The Wiskott-Aldrich syndrome (WAS) is characterized by defective cytoskeletal dynamics affecting multiple immune cell lineages, and leading to immunodeficiency and autoimmunity. The contribution of dendritic cell (DC) dysfunction to the immune dysregulation has not been defined, although both immature and mature WAS knockout (KO) DCs exhibit significant abnormalities of chemotaxis and migration. To exclude environmental confounders as a result of WAS protein (WASp) deficiency, we studied migration and priming activity of WAS KO DCs in vivo after adoptive transfer into wild-type recipient mice. Homing to draining lymph nodes was reduced and WAS KO DCs failed to localize efficiently in T-cell areas. Priming of both CD4+ and CD8+ T lymphocytes by WAS KO DCs preloaded with antigen was significantly decreased. At low doses of antigen, activation of preprimed wild-type CD4+ T lymphocytes by WAS KO DCs in vitro was also abrogated, suggesting that there is a threshold-dependent impairment even if successful DC–T cell colocalization is achieved. Our data indicate that intrinsic DC dysfunction due to WASp deficiency directly impairs the T-cell priming response in vivo, most likely as a result of inefficient migration, but also possibly influenced by suboptimal DC-mediated cognate interaction.
Introduction
Dendritic cells (DCs) are professional antigen-presenting cells that survey tissues for foreign antigen, which is normally transported for presentation in a recognizable processed configuration to T cells in secondary lymphoid tissue.1 A similar process may participate in the maintenance of peripheral tolerance to self-antigens.1 Both the spatial and temporal distribution of DCs crucially impact the efficacy of immune reactions. DCs need to home via lymphatics to the draining lymphoid tissue to engage T lymphocytes. This is controlled in part by changes in the responsiveness of DCs to combinations of chemokines and by alteration in motile characteristics. After antigen uptake, and following stimulation from inflammatory mediators, DCs up-regulate costimulatory molecules and change their chemokine receptor expression profile.1,2 As a result, they express molecules such as CCR7, which enables migration via CCL21-expressing lymphatic vessels. In the draining lymphoid tissue, both CCL21 and CCL19 are expressed specifically in T-cell areas, thereby guiding the delivery of incoming DCs to immunologically relevant locations.3,–5 Within the T-cell areas, DCs make repeated contacts with T cells, some of which are sustained, presumably reflecting the successful assembly of a cognate immunologic synapse.6,7 As well as immunophenotypic differentiation, the maturation of DCs is associated with gross changes in the actin cytoskeleton—including an increase in cell motility—and down-regulation of specialized adhesion structures known as podosomes that are presumed to play an important role in early translocation.8 This whole process is complex, and requires coordinated regulation to achieve safe and efficient intercellular interaction for normal immunologic homeostasis and effective response to infection
The Wiskott-Aldrich syndrome (WAS) is a rare X-linked immunodeficiency disorder that is characterized by microthrombocytopenia, eczema, recurrent infections, and increased risk of autoimmunity and malignancies.9,10 WAS is caused by mutations in the gene encoding the Wiskott-Aldrich syndrome protein (WASp) that is exclusively expressed in nonerythroid hematopoietic cells. WASp is a key member of a family of proteins that plays an important role in transferring signals from cell surface receptors to the actin cytoskeleton by activating Arp2/3-mediated actin polymerization.9,10 Lack of WASp expression results in a wide range of cellular defects including impaired T-cell proliferation, reduced T-cell receptor capping, impaired macrophage phagocytosis, and impaired immune cell migration.10,11 Migration of multiple immune cell lineages including lymphocytes and myeloid cells has been shown to be defective in both human and murine models of WASp deficiency.12,,–15 These abnormalities have been variously ascribed to processes of chemotaxis, chemokinesis, and adhesion through specialized structures such as podosomes.16,,–19
The contribution of individual cell lineages to the immunodeficiency of WAS remains unclear and has been difficult to dissect due to the panhematopoietic expression pattern of WASp. Here, we examined migration of WAS knockout (KO) DCs in vivo and their impact on the activation of T cells. To exclude environmental confounders as a result of WASp deficiency in other cells, all experiments were conducted using wild-type C57BL/6 recipient mice. Our results suggest that dysfunction of WAS KO DCs is associated with a reduced capacity to prime CD4+ and CD8+ T cells.
Materials and methods
Mice
WAS KO mice that were backcrossed at least 10 times with C57BL/6 were kindly provided by Dr T. Strom (Memphis VA Medical Center, Memphis, TN) and bred in our own facilities. Control wild-type C57BL/6 mice were obtained from Charles River (Kent, United Kingdom). OT-I/Rag1−/− mice, expressing the ovalbumin (OVA) peptide257-264 (SIINFEKL) T-cell receptor, were kindly provided by Dr G. Bendle (Royal Free Hospital, UCL, London, United Kingdom). Mice were used at 6 to 12 weeks of age, and all experiments were approved by and performed according to Home Office Animal Welfare Legislation.
Cells
Mice were killed with CO2 and bone marrow was extracted from femora. DCs were cultured from bone marrow cells in RPMI medium 1640 supplemented with 10% fetal bovine serum, 100 units/mL penicillin, and 100 μg/mL streptomycin (Gibco, Paisley, United Kingdom) in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF, 20 ng/mL; BioSource, Nivelles, Belgium) for 7 days and, for some experiments, were matured overnight with LPS (100 ng/mL; Sigma, Steinheim, Germany). For in vivo migration studies, the matured cells were labeled for 15 minutes with CFSE (1 μM; Molecular Probes, Leiden, the Netherlands). For T-cell priming experiments, the immature cells were pulsed overnight with ovalbumin (OVA, 100 μg/mL; Sigma) and LPS. CD4+ T cells were isolated from spleen using magnetic bead separation (Miltenyi Biotech, Bergisch Gladbach, Germany) according to manufacturer's instructions. OT-I cells were isolated from spleen after lysing of the erythrocytes and made into a single-cell suspension by application through a 70-μm cell strainer (BD Falcon, Erembodegem, Belgium). As the OT-I mice were on a Rag1−/− background, no further purification of CD8+ T cells was required. RMA-S cells were kindly provided by Dr A. McNicol (Institute of Child Health, UCL, London, United Kingdom).
In vitro migration
For transwell migration, transwells of 6.5 mm diameter, with 5-μm pore filters (24 wells; Costar, Corning, NY) were coated with fibronectin (10 μg/mL; Sigma) for at least 2 hours at 37°C. DCs (1 × 105 cells) were added to the upper compartment of the transwell and CCL3 or CCL21 (both 100 ng/mL; Peprotech, London, United Kingdom) added to the lower compartment. After incubation for 90 minutes at 37°C, the cells from the lower compartment were collected, flowcount beads (Beckman Coulter, High Wycombe, United Kingdom) added for quantification, and the cells counted on a Cyan cytometer (Dako Cytomation, Glostrup, Denmark). The assay was performed in duplicate, and negative (no chemokine in lower compartment) and chemokinesis (chemokine in both compartments) controls were included. For migration with Dunn chambers (Hawksley, Lancing, United Kingdom), DCs (25 × 103) were allowed to adhere for at least 1 hour to fibronectin-coated coverslips at 37°C. Inner wells of the Dunn chambers were filled with culture medium without chemokine and outer wells, with chemokine (CCL3 or CCL21). Dunn chambers were maintained at 37°C on a microscope stage of an inverted microscope (Zeiss Axiovert 135; Zeiss, Hertfordshire, United Kingdom) and cell motility recorded using a 10×/0.25 NA lens (Zeiss). Images were acquired every 10 minutes over a 5-hour period using a Hamamatsu digital camera (C4742-95) and Openlab 4.04 software (Improvision, Coventry, United Kingdom). Images were analyzed using Volocity 4.0 software (Improvision). Migration was determined by tracing individual cells. For spreading, the area and perimeter of individual cells were recorded by manually selecting the outline of the cells, and data are shown as an index of area divided by perimeter.
In vivo migration
DCs were prepared and labeled with CFSE as described above. DCs (2 × 106) were injected subcutaneously in the tail base and the draining inguinal lymph node was harvested at the indicated times. Lymph nodes were then either made into a single-cell suspension by applying them over a 70-μm cell strainer or embedded in Tissue-Tek OCT compound (Sakura, Zoeterwoude, the Netherlands) and subsequently snap-frozen in liquid nitrogen and stored at −80°C until further use. Lymph node single-cell suspensions were stained with APC-conjugated anti-CD11c (HL3; BD Pharmingen, Erembodegem, Belgium) and analyzed on a Cyan cytometer with Summit 4.3 software (Dako Cytomation). To visualize localization of the CFSE+ DCs in 3D, whole lymph nodes were visualized with a Leica TCS SP2 MP multiphoton microscope (Leica, Milton Keynes, United Kingdom) fitted with a Tsunami pulsed laser (Spectra Physics, Didcot, United Kingdom) using excitation wavelength of 850 nm. Images were acquired using a 40×/0.8 NA water-immersed objective lens (Leica) and processed using Volocity 4.0 software (Improvision).
Immunohistochemistry
Cryostat sections of 7-μm thickness were cut on poly-l-lysine–coated slides (VWR, Leuven, Belgium) and fixed for 20 minutes with 1% paraformaldehyde (BDH, Poole, United Kingdom) in PBS and rinsed in PBS. Slides were blocked with 2% normal mouse serum (Dako Cytomation), and then incubated with avidin and biotin (Vector Laboratories, Burlingame, CA) to block endogenous biotin followed by incubation for at least one hour with primary antibodies specific for CD11c (biotinylated N418; eBioscience, San Diego, CA), CD3 (biotinylated 145-2C11; BD Pharmingen), or B220 (RA3-6B2; BD Pharmingen). Subsequently, slides were washed thoroughly with PBS and incubated with Alexa Fluor 555–conjugated streptavidin (Molecular Probes) or Cy5-conjugated F(ab′)2 fragment mouse anti–rat IgG (Jackson Immunoresearch, West Grove, PA) for 30 to 45 minutes. After thoroughly washing in PBS, the slides were mounted in DAPI-containing vectashield (Vector Laboratories) and examined using a laser scanning spectral confocal microscope (TCS SP2; Leica). Images were processed with Adobe Photoshop software for publication (Adobe Systems, San Jose, CA). Quantification was performed on images using ImageJ software (version 1.37v; NIH, http://rsb.info.nih.gov/ij/). T-cell areas were arbitrarily divided into 50-μm-wide sections; the number of CFSE+ DCs per section was counted and calculated as percentage of total CFSE+ DCs.
T-cell priming
DCs were generated and primed with OVA as described above. OVA-pulsed DCs (2 × 106) were injected subcutaneously in the tail base of wild-type C57BL/6 mice and the draining lymph nodes (inguinal) harvested after 7 days. For CD4+ T-cell experiments, the CD4+ T cells were isolated using magnetic bead separation (negative selection; Miltenyi Biotech) and cocultured with OVA-pulsed DCs for 3 days. For the last 16 hours of culture, 3H-thymidine (0.5 μCi [0.0185 MBq]; GE Healthcare, Buckinghamshire, United Kingdom) was added and plates were harvested using a 96-well plate harvester (Tomtec, Hamden, CT). Proliferation was assessed as 3H-thymidine incorporation and measured using a betaplate liquid scintillation counter (Perkin Elmer, Bucks, United Kingdom). To determine in vitro T-cell priming, OVA-pulsed DCs were cocultured with CD4+ T cells isolated from wild-type C57BL/6 mice that were immunized with OVA prior to the in vitro proliferation. Briefly, OVA (final concentration, 1 μg/mL) was emulsified with complete Freund adjuvant (1:1) and 50 μL injected at both left and right side of the tail base. After 7 days, the inguinal lymph nodes were harvested and CD4+ T cells isolated as described above. The isolated CD4+ T cells were cocultured with OVA-pulsed DCs and proliferation was measured. Data are averages of triplicate values and presented as an index relative to the medium control or as counts per minute (cpm).
For priming of CD8+ T cells, wild-type C57BL/6 mice were subcutaneously injected with OVA-pulsed DCs (2 × 106) and the spleen and draining lymph nodes harvested at the indicated time points. Lymph node and spleen were made into single-cell suspensions and stained with FITC-conjugated CD3 (BD Pharmingen), PerCP-conjugated CD8 (BD Pharmingen), and APC-conjugated pentamers specific for the OVA peptide257-264 (SIINFEKL) T-cell receptor (Proimmune, Oxford, United Kingdom). To study the IFN-γ production, lymph node and spleen cells were cocultured with SIINFEKL peptide (2 μM; Proimmune) and RMA-S cells in the presence of brefeldin A (5 μg/mL; Sigma). The RMA-S cells were incubated overnight at 26°C prior to the experiment to establish expression of empty MHC class I molecules on the cell surface,20 which during the culture with lymph node or spleen cells will present the SIINFEKL peptide. After 4 hours, the cells were stained with FITC-conjugated CD3 and PerCP-conjugated CD8, and then permeabilized (BD Perm/Wash; BD Pharmingen) and stained with PE-conjugated IFN-γ (eBioscience). The cells were analyzed on a Cyan flow cytometer. For priming of OT-I cells, spleen was isolated from OT-I/Rag1−/− mice and made into single-cell suspension. OT-I cells were labeled with CFSE (1 μM) and 2 × 106 cells injected intravenously into wild-type C57BL/6 recipients one day prior to injection of OVA-pulsed DCs. Four days after DC administration, draining lymph nodes and spleen were isolated and the cells stained with PE-conjugated CD3 and PerCP-conjugated CD8. Cells were analyzed with a Cyan flow cytometer and data expressed as percentage of CD8+ T cells.
Results
Impaired migration and polarization in vitro
We and others have shown decreased migration of mature murine bone marrow–derived WAS KO DCs (Sv129SvEv background) in vitro in response to CCL19 or CCL21.12,14 As we wanted to use WAS KO mice on a C57BL/6 background for immunologic studies, we verified whether DC migration was also defective in this context. In addition, we wanted to determine whether chemotaxis or chemokinesis was compromised in immature DCs, as there are significant changes in the organization of the actin cytoskeleton following maturation.8 In transwell systems, chemotaxis of mature WAS KO DCs to CCL21 was abrogated as shown previously (data not shown). Similarly, chemotaxis of immature WAS KO DCs to CCL3 was significantly reduced compared with control C57BL/6 DCs (Figure 1A). To assess the migration defects in more detail, immature DCs were visualized individually in a Dunn chamber using video microscopy. The chemokinetic response of immature WAS KO DCs to CCL3 (activation resulting in increased motility and consequently increased migration) was also significantly reduced, although no difference was observed in chemokine receptor expression (Figure 1B and data not shown, respectively). Furthermore, in contrast to control DCs, WAS KO DCs failed to form persistent leading edges and did not spread normally, resulting in reduced translocation and seemingly random movement around their point of origin (Figure 1C; Video S1A,B, available on the Blood website; see the Supplemental Materials link at the top of the online article). These findings demonstrate significant motility and migratory defects in both immature and mature DCs of C57BL/6 WAS KO mice.
Decreased in vitro migration of WAS KO DCs in vitro. (A) Chemotaxis of immature WAS KO DCs toward CCL3 was reduced when assessed with transwells; c-CCL3 indicates chemokine control (chemokine in both upper and lower compartment). (B) Similarly using Dunn chambers, WAS KO show reduced DC motility. Chemokine receptor engagement does increase motility of WAS KO DCs, but to a lesser degree. (C) Cytoskeletal rearrangements measured as an index of the area and perimeter of individual cells is impaired in WAS KO DCs. Transwell migration (A) shows average plus or minus SEM of 4 mice per group and Dunn chamber migration; (B) averages plus or minus SEM of at least 10 cells responding to medium and at least 40 cells responding to CCL3 from 2 independent experiments are shown. Spreading (C) is shown as averages plus or minus SEM of at least 13 cells from both strains.
Decreased in vitro migration of WAS KO DCs in vitro. (A) Chemotaxis of immature WAS KO DCs toward CCL3 was reduced when assessed with transwells; c-CCL3 indicates chemokine control (chemokine in both upper and lower compartment). (B) Similarly using Dunn chambers, WAS KO show reduced DC motility. Chemokine receptor engagement does increase motility of WAS KO DCs, but to a lesser degree. (C) Cytoskeletal rearrangements measured as an index of the area and perimeter of individual cells is impaired in WAS KO DCs. Transwell migration (A) shows average plus or minus SEM of 4 mice per group and Dunn chamber migration; (B) averages plus or minus SEM of at least 10 cells responding to medium and at least 40 cells responding to CCL3 from 2 independent experiments are shown. Spreading (C) is shown as averages plus or minus SEM of at least 13 cells from both strains.
Impaired migration and localization in vivo
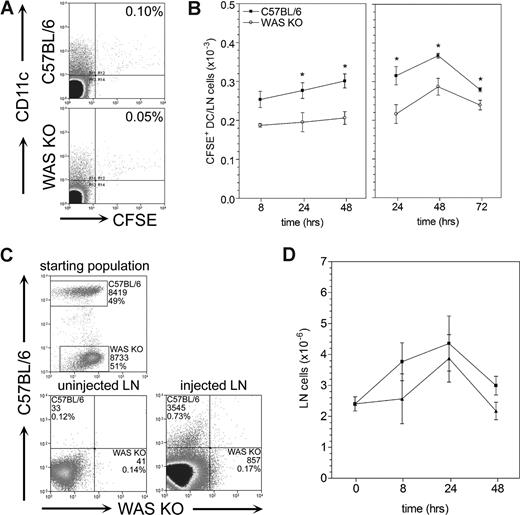
Migration of Langerhans cells to draining lymph nodes has been shown to be compromised in WAS KO mice after FITC skin painting.12,14 To avoid potential confounding influences on this process from defects in other cell lineages or secondary abnormalities in lymphoreticular architecture, we subcutaneously injected fluorescently labeled mature DCs into control C57BL/6 mice. DCs from either C57BL/6 or WAS KO mice showed similar expression of chemokine receptors and maturation markers (data not shown and Figure S1, respectively). We analyzed the draining lymph node for the number and localization of the migrating DCs. As expected, WAS KO DCs showed a decreased migratory efficiency compared with control C57BL/6 DCs (Figure 2A,B). Priming of the injection site with TNF-α 24 hours prior to injection of DCs increased the number of migrating cells in both C57BL/6 and WAS KO mice, although the level of migration of WAS KO DCs remained significantly lower compared with control C57BL/6 DCs (Figure 2B). Similarly, when WAS KO and control C57BL/6 DCs were labeled with different fluorescent dyes and mixed 1:1 before injection into C57BL/6 recipients, the number of WAS KO DCs that reached the draining lymph node after 24 hours was significantly reduced compared with that of C57BL/6 DCs (Figure 2C). It has previously been reported that a general increase in lymph node cellularity occurs after administration of migrating DCs.21,22 In mice receiving WAS KO DCs, the increase was reduced, although this did not reach significance (Figure 2D).
Decreased in vivo migration of WAS KO DCs compared with C57BL/6 DCs. DCs were CFSE labeled in vitro and injected subcutaneously into wild-type C57BL/6 recipients. DC migration was assessed by quantifying the number of CFSE+ DCs in the draining lymph nodes. (A) WAS KO DCs showed a decreased ability to migrate to the draining lymph nodes. (B) Quantification showed that both in unprimed and TNF-α–primed mice, WAS KO DCs have decreased migration in vivo. (C) Injection of a 1:1 mixture of C57BL/6 CFSE+ DCs and WAS KO DiI+ DCs confirmed that migration of WAS KO DCs was reduced compared with C57BL/6 DCs. (D) A similar increase in lymph node cellularity induced by migration was observed for both WAS KO and C57BL/6 DCs. Fluorescence-activated cell sorting (FACS) plots shown in panel A are after 24 hours and representative of 6 mice. Data shown in panels B and D are averages plus or minus SEM of 2 to 6 mice per group, *P < .05. Data shown in panel C are representative of 2 mice.
Decreased in vivo migration of WAS KO DCs compared with C57BL/6 DCs. DCs were CFSE labeled in vitro and injected subcutaneously into wild-type C57BL/6 recipients. DC migration was assessed by quantifying the number of CFSE+ DCs in the draining lymph nodes. (A) WAS KO DCs showed a decreased ability to migrate to the draining lymph nodes. (B) Quantification showed that both in unprimed and TNF-α–primed mice, WAS KO DCs have decreased migration in vivo. (C) Injection of a 1:1 mixture of C57BL/6 CFSE+ DCs and WAS KO DiI+ DCs confirmed that migration of WAS KO DCs was reduced compared with C57BL/6 DCs. (D) A similar increase in lymph node cellularity induced by migration was observed for both WAS KO and C57BL/6 DCs. Fluorescence-activated cell sorting (FACS) plots shown in panel A are after 24 hours and representative of 6 mice. Data shown in panels B and D are averages plus or minus SEM of 2 to 6 mice per group, *P < .05. Data shown in panel C are representative of 2 mice.
Immunohistochemical analysis of the localization of the migrated DCs revealed that WAS KO DCs did not efficiently migrate into the T-cell areas of the lymph node. Most WAS KO DCs seemed to remain in the subcapsular region and in the conduit system of the lymph node, whereas control C57BL/6 DCs penetrated the T-cell area (Figure 3A). This was quantified by arbitrarily dividing the T-cell area into 50-μm-wide sections. While C57BL/6 DCs were found as deep as 150 to 200 μm in the T-cell area, most WAS KO DCs were found in the first 50 μm and just a few managed to reach 50 to 100 μm (Figure 3B). We next analyzed the DCs that entered the lymph nodes using multiphoton laser microscopy. Video S2 shows the 3D reconstruction of a 50-μm lymph node section 24 hours after injection of CFSE+ C57BL/6 (Video S2A) or WAS KO DCs (Video S2B). Again, we observed that WAS KO DCs failed to migrate efficiently beyond the subcapsular region, while C57BL/6 DCs were found deeper in the lymph node at an equivalent time point.
Decreased in vivo migration and localization of WAS KO DCs. (A) Immunofluorescent analysis of draining lymph nodes after in vivo migration showed that the WAS KO DCs that reached the lymph nodes did not penetrate into the T-cell area as deeply as C57BL/6 DCs. (B) Quantification of DC localization showed that C57BL/6 DCs penetrated deeper into the T-cell area, compared with WAS KO DCs. Pictures in panel A are representative of 4 mice and of at least 2 independent experiments. CFSE+ DCs are green; T cells, red; and B cells, blue. Arrows point to cells that have migrated into the T-cell area. Scale bars are 75 mm. Data shown in panel B are averages plus or minus SEM of 2 to 4 mice per group.
Decreased in vivo migration and localization of WAS KO DCs. (A) Immunofluorescent analysis of draining lymph nodes after in vivo migration showed that the WAS KO DCs that reached the lymph nodes did not penetrate into the T-cell area as deeply as C57BL/6 DCs. (B) Quantification of DC localization showed that C57BL/6 DCs penetrated deeper into the T-cell area, compared with WAS KO DCs. Pictures in panel A are representative of 4 mice and of at least 2 independent experiments. CFSE+ DCs are green; T cells, red; and B cells, blue. Arrows point to cells that have migrated into the T-cell area. Scale bars are 75 mm. Data shown in panel B are averages plus or minus SEM of 2 to 4 mice per group.
Reduced priming of T lymphocytes in vivo
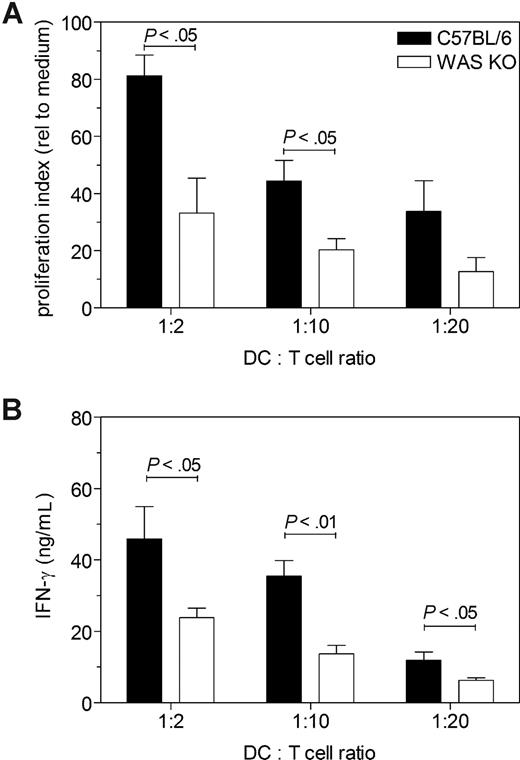
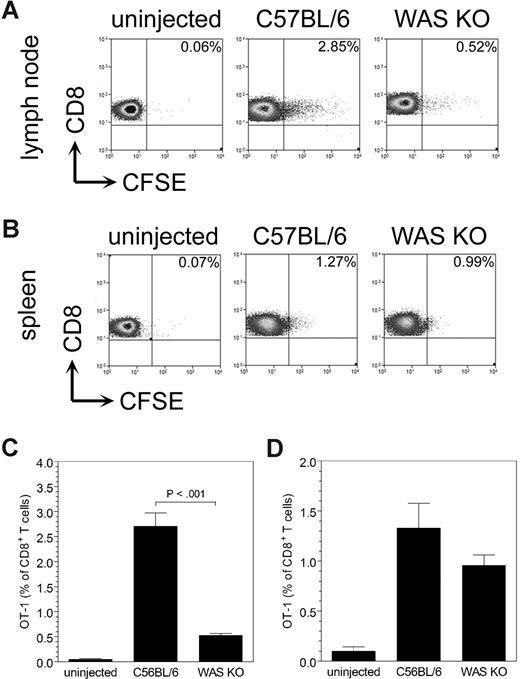
Because of the reduced numbers of WAS KO DCs that were able to reach the T-cell area of the lymph nodes, we hypothesized that this would affect T-cell priming. To address this, we loaded the DCs with ovalbumin (OVA) before injection, removed the lymph nodes after 7 days, and isolated the CD4+ T lymphocytes. The purified CD4+ T cells were then cocultured with OVA-pulsed control C57BL/6 DCs, and assessed for T-cell proliferation and IFN-γ production. Compared with the proliferation of CD4+ T cells induced by migrating C57BL/6 DCs, proliferation of WAS KO DC–primed CD4+ T cells was significantly reduced (Figure 4A). Furthermore, IFN-γ production by WAS KO DC–primed CD4+ T cells was decreased compared with that of CD4+ T cells that were primed by C57BL/6 DCs (Figure 4B). We also tested priming of CD8+ T cells, but as we could detect only a small number of OVA-specific CD8+ T cells primed by the migrating DCs (0.1%-0.3% of all CD8+ T cells, data not shown), we used a more sensitive method by injecting CFSE-labeled OT-I cells 24 hours prior to DC administration (OT-I T cells [H-2b] recognize the OVA peptide257-264 [SIINFEKL] in association with MHC class I). After 4 days, the draining lymph nodes and spleen were isolated and analyzed for CFSE+ OT-I cells. As shown in Figure 5, WAS KO DCs induced less proliferation of OT-I cells compared with C57BL/6 DCs. Both in the draining lymph node (Figure 5A,C) and in spleen (Figure 5B,D), we observed a reduced proliferation of OT-I CD8+ T cells when the mice received WAS KO DCs. These findings indicate that WAS KO DCs are compromised in their ability to prime both CD4+ and CD8+ T cells in vivo.
Decreased CD4+ T-cell priming in vivo by migrating WAS KO DCs. (A) Administration of OVA-pulsed WAS KO DCs primed less CD4+ T cells in vivo, as determined by in vitro proliferation and (B) IFN-γ production. Data in panel A are representative of 2 independent experiments with 3 mice each receiving C57BL/6 DC and 3 mice each receiving WAS KO DCs; averages plus or minus SEM are shown. Data in panel B represent the averages plus or minus SEM of 6 mice per group.
Decreased CD4+ T-cell priming in vivo by migrating WAS KO DCs. (A) Administration of OVA-pulsed WAS KO DCs primed less CD4+ T cells in vivo, as determined by in vitro proliferation and (B) IFN-γ production. Data in panel A are representative of 2 independent experiments with 3 mice each receiving C57BL/6 DC and 3 mice each receiving WAS KO DCs; averages plus or minus SEM are shown. Data in panel B represent the averages plus or minus SEM of 6 mice per group.
Decreased in vivo migration of WAS KO DCs induces reduced OT-1 proliferation. CD8+ OT-I T-cell proliferation was determined after injection of WAS KO or C57BL/6 DCs. In both the draining lymph node (A) and in spleen (B), a reduced proliferation of OT-I cells was observed when WAS KO was injected. Quantification of the data shows that in panel C lymph node a strong reduction is observed, while in panel D spleen the effect is much smaller. Data shown are representative of 3 mice in panels A,B, and averages plus or minus SD in panels C,D.
Decreased in vivo migration of WAS KO DCs induces reduced OT-1 proliferation. CD8+ OT-I T-cell proliferation was determined after injection of WAS KO or C57BL/6 DCs. In both the draining lymph node (A) and in spleen (B), a reduced proliferation of OT-I cells was observed when WAS KO was injected. Quantification of the data shows that in panel C lymph node a strong reduction is observed, while in panel D spleen the effect is much smaller. Data shown are representative of 3 mice in panels A,B, and averages plus or minus SD in panels C,D.
Impaired functionality of DC–T-cell engagement
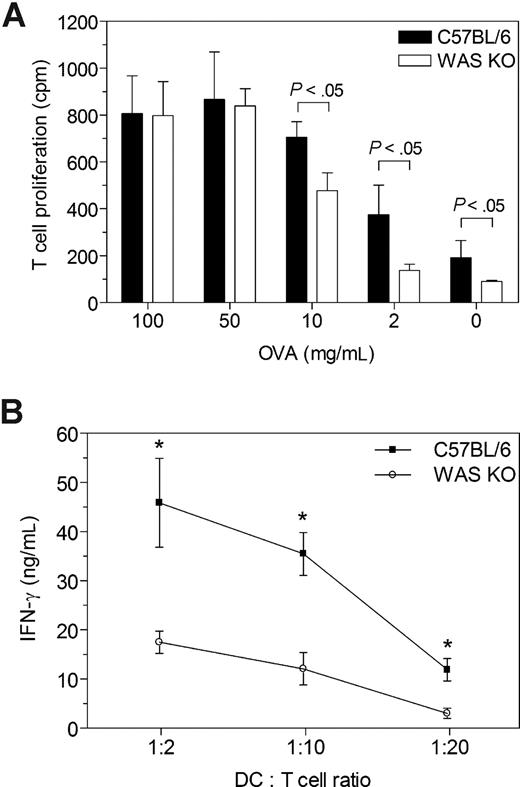
To assess whether the reduced priming capability was due solely to an impairment in migration and therefore colocalization of DC and T cells, we determined the in vitro ability of WAS KO DCs to stimulate T-cell proliferation. For this purpose, C57BL/6 mice were immunized with OVA in complete Freund adjuvant and 1 week later the CD4+ T cells harvested from the draining lymph nodes. These cells were then cocultured with WAS KO DCs or C57BL/6 DCs in the presence of OVA. No difference was observed in T-cell proliferation when high concentrations of OVA (50-100 μg/mL) were used, but using lower concentrations (2-10 μg/mL), WAS KO DCs were less efficient (Figure 6A). Similarly, WAS KO DCs were less able to stimulate IFN-γ production from purified CD4+ T cells that had been primed in vivo by OVA-pulsed control C57BL/6 DCs (Figure 6B). Therefore, WAS KO DCs exhibit defective T-cell stimulation in vitro, suggesting that in addition to likely abnormalities arising from mislocalization, there are defects of antigen presentation or functionality of DC–T-cell engagement.
Reduced priming capability of WAS KO DCs. (A) CD4+ T cells were isolated from OVA/CFA-immunized C57BL/6 mice and in vitro proliferation was determined after stimulation with OVA and DCs of either C57BL/6 or WAS KO mice. Similar T-cell stimulatory capacity was observed between the strains for the higher range of OVA, but with lower OVA concentrations, WAS KO DCs were less capable of inducing T-cell proliferation. (B) Ex vivo stimulation of CD4+ T cells, isolated from mice that were immunized with OVA-pulsed C57BL/6 DCs, showed that WAS KO DCs were less capable of inducing IFN-γ production, compared with C57BL/6 DCs. Data in panel A are representative of 2 independent experiments with DCs from 2 mice per group and pooled CD4+ T cells from 3 OVA/CFA-immunized C57BL/6 mice; averages plus or minus SD are shown. Data in panel B represent the averages plus or minus SEM of 6 mice per group.
Reduced priming capability of WAS KO DCs. (A) CD4+ T cells were isolated from OVA/CFA-immunized C57BL/6 mice and in vitro proliferation was determined after stimulation with OVA and DCs of either C57BL/6 or WAS KO mice. Similar T-cell stimulatory capacity was observed between the strains for the higher range of OVA, but with lower OVA concentrations, WAS KO DCs were less capable of inducing T-cell proliferation. (B) Ex vivo stimulation of CD4+ T cells, isolated from mice that were immunized with OVA-pulsed C57BL/6 DCs, showed that WAS KO DCs were less capable of inducing IFN-γ production, compared with C57BL/6 DCs. Data in panel A are representative of 2 independent experiments with DCs from 2 mice per group and pooled CD4+ T cells from 3 OVA/CFA-immunized C57BL/6 mice; averages plus or minus SD are shown. Data in panel B represent the averages plus or minus SEM of 6 mice per group.
Discussion
The involvement of WASp in dynamic reorganization of the cytoskeleton has led to the suggestion, supported by experimental evidence, that the trafficking capability of hematopoietic cells may be compromised.12,14,,–17,23,,–26 We have been interested in motility mechanisms of dendritic cells in particular, as regulated migration is assumed to play an important part of their response to antigenic stimuli and maintenance of normal immune homeostasis. In the context of WASp deficiency, previous studies have demonstrated impaired migration of mature DCs toward the CCR7 ligands CCL19 and CCL21 in vitro, which are responsible for normal trafficking of DCs via lymphatics to lymph nodes.12,14 Here, we have shown that immature murine WAS KO DCs also exhibit an abnormal chemotactic response to CCL3, which is important for mobilization to inflammatory sites. The cytoskeletal abnormalities resulted in an inability to form a persistent lamella or leading edge and to properly polarize or spread, as has been reported for DCs derived from WAS patients.16,17 In part, these features may relate to a lack of podosomes that normally form behind the leading edge of migrating cells and are thought to mediate adhesion to the substratum where they provide anchorage for further movement.17,18,27 Deficiencies of both chemotaxis and chemokinesis therefore contribute to the overall motility deficiencies of the DC lineage.
More importantly, impaired migration of WAS KO DCs in vivo was associated with defective CD4+ and CD8+ T-cell priming. As the DCs were injected into wild-type C57BL/6 recipients, this is a reflection of intrinsic DC dysfunction resulting from WASp deficiency. The impairment of migration was observed both in terms of the absolute number of injected DCs that were able to reach draining lymph nodes and also by the mislocalization to the subcapsular areas indicating that there were defects of dispersion. Migratory DCs that enter the lymph node normally disperse rapidly before integrating into the existing network of DCs.28 This network enables antigen transfer to lymph node–resident DCs and provides an enormous surface area to establish DC–T-cell contacts and to initiate immune responses. Interestingly, in a previous study, we found that resident splenic DCs were inefficient at relocalization into T-cell areas following microbial stimulation.12 Therefore, there seem to be multiple defects of WAS KO DC trafficking to the lymph nodes and within the T-cell areas of secondary lymphoid organs.
The defects of WAS KO DC priming of CD8+ T cells are reminiscent of those demonstrated in CCR7-deficient mice in which DC localization has been shown to be important.29,30 Administration of antigen-specific CCR7−/− DCs failed to prime CCR7-competent CD8+ T cells, but in CCR7−/− mice (both DCs and T cells were CCR7-deficient), a delayed CD8+ T-cell response could be induced.30 Priming of the CD8+ T cells could occur outside the T-cell area when both DCs and T cells were CCR7-deficient; however, the chance of the CCR7-competent CD8+ T cells encountering CCR7-deficient DCs was significantly reduced, resulting in less priming.30 Similarly, impaired localization of WAS KO DCs to the T-cell area could negatively affect the chance of the DCs meeting antigen-specific T cells and initiating a proper immune response. As it is thought that most priming of CD8+ T cells by migratory DCs occurs through transfer of the antigen to lymph node–resident CD8+ DCs, it is likely that failure of WAS KO DCs to migrate efficiently from peripheral sites and to localize properly within the lymphoid tissue, itself, have a combined deleterious effect.31,32
Previous studies have indicated that WAS KO T cells fail to proliferate or cap normally in response to CD3 coreceptor cross-linking.33,–35 More recently, WASp has been suggested to play a role specifically in the reformation of the immunologic synapse, thereby allowing the T cell to encounter multiple antigen-presenting cells in between short migratory events.36 However, the reorganization of the DC cytoskeleton during this process has not been studied in detail and its role is therefore undetermined. Here we have found that there is impairment in the ability of WAS KO DCs to stimulate normal T cells, suggesting that this cognate interaction is also compromised by DC dysfunction. Antigen-specific stimulation of normal T cells by WAS KO DCs was reduced in vitro when measured by proliferation and production of IFN-γ, but only at low antigen concentrations, indicating that the defect is incomplete. Abnormalities of antigen uptake and processing by the DCs could partly explain this effect, and although WAS KO DCs have been shown to exhibit impaired phagocytosis of particulate antigens, endocytosis and processing of soluble antigens such as OVA have been reported to be normal.37,–39 This aspect of DC function deserves further study, but it suggests that the ability of the DCs to signal to T cells is intrinsically reduced. However, we favor a prominent contribution of impaired migration to the reduction in T-cell priming because (1) previous studies have shown that the efficacy of T-cell activation is dependent on the numbers of antigen-pulsed DCs that are transferred21,40 ; (2) DCs of CCR7−/− mice that do not respond to CCL19 and CCL21 also show impaired DC migration and reduced T-cell priming as a result of impaired localization of DCs to the T-cell area29,30 ; and (3) migrating and resident WAS KO DCs showed impaired homing to the T-cell area of draining lymph nodes. It therefore seems likely that proper activation of T cells will not be accomplished normally as the DCs are physically delocalized from normal T-cell areas of secondary lymphoid organs.
The contribution of different cell lineages to the complex immune dysregulation of the WAS is of considerable importance for advancing our understanding of pathogenesis and also for optimizing therapies in patients. As an example, recent evidence suggests that mixed chimerism in some patients following allogeneic hematopoietic stem-cell transplantation (where T lymphocytes are usually donor and the myeloid compartment including DCs remains host derived) is associated with an increased incidence of autoimmunity (Ozsahin et al41 ). It is therefore possible that defective DC function alone is a significant contributor to both immunodeficiency and autoimmune complications. Here we have shown that a lack of WASp compromises the ability of DCs to prime normal T cells. Failure to correct this lineage following either stem cell transplantation or gene therapy may result in a suboptimal clinical response.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Wellcome Trust (057965/Z/99/B; A.J.T. and G.B.) and the European Union (040855, WASpTrafficDC; G.B.). S.B. was sponsored by the Primary Immunodeficiency Association, the Academy of Medical Sciences, and the Institute of Child Health, University College London.
Wellcome Trust
Authorship
Contribution: G.B. designed and performed the research, analyzed the data, and wrote the paper together with A.J.T.; S.B. provided critical reading of this paper and participated in writing the paper; A.J.T. designed the experiments and wrote the paper together with G.B.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Adrian Thrasher, Institute of Child Health, University College London, Molecular Immunology, 30 Guilford Street, London WC1N 1EH, United Kingdom; e-mail:a.thrasher@ich.ucl.ac.uk.