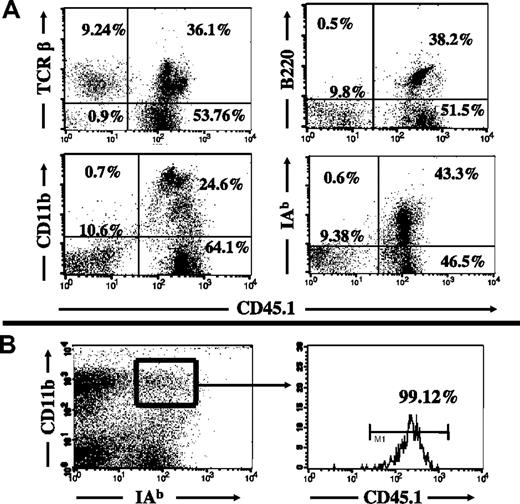
To evaluate the extent of KC replacement within livers following bone marrow transplantation, B6.45.1 (CD45.1 background) recipients of C57BL/6 bone marrow transplants were killed 4 and 12 weeks after bone marrow transplantation, and the intrahepatic leukocytes were isolated for flow-cytometry analysis. The congenic cell-surface markers CD45.1/CD45.2, together with lineage markers for macrophages (MHC II, F4/80, CD11b), were used to identify the recipient-derived (sessile) KC population (CD45.2+) and KCs that arose from the bone marrow transplant (CD45.1+). Four weeks after bone marrow transplantation, isolated intrahepatic leukocytes showed a 90% replacement by bone marrow–derived leukocytes (Figure 1A). The remaining 10% of the total intrahepatic leukocytes were found to be of recipient origin; however, these cells were almost exclusively T cells and accounted for about 20% of the intrahepatic T cells. However, within the KC population, there was a 99% replacement by bone marrow–derived cells based on costaining with macrophage lineage markers (Figure 1B). While the complete replacement of KCs was unaffected by variation of the radiation dose from 9 Gy up to 15 Gy (2 × 7.5 Gy within 12 hours), the rate of T-cell replacement increased with higher radiation doses (Table 1). The replacement of intrahepatic leukocytes remained stable up to 12 weeks following bone marrow transplantation (data not shown).
Flow-cytometric analysis of recipient leukocyte replacement in radiation bone marrow chimeras. The replacement of various leukocyte populations in B6.CD45.2 mice was analyzed 4 weeks after 10-Gy radiation and congenic bone marrow transplantation from B6.CD45.1 bone marrow donors. (A) Approximately 90% of intrahepatic leukocytes were replaced by donor-type leukocytes. The remaining 10% were almost entirely TCR-positive (top left) and negative for CD45R/B220, CD11b, and MHC class II, indicating that they were non-Kupffer cells. (B) The origin of isolated intrahepatic macrophages was greater than 99% donor BM-derived, based on expression of CD11b and MHC class II (IAb). Numbers on plots are percentages of lymphocytes.
Flow-cytometric analysis of recipient leukocyte replacement in radiation bone marrow chimeras. The replacement of various leukocyte populations in B6.CD45.2 mice was analyzed 4 weeks after 10-Gy radiation and congenic bone marrow transplantation from B6.CD45.1 bone marrow donors. (A) Approximately 90% of intrahepatic leukocytes were replaced by donor-type leukocytes. The remaining 10% were almost entirely TCR-positive (top left) and negative for CD45R/B220, CD11b, and MHC class II, indicating that they were non-Kupffer cells. (B) The origin of isolated intrahepatic macrophages was greater than 99% donor BM-derived, based on expression of CD11b and MHC class II (IAb). Numbers on plots are percentages of lymphocytes.
Kupffer cell turnover following bone marrow transplantation: analysis by immunohistology
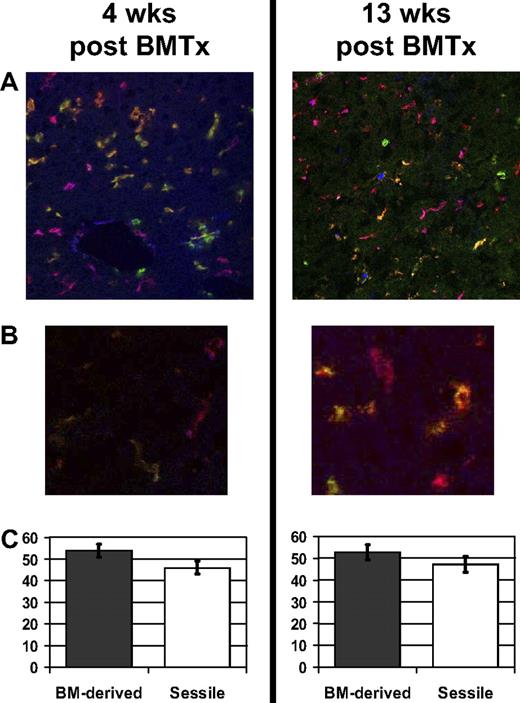
In order to validate the quantitative flow-cytometric results, frozen liver sections of bone marrow recipients were stained for immunohistologic analysis. To identify terminally differentiated KCs, the monoclonal antibody F4/80 was used together with the congenic markers for the bone marrow donor (CD45.2) and recipient (CD45.1) in a triple staining procedure for confocal immunohistology. Each analyzed group consisted of 3 to 5 animals; 10 sections were taken from each animal (400× magnification), and the experiments were repeated 3 or more times. Surprisingly, in contrast to the flow-cytometric results, a high percentage of F4/80-positive cells were of recipient origin (Figure 2A,B left panel, orange). Quantitative analysis revealed that 46% (± 3%) of the KCs in situ remained of recipient origin 4 weeks after bone marrow transplantation (a total of 1118 KCs were counted). This recipient-to-donor ratio remained stable for up to 13 weeks after bone marrow transplantation (Figure 2A,B right panel and 2C; a total of 769 KCs were counted).
Immunohistologic staining for Kupffer cells in radiation bone marrow chimeras. B6.CD45.2 recipient mice were irradiated (10 Gy) and reconstituted with B6.CD45.1 bone marrow. Frozen tissue sections were stained for macrophages (F4/80), CD45.1+ BM-derived cells, and CD45.2+ recipient-derived cells, revealing Kupffer cells derived from donor BM (purple-red), sessile recipient-type KCs (orange-green), BM-derived non-KC leukocytes (blue), and recipient-type non-KC leukocytes (green). (A) Liver tissue sections 4 weeks (left) and 13 weeks (right) after bone marrow transplantation, showing an equal distribution of BM-derived and sessile KCs. Original magnification, ×200. (B) Higher magnification of BM-derived and sessile KCs 4 weeks (left) and 13 weeks (right) after bone marrow transplantation. Original magnification, ×400. (C) Quantitative analysis of the 2 KC subsets revealed that 46% (± 3%) were sessile KCs of recipient origin. Error bars represent SEM.
Immunohistologic staining for Kupffer cells in radiation bone marrow chimeras. B6.CD45.2 recipient mice were irradiated (10 Gy) and reconstituted with B6.CD45.1 bone marrow. Frozen tissue sections were stained for macrophages (F4/80), CD45.1+ BM-derived cells, and CD45.2+ recipient-derived cells, revealing Kupffer cells derived from donor BM (purple-red), sessile recipient-type KCs (orange-green), BM-derived non-KC leukocytes (blue), and recipient-type non-KC leukocytes (green). (A) Liver tissue sections 4 weeks (left) and 13 weeks (right) after bone marrow transplantation, showing an equal distribution of BM-derived and sessile KCs. Original magnification, ×200. (B) Higher magnification of BM-derived and sessile KCs 4 weeks (left) and 13 weeks (right) after bone marrow transplantation. Original magnification, ×400. (C) Quantitative analysis of the 2 KC subsets revealed that 46% (± 3%) were sessile KCs of recipient origin. Error bars represent SEM.
The discrepancy between the immunohistology and the flow-cytometric data could possibly have been explained by the loss of the sessile KCs during the cell isolation process. To attempt to circumvent this, various modifications were made in the isolation method for intrahepatic nonparenchymal cells (NPCs), including the use of different density gradients (Lympholyte-M, Ficoll-Hypaque, and Percoll step density gradients). We also omitted the collagenase digestion step, replacing it by an entirely mechanical process of cell separation using a Stomacher device (Seward, Norfolk, United Kingdom). In another variant, we omitted the density gradient centrifugation steps, using direct fluorescence-activated cell sorter (FACS) sorting of the NPC suspension. These approaches did not reveal the sessile KC population that was clearly seen in situ by immunohistology. These data are not shown in detail, since they are exclusively negative (but see Figure 1 for typical examples). However, we think that the identification of sessile KCs is completely convincing because these cells (1) are abundant, intrasinusoidal cells with an elongated morphology, (2) express the molecules F4/80 and CD45, (3) are phagocytic, and (4) are eliminated by treatment with clodronate liposomes (see Figure 5). None of the other liver cell types, apart from KCs, share these attributes. The specificity of the CD45.1/CD45.2 KC staining is demonstrated in Figure S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article) for non–bone marrow chimeric control animals as well as other controls of the immunohistology staining protocol.
Functions of bone marrow–derived and sessile KCs
With in situ evidence for a mixed population of sessile, recipient-derived and bone marrow–derived KCs, we wanted to analyze the biologic function of the 2 different populations. The hallmark of macrophage function is phagocytosis of particles, debris, and pathogens followed by their processing and presentation as antigen, which is often accompanied by the release of inflammatory cytokines causing the activation or recruitment of other cells of the immune system.
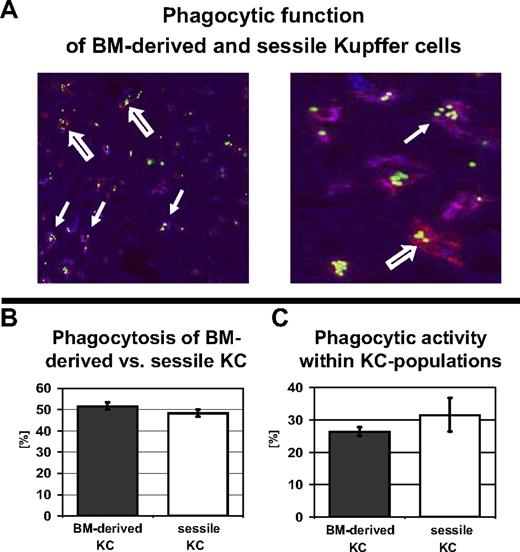
Phagocytosis was tested by intravenous injection of fluorescent carboxylate–modified microspheres, which are readily cleared from the circulation by terminally differentiated macrophages but can be detected within macrophages by immunohistology.16 To evaluate the functional capacity of the 2 KC populations, liver sections for immunohistologic staining were harvested 3 hours after injection of fluorescent latex beads and the percentage of bead-carrying bone marrow–derived KCs and sessile KCs was determined by quantitative analysis. Four weeks after bone marrow transplantation, 52% (± 1.7% SEM) of all bead-carrying KCs were bone marrow–derived, whereas 48% of the phagocytically active intrahepatic macrophages were sessile cells with a recipient phenotype (Figure 3A-B). There was no significant difference in the overall phagocytic activity of the 2 KC subsets; of all bone marrow–derived KCs, 26% (± 1.7% SEM) had engulfed fluorescent beads compared with 32% (± 5.3% SEM) of the sessile KCs (Figure 3C; a total of 492 Kupffer cells were counted).
Sessile and BM-derived KCs have equal phagocytic capacity. To evaluate the phagocytic capacity of BM-derived and sessile KCs, radiation bone marrow chimeras (10-Gy radiation dose) were injected with fluorescent beads, which underwent rapid phagocytosis by macrophages. (A, left) The distribution of fluorescent beads in BM-derived and sessile KCs was assessed by costaining with a fluorescent anti-CD45.1 mAb. Double-positive CD45.1 KCs appeared purple (closed arrow), whereas sessile (CD45.2+) KCs only stained with F4/80 (red, open arrow). Original magnification, ×200 (left). Higher magnification, ×400 (right). (B) Quantitative analysis of bead-carrying KCs revealed an almost equal distribution of phagocytic BM-derived and sessile KCs (52% ± 1.7% vs 48% ± 1.7%). (C) Phagocytic activity of BM-derived (26% ± 1.7%) versus sessile KCs (32% ± 5.3%) expressed as a fraction of the total KCs.
Sessile and BM-derived KCs have equal phagocytic capacity. To evaluate the phagocytic capacity of BM-derived and sessile KCs, radiation bone marrow chimeras (10-Gy radiation dose) were injected with fluorescent beads, which underwent rapid phagocytosis by macrophages. (A, left) The distribution of fluorescent beads in BM-derived and sessile KCs was assessed by costaining with a fluorescent anti-CD45.1 mAb. Double-positive CD45.1 KCs appeared purple (closed arrow), whereas sessile (CD45.2+) KCs only stained with F4/80 (red, open arrow). Original magnification, ×200 (left). Higher magnification, ×400 (right). (B) Quantitative analysis of bead-carrying KCs revealed an almost equal distribution of phagocytic BM-derived and sessile KCs (52% ± 1.7% vs 48% ± 1.7%). (C) Phagocytic activity of BM-derived (26% ± 1.7%) versus sessile KCs (32% ± 5.3%) expressed as a fraction of the total KCs.
Another important effector function of macrophages is the secretion of cytokines and chemokines, which often results in the recruitment of other inflammatory cells. The potential of the 2 KC populations to generate an inflammatory environment was tested using 2 different models of focal intrahepatic inflammation. Activated CD8+ T lymphocytes were generated in 2 different ways: first, using the systemic presentation of peptide antigen to adoptively transferred T-cell receptor transgenic OT-I cells; and second, using the extrahepatic activation of endogenous CD8+ T cells during pulmonary influenza infection. The first results in presentation of antigen by all cells, including KCs,17 whereas the second does not involve antigen presentation in the liver.13 Both experimental models resulted in intrahepatic formation of inflammatory foci, a process that is KC dependent.13,18
The participation of the 2 KC populations in the formation of intrahepatic inflammatory foci was analyzed by immunohistology 5 days after initial T-cell activation, when focus formation was at a maximum. Only bone marrow–derived KCs were found to engage in focus formation. Sessile KCs were found throughout the tissue sections comparable to previous experiments but were not detectable within the inflammatory foci (Figure 4A,B). This was confirmed using 2-color analysis of the same sections with only 1 congenic CD45 marker (Figure 4C,D) to eliminate overlap of the abundant bone marrow–derived CD45.1+ KCs.
Only BM-derived KCs participate in focal intrahepatic inflammation. Liver sections of bone marrow transplant recipients were stained with CD45.2-FITC (green), CD45.1-Cy5 (displayed in false color blue), and F4/80-PE (red). Inflammatory foci developed during peptide-activated intrahepatic accumulation of adoptively transferred TCR-tg OT-I cells (A-D) or with accumulation of endogenous CD8+ T cells during pulmonary influenza infection (E-H). (A,E) In both models, sessile KCs (orange, closed arrow) were only detectable outside the foci. Kupffer cells within the foci are all BM derived (purple) with multiple BM-derived and recipient-type non-KC leukocytes within the foci (blue and green). Original magnification, ×200. (B,F) Enlarged (×400) inflammatory focus following adoptive OT-I transfer and peptide activation (B) or during pulmonary influenza infection (F). (C,D,G,H) Two-color reproductions of the same foci with F4/80-CD45.1 costaining for BM-derived KCs (C,G) and F4/80-CD45.2 costaining for sessile KCs (D,H) confirmed the absence of sessile KCs within inflammatory foci.
Only BM-derived KCs participate in focal intrahepatic inflammation. Liver sections of bone marrow transplant recipients were stained with CD45.2-FITC (green), CD45.1-Cy5 (displayed in false color blue), and F4/80-PE (red). Inflammatory foci developed during peptide-activated intrahepatic accumulation of adoptively transferred TCR-tg OT-I cells (A-D) or with accumulation of endogenous CD8+ T cells during pulmonary influenza infection (E-H). (A,E) In both models, sessile KCs (orange, closed arrow) were only detectable outside the foci. Kupffer cells within the foci are all BM derived (purple) with multiple BM-derived and recipient-type non-KC leukocytes within the foci (blue and green). Original magnification, ×200. (B,F) Enlarged (×400) inflammatory focus following adoptive OT-I transfer and peptide activation (B) or during pulmonary influenza infection (F). (C,D,G,H) Two-color reproductions of the same foci with F4/80-CD45.1 costaining for BM-derived KCs (C,G) and F4/80-CD45.2 costaining for sessile KCs (D,H) confirmed the absence of sessile KCs within inflammatory foci.
To eliminate the possibility that local antigen expression within the liver contributed to the preferential recruitment of bone marrow–derived KCs, we used an infectious-inflammatory model of pulmonary influenza infection, which results in intrahepatic inflammatory lesions without any intrahepatic antigen presentation.13 Pulmonary influenza infection results in a large number of activated CD8+ T cells that also accumulate in the liver in the form of inflammatory foci. Comparable with the peptide-activation model, only bone marrow–derived KCs were recruited into the foci at the peak of focus formation 9 days after influenza infection (Figure 4E,F). Sessile KCs were only detectable outside the foci as demonstrated by separate 2-color analysis of the congenic CD45 markers on each KC population (Figure 4G,H).
Bone marrow–derived KCs that reside in the liver are recruited during inflammation
The most plausible explanation for the predominance of bone marrow–derived KCs within the inflammatory foci was that these cell derive directly from circulating monocytes, which are recruited into inflammatory sites before they differentiate into macrophages in situ. To test whether differentiated KCs that had already populated the liver actively migrated into inflammatory foci, we injected fluorescent latex beads into mice that were subsequently infected with influenza virus. While the latex beads were cleared from the circulation within several hours, focus formation in the liver did not occur until day 4 after infection. Thus, the presence of bead-carrying KCs within the hepatic inflammatory foci would document the participation of locally residing phagocytic KCs. Five days after intravenous injection of fluorescent latex beads and pulmonary influenza infection, bead-carrying KCs explicitly accumulated within the inflammatory foci (Figure 5A). Our preferred interpretation of this result is that the bone marrow (BM)–derived KCs were labeled in situ with the latex beads, and then during the influenza infection these cells migrated locally to contribute to the inflammatory focus. We cannot exclude the possibility that some of these KCs had in fact acquired their beads indirectly, for example through the phagocytosis of cell debris arising due to the apoptosis of other KCs, possibly including some sessile KCs. However, KC apoptosis is not a major feature of this mechanism of immunopathology. In a previous study we found that apoptosis during hepatic focus formation affected both the hepatocytes and the intrahepatic T-cell population, but there was no detectable KC apoptosis.13 Even if a subset of the KCs in the foci had acquired their beads indirectly, they nevertheless must have migrated locally from their normal dispersed configuration in the sinusoids to the dense KC aggregates in the foci.
Inflammatory BM-derived KCs were not directly recruited from the bone marrow. (A) Bone marrow chimeras were injected with fluorescent microspheres and then infected with influenza. Microspheres were immediately removed from the circulation by phagocytes, but focus formation within the livers did not occur until 5 days after infection. The accumulation of bead-carrying KCs indicated that KCs engaging in focus formation had already populated the liver several days earlier and were not derived from circulating monocytes. KCs (F4/80-Cy5) are displayed in false color blue, DAPI-stained nuclei are red, and fluorescent microspheres green. (B) To evaluate if the sessile KC population was derived from radioresistant intrahepatic precursor cells, hepatic KCs were entirely eliminated by clodronate liposome injection 3 weeks after bone marrow transplantation. Three weeks or 9 weeks later, sections from bone marrow chimeras were stained for BM-derived (CD45.1, blue) and sessile (CD45.2, green) KCs (F4/80, red). While recipient CD45.2 non-KC leukocytes (lymphocytes, granulocytes, etc) were abundant 3 and 9 weeks after clodronate injection, there were only BM-derived KCs in the liver sections of these animals; sessile KCs were not detectable. Original magnification, ×400.
Inflammatory BM-derived KCs were not directly recruited from the bone marrow. (A) Bone marrow chimeras were injected with fluorescent microspheres and then infected with influenza. Microspheres were immediately removed from the circulation by phagocytes, but focus formation within the livers did not occur until 5 days after infection. The accumulation of bead-carrying KCs indicated that KCs engaging in focus formation had already populated the liver several days earlier and were not derived from circulating monocytes. KCs (F4/80-Cy5) are displayed in false color blue, DAPI-stained nuclei are red, and fluorescent microspheres green. (B) To evaluate if the sessile KC population was derived from radioresistant intrahepatic precursor cells, hepatic KCs were entirely eliminated by clodronate liposome injection 3 weeks after bone marrow transplantation. Three weeks or 9 weeks later, sections from bone marrow chimeras were stained for BM-derived (CD45.1, blue) and sessile (CD45.2, green) KCs (F4/80, red). While recipient CD45.2 non-KC leukocytes (lymphocytes, granulocytes, etc) were abundant 3 and 9 weeks after clodronate injection, there were only BM-derived KCs in the liver sections of these animals; sessile KCs were not detectable. Original magnification, ×400.
Sessile KCs do not differentiate from local radioresistant precursors
Since the sessile KC population remained stable from 4 weeks after bone marrow transplantation for more than 12 weeks, we were interested in the origin of this KC population. To investigate whether sessile KCs derived from intrahepatic or extrahepatic precursor cells, or from already differentiated KCs, the intrahepatic macrophage population of radiation bone marrow chimeras was depleted by injection of dichloromethylene-bisphosphonate (clodronate) liposomes 3 to 4 weeks after bone marrow transplantation, a time point at which the number of sessile- and bone marrow–derived KCs was equal. Intravenous injection of 250 μL of clodronate-loaded liposomes resulted in complete depletion of macrophages from the livers of bone marrow chimeras within 36 hours after injection, based on F4/80 staining in frozen sections (data not shown). The repopulation by either sessile or bone marrow–derived KCs was analyzed by immunohistology, 3 and 9 weeks after KC depletion. As clodronate liposomes induced apoptosis only in differentiated macrophages capable of phagocytosis, potential hematopoietic precursor cells in the liver were spared and could have been a source of KC repopulation. However, at 3 and 9 weeks after KC depletion, only bone marrow–derived KCs were found in the livers of bone marrow chimeras (Figure 5B). Only 1% (± 0.45% SEM) of all the KCs stained double-positive for F4/80 and CD45.2, the congenic marker of the bone marrow recipient (a total of 1364 Kupffer cells were counted). However, these cells did not display diffuse F4/80 staining but instead a punctate pattern, suggesting that even these were not intact, host-derived sessile KCs but instead were bone marrow–derived KCs in the process of phagocytosis of a residual radioresistant cell such as a T lymphocyte. This experiment therefore argues that cell division of already differentiated cells, rather than the differentiation of intrahepatic precursors, was the source of the sessile KC population.
Sessile KCs in orthotopic liver transplantation
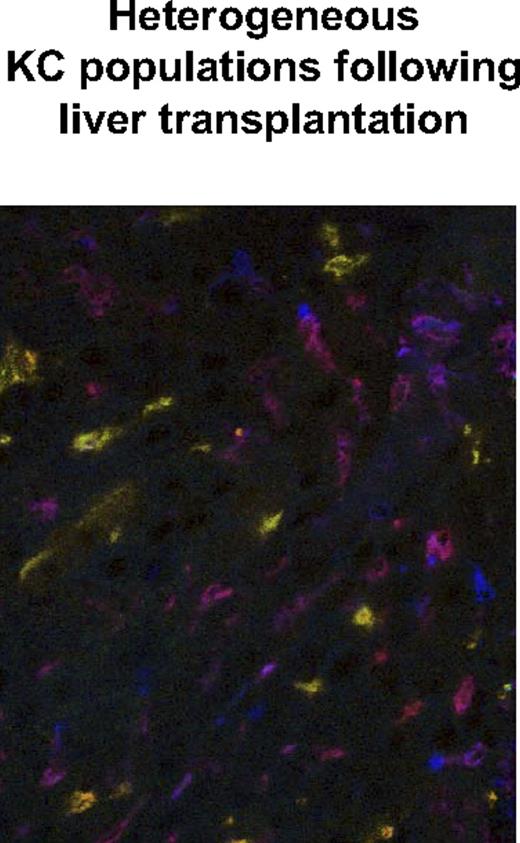
The discovery of distinct sessile and bone marrow–derived KC populations was based on experiments using bone marrow transplantation following sublethal irradiation. In order to determine whether the appearance of these 2 populations was due to an effect of radiation per se, we replaced the livers of CD45.1 recipients by liver grafts from congenic CD45.2 mice by orthotopic mouse liver transplantation. Four and 8 weeks after liver transplantation, the repopulation of the liver transplants by CD45.1− bone marrow–derived cells from the transplant recipient were analyzed by flow cytometry and immunohistology. Four weeks after orthotopic liver transplantation, flow-cytometric analysis of the intrahepatic leukocyte population revealed the almost-complete replacement of the isolated KC population by bone marrow–derived cells (99.93% ± 0.26%). This percentage remained unchanged in the late phase after liver transplantation (99.96% ± 0.23%). A relatively small population of residual TCR-positive cells was noted 4 and 8 weeks after liver transplantation (Figure 6; Table 2). For in situ analysis of the KC replacement, sections from the same animals were taken for immunohistologic staining. Similar to the immunohistologic results following bone marrow transplantation, a nearly equal distribution of bone marrow–derived and sessile KCs was observed 4 weeks after liver transplantation (49.4% of BM-derived KCs vs 50.6% ± 13.2% of sessile KCs; Figure 7; a total of 648 Kupffer cells were counted). The phagocytic activity in both populations evaluated by injection of fluorescent beads and confocal microscopy was found to be equal (52.1% of BM-derived KCs vs 48.9% ± 6.7% of sessile KCs; data not shown). This indicated the presence of 2 fully functional KC populations and confirmed the immunohistologic results in animals following bone marrow transplantation.
Flow-cytometric analysis of recipient leukocyte replacement in orthotopic liver transplant recipients. As an alternative approach to the analysis of KC turnover, livers from B6.CD45.2 animals were transplanted orthotopically into B6.CD45.1 recipients. By this experimental design, the bone marrow compartment of the recipient was spared from experimental manipulation. (A) The replacement of CD45.2 intrahepatic leukocytes by CD45.1 BM-derived cells was evaluated 30 and 60 days after liver transplantation by flow-cytometric analysis. Similar to bone marrow chimeras, CD11b/F4/80-positive KCs were all CD45.1+ in cell isolates from intrahepatic leukocytes. This indicated their origin from the bone marrow of the liver transplant recipient. (B) As seen in radiation bone marrow chimeras, a small percentage of CD45.2/TCR+ cells remained within the transplanted livers. Numbers in plots are percentages of lymphocytes.
Flow-cytometric analysis of recipient leukocyte replacement in orthotopic liver transplant recipients. As an alternative approach to the analysis of KC turnover, livers from B6.CD45.2 animals were transplanted orthotopically into B6.CD45.1 recipients. By this experimental design, the bone marrow compartment of the recipient was spared from experimental manipulation. (A) The replacement of CD45.2 intrahepatic leukocytes by CD45.1 BM-derived cells was evaluated 30 and 60 days after liver transplantation by flow-cytometric analysis. Similar to bone marrow chimeras, CD11b/F4/80-positive KCs were all CD45.1+ in cell isolates from intrahepatic leukocytes. This indicated their origin from the bone marrow of the liver transplant recipient. (B) As seen in radiation bone marrow chimeras, a small percentage of CD45.2/TCR+ cells remained within the transplanted livers. Numbers in plots are percentages of lymphocytes.
Immunohistologic staining of liver transplants reveals equal numbers of BM-derived and sessile KCs. Frozen tissue sections from liver transplants (B6.CD45.2 liver transplanted into B6.CD45.1 recipient) were stained for macrophages (F4/80 PE), CD45.1 Cy5 (displayed in false color blue) BM-derived cells, and CD45.2 FITC (green) liver transplant-derived cells. Equal numbers of BM-derived ‘KCs (purple-red) and sessile KCs (orange-green) were detected by confocal microscopy.
Immunohistologic staining of liver transplants reveals equal numbers of BM-derived and sessile KCs. Frozen tissue sections from liver transplants (B6.CD45.2 liver transplanted into B6.CD45.1 recipient) were stained for macrophages (F4/80 PE), CD45.1 Cy5 (displayed in false color blue) BM-derived cells, and CD45.2 FITC (green) liver transplant-derived cells. Equal numbers of BM-derived ‘KCs (purple-red) and sessile KCs (orange-green) were detected by confocal microscopy.
Increased expression of CD80 (B7–1) on bone marrow–derived KCs
The cell-surface phenotypes of the 2 KC populations were defined by immunohistologic staining for macrophage differentiation markers, cell-surface receptors, and costimulatory molecules. Coexpression of the different markers together with F4/80 and the congenic markers CD45.1 and CD45.2 was used to identify surface expression on either bone marrow–derived or sessile KCs (Table 3). There was no detectable difference in the expression of differentiation markers and homing receptors on the 2 KC subsets. Likewise, the expression of the endotoxin receptors CD14 and TLR4 was similar in both KC populations in naive animals. Interestingly, there was increased expression of the costimulatory molecule CD80 (B7-1) on bone marrow–derived KCs. However, this expression was not exclusive for these cells but was occasionally detectable on sessile KCs. This was not due to a generally higher level of the expression of costimulatory ligands on bone marrow–derived KCs, since CD86 (B7-2) expression was similar on the 2 KC subsets.
Discussion
The mononuclear phagocyte system (MPS) element of the innate immune system consists of several different populations of tissue macrophages, each of them with distinct functions based on the local particularities of the tissue type. As a common feature, all of the cells of the MPS initiate immune responses by the uptake, processing, and presentation of antigen, which mobilizes other leukocytes, both of the innate and adaptive immune system. Therefore macrophages act as sentinels of the immune system in tissues that form a barrier to the extracorporeal environment. Kupffer cells in the liver are constantly exposed to harmless food antigens and bacterial products of the commensal gut bacteria but also to bacterial and viral pathogens and parasites. Several phenomena in liver immunobiology, most notably the observation of oral and portal venous tolerance, illustrate that antigen processing and presentation within the liver does not always result in inflammatory responses. Although it is generally accepted that the MPS is of hematopoietic origin, it is unclear whether macrophage populations are maintained by infiltration of circulating monocytes directly from the bone marrow19,20 or by proliferation of local precursor cells or cell division of differentiated cells.21-23 The hypothesis that KCs are derived from the local proliferation of a largely self-renewing population of precursor cells came from studies in injury models, such as partial hepatectomy. Here, there was extensive mitosis of KCs during the regeneration phase.24 The importance of local self-renewal in KC turnover was also argued on the basis that cells intermediate between monocytes and KCs were difficult to find in the liver,25 but contested on the basis that 3H-thymidine labeling of KCs during the reconstitution of irradiated hosts was closely linked to the frequency of 3H-thymidine–labeled blood monocytes. The increase in 3H-thymidine labeling in KCs in X-irradiated mice in which the hind limbs had been shielded argued for a bone marrow origin of these cells, at least during recovery from radiation.8,26
Support for the local precursor model came from a series of studies in which strontium-89 (89Sr) was used to selectively irradiate the bone marrow and cause severe leucopenia in vivo. In these experiments, local proliferation of KCs was detected using autoradiography with 3H-thymidine.27 Under these conditions, both intravascular and intrahepatic myeloid precursor activity was severely depleted, arguing that the KCs arose from cells that were not detectable in a myeloid precursor assay, supporting the case for a distinctive local cell population with the capacity for self-renewal.28 These experiments inferred the lack of bone marrow precursors principally from the toxic effects of 89Sr and did not directly trace precursor-product relationships. More recently, the ROSA26 transgenic mouse, which expressed β-galactosidase in all tissues, was used as the donor in bone marrow transplantation experiments to determine the origin of different macrophage populations.29 The kinetics of replacement was most rapid for splenic macrophages and much slower for microglia and KCs. In particular, the KCs appeared to be only 50% derived from the bone marrow donor, 1 year after irradiation reconstitution. However, these experiments did not achieve an exact minor transplantation match between the donor and recipient, and the detection of donor cells was strictly dependent on the expression of the transgene. Thus, it is possible that donor-derived cells were underdetected due to variable transgene expression or that KC turnover was complicated by a low-level immune response to minor transplantation antigens and/or the ROSA26 marker. The latter issue can only be avoided in syngeneic or congenic cell tracing experiments. When bone marrow cells labeled with the fluorescent dye PKH26, but genetically identical to the host, were infused into intact mice, the donor cells expressed markers characteristic of KCs in a few days, and some of the changes in cell-surface markers were evident in 4 hours.30 This supports the hypothesis that KCs are derived from an immediate hematogenous precursor.
An alternative approach to determine the hematogenous origin of a cell population was parabiosis. Such an experiment used rats in which dividing cells had been labeled in vivo with 3H-thymidine, surgically joined by a skin bridge and vascular anastomoses to rats that had been saturated with unlabeled thymidine. The appearance of 3H-thymidine–labeled KCs in the nonlabeled parabiont was used to estimate a 12.4-day half-life for these cells, based on replacement via the circulation.31
The issue of KC replacement for hematogenous sources has also been addressed in liver transplant recipients. In allogeneic liver transplantation in rats, the replacement of donor by host KCs was not seen during the early postoperative period. However, such replacement increased during a rejection crisis following withdrawal of cyclosporine A immunosuppressive treatment and was extensive in stable allografts, based on staining for host MHC class II antigens.32 Similarly, in human liver allografts using either Y-chromosome sequences or microsatellite analysis, the KC population was found to be chimeric, with an increase in recipient KCs over several hundred days.33,34 The interpretation of these studies as models of normal KC turnover is complicated, since liver allografts elicit an immune response often followed by apoptosis of the infiltrating T cells, leading to “liver tolerance” and a stable allograft.35,36 A further complication is that transplant recipients are usually treated with immunosuppression to prevent rejection of the graft.37 The influence of any of these events on KC dynamics is hard to predict.
An extensive literature supports each of the 2 models for the origin on KCs. In the present analysis of this problem, we used both bone marrow transplantation and liver transplantation to evaluate KC turnover. To avoid the problems of inconsistent expression of transgenic cell tracers, we used the difference between the 2 allelic variants of CD45 (formerly known as Ly-5). This molecule is an abundant cell-surface protein expressed on all bone marrow–derived cells, and the difference between the CD45.1 and CD45.2 molecules elicits no immune response. In both the irradiation and the transplantation model, flow-cytometric analysis of liver leukocytes suggested that KCs are essentially completely (99%) replaced from a hematogenous source within 4 weeks, but this conclusion was misleading. Instead, analysis by immunohistology revealed distinct populations of bone marrow–derived and sessile KCs. This strongly supports the conclusions of many studies over the past 30 years but argues against the conclusions of many others.
Our experiments suggest that a population of sessile KCs is distinct from the bone marrow–derived KCs in terms of their turnover in response to irradiation and reconstitution, in terms of local migration to participate in inflammatory foci, and in their expression of CD80. However, we failed to detect any evidence that these cells arise from a local, nonphagocytic, radioresistant precursor cell, since the persistence of sessile KCs was disrupted after eradication of differentiated intrahepatic macrophages using clodronate liposomes. What, then, is the most likely origin of these cells? The data are fully consistent with a single-lineage model, in which the sessile KCs are ultimately derived from hematogenous progenitors but have acquired both a long lifespan and a resistance to irradiation in the liver environment compared with other tissues. In the future it will be important to explore the microanatomy, toxicology, and role in host defense of this distinctive KC population.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health grant numbers AI063353 and AI054517 (I.N.C.), AJ021970 (D.J.T.), and AI055888 and AI064463 (R.H.P.) and the German Research Foundation (DFG) grant number KL1403/2-1 (I.K.).
National Institutes of Health
Authorship
Contribution: I.K. designed and performed the research, collected and analyzed the data, and wrote the paper. J.C.C., N.K.P., B.J., and S.A.W. designed and performed the research and collected and analyzed data. D.J.T. and R.H.P. designed the research and collected and analyzed data. I.N.C. designed the research, analyzed the data, and cowrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ingo Klein, Department of General Surgery, University Hospital Wuerzburg, Oberduerrbacher Str 6, D-97080 Wuerzburg, Germany; e-mail: klein_i@chirurgie.uni-wuerzburg.de.