Foxp3+CD25+CD4+ regulatory T cells are produced in the thymus (natural T regs) but can also differentiate from peripheral Foxp3−CD4+ precursors (induced or adaptive T regs). We assessed antigen presenting cell (APC) requirements for the latter differentiation. With added transforming growth factor (TGF)-β, both immature and mature populations of dendritic cells (DCs) induced antigen-specific Foxp3+ T regs from Foxp3− precursors. Using endogenous TGF-β, DCs from gut-associated mesenteric lymph nodes were capable of differentiating Foxp3+T regs. Spleen DCs were 100-fold more potent than DC-depleted APCs for the induction of T regs and required 10-fold lower doses of peptide antigen. Interleukin-2 (IL-2) was essential, but could be provided endogenously by T cells stimulated by DCs, but not other APCs. The required IL-2 was induced by DCs that expressed CD80/CD86 costimulatory molecules. The DC-induced Foxp3+T regs divided up to 6 times in 6 days and were comprised of CD62L and CD103 positive and negative forms. The induced Foxp3+T regs exerted suppression in vitro and blocked tumor immunity in vivo. These results indicate that DCs are specialized to differentiate functional peripheral Foxp3+T regs and help set the stage to use DCs to actively suppress the immune response in an antigen-specific manner.
Introduction
The thymus produces naive CD4+ lymphocytes that can differentiate along several distinct pathways including Th1 cells that make interferon (IFN)-γ for resistance to many intracellular infections and tumors,1,2 Th2 cells that produce interleukin-4 (IL-4), 5, and 13 for resistance to helminths,3 and Th17 cells that produce IL-17 for resistance to extracellular infections.4,5 These pathways for T-cell differentiation can also be inappropriately induced, leading to autoimmunity and allergy. In addition, CD4+ T cells can differentiate to exert suppressive roles on immunity. Such regulatory T cells, or T regs, can take on several forms, including CD25+ cells that express a critical transcription factor, Foxp3,6,–8 and IL-10 producing Foxp3− Tr1 cells.9,10 The outcome of an antigen-specific immune response therefore varies markedly with the pathway of T-cell differentiation that is selected
T-cell differentiation is influenced by cytokines such as IL-12 for Th1 cells,11 IL-412 and thymic stromal lymphopoietin (TSLP) for Th2 cells,13 TGF-β and IL-6 for mouse Th17 cells14,–16 and TGF-β itself for Foxp3+ T regs. The latter “induced” or “adaptive” T regs develop from CD25−CD4+ T cells with a combination of TCR ligation and TGF-β supplementation.17,,–20 T regs induced by anti-CD3 and anti-CD28 stimulation are suppressive, including the inhibition of airway hypersensitivity and type 1 diabetes.17,21 To understand the differentiation of T regs, and to have larger numbers of antigen-specific T regs that could suppress disease in an antigen specific manner, it is necessary to define antigen presenting cells (APCs) requirements.
Dendritic cells (DCs) are professional APCs that control T-cell responses, including the differentiation of Th1,22,23 Th2,13,24 Th1725 and IL-10 producing Tr1 cells.10,26 Recently, Luo et al found that DCs can differentiate Foxp3+ T regs in the presence of TGF-β, from BDC2.5 RAG sufficient NOD CD25−CD4+ T cells, and that these cells could suppress autoimmune rejection of pancreatic islets in diabetic NOD mice.27 In contrast, it is reported that DCs are not as effective as B cells to differentiate Foxp3+ T regs by TGF-β using Foxp3− precursors from Foxp3-GFP knockin mice.28 Therefore, the relative roles of DCs and other APCs in the differentiation of Foxp3+ T regs from Foxp3− precursors remain to be explored.
Here, we have pursued the APC requirements for the differentiation of functional OVA-specific. Foxp3+ T regs from RAG −/− DO11.10 mice or from OT-II- RFP Foxp3 knockin, CD4+ Foxp3− precursors. The use of RAG−/− and Foxp3 knockin mice allowed us to exclude a contribution from the minor population of CD25− Foxp3+ T cells in RAG+/+ mice.29 We will describe the efficacy of DCs relative to non-DCs in the induction of antigen-specific T regs, and the capacity of these T regs to suppress tumor immunity. We also find that DCs require much lower doses of peptide than DC-depleted APCs, that some DCs induce Foxp3+ T regs without exogenous TGF-β, and that DCs induce the formation of the required IL-2 from T cells through CD80/CD86 costimulation.
Materials and methods
Mice
Six- to eight-week-old specific pathogen-free, female, C57BL/6 (B6), BALB/c were purchased from Taconic (Germantown, NY). IL-2−/− and CD80/CD86−/− mice were from Jackson Laboratories (Bar Harbor, Maine). DO11.10 RAG−/− mice were obtained through Taconic, the National Institute of Allergy and Infectious Diseases (NIAID) Exchange Program (National Institutes of Health [NIH], Bethesda, MD),30 while DO11.10 RAG+/+ mice were kindly provided by Dr P. Marrack (National Jewish Medical and Research Center, Denver, CO). OT-II mice were from Dr F. Carbone (University of Melbourne, Melbourne, Australia). Foxp3-IRES-RFP (FIR) knockin mice were generous gifts from R. Flavell (Yale University, New Haven, CT).31 All mice were used according to guidelines of our institutional animal care and use committee.
Antibodies and reagents
mAbs for CD8 (TIB211, 3–155), CD4 (TIB207, GK1.5), CD3(2C11), B220 (TIB146, RA3–6B2), and MHC II (TIB120, M5/114) were from American Type Culture Collection (Manassas, VA). PE, FITC, or APC conjugated anti-CD25 (7D4), CD25 (PC61), CD4 (H129.19), CD62L (MEL-14), CD103, GITR, CTLA-4, CD45RB, isotype rat IgG2a, hamster IgG, mouse IgG1, streptavidin, and purified anti-CD16/CD32 (2.4G2), anti–IL-2 mAbs were from BD PharMingen (San Diego, CA). Human IL-2 was from Chiron (Emeryville, CA). Anti-CD11c, FITC, streptavidin, and PE microbeads were from Miltenyi Biotec (Gladbach, Germany). Carboxyfluorescein diacetate succinimidyl ester (CFSE), TOPORO-3, and streptavidin Pacific blue were from Molecular Probes (Eugene, OR). Anti–mouse Foxp3 (FJK-16s) staining kit, anti–CD127-APC, and anti–CD4-Alexa700 were from eBioscience (San Diego, CA). Human TGF-β1, anti–mouse TGF-β mAb (1D11), and isotype control were from R&D Systems (Minneapolis, MN). Anti-KJ1.26 Ab was from Caltag/Invitrogen (Carlsbad, CA). Lipopolysaccharide (LPS) was from Sigma-Aldrich (St Louis, MO).
T cells and DCs
CD4+ T cells were negatively separated by MACS beads from lymph nodes and spleen cell suspensions (> 90%) (Miltenyi Biotech) or purified by flow cytometry (> 99%). Bone marrow DCs (BM-DCs) were derived with GM-CSF.32 Spleen or lymph node CD11c+ DCs were selected with anti-CD11c beads (> 90%),32 and CD11c− splenic cells were also tested. The APCs were irradiated (15 Gy) prior to coculture with T cells. The purified T cells and APCs were cultured in 96-well plates (Corning Coster, Corning, NY) with or without exogenous TGF-β (2 ng/mL) or IL-2 (100 U/mL) in the presence of OVA peptide (323–336). Live cell numbers per culture were counted by trypan blue exclusion or calculated from the yield from flow cytometry.
Flow cytometry
Cultured cells were first stained with anti-CD4, CD25, KJ1.26 clonotype with or without the other Abs and after fixation, intracellular Foxp3 was stained. Intracellular staining was also done for CTLA-4. A BDLSRII, FACSort, FACSAria, or FACSdiva was used (Becton Dickinson, Franklin Lakes, NJ). To assess cell numbers, all cells were acquired from each culture and analyzed with Flow Jo software (Tree Star, OR).
Cytokine multiplex analysis
The concentrations of cytokines were measured in cell culture supernatants by Luminex (Upstate, Charlottesville, VA), according to the manufacturer's protocol: 50 μL samples were incubated for 2 hours with Beadmates coated with anti-cytokine mAbs. The fluid was aspirated and biotin-conjugated anti-cytokine mAbs were added for 1.5 hours, followed by 30 minutes of incubation with Beadlyte streptavidin-PE. Samples were acquired in duplicates by Luminex and analyzed using Beadview software (Upstate).
Suppression of tumor immunity
OVA-expressing A20 tumor was kindly provided by Dr A. Marshak-Rothstein (Boston University, Boston, MA).33 They were cultured with G418. To assess resistance to the tumor, BALB/c mice were sublethally irradiated (4.5 Gy) and 6 hours later, injected intradermally with OVA-A20 tumor (4 × 106) with or without effector Foxp3−CD25−CD4+ T cells (2 × 106 intravenously) from RAG−/− DO11.10 OVA transgenic mice. To suppress resistance by Foxp3−CD25−CD4+ T cells, we coinjected DC-induced, Foxp3+CD25+CD4+ T reg. Tumor growth was assessed every 2 to 3 days.
Results
Exogenous TGF-β allows immature and mature DCs to induce Foxp3+ T regs from Foxp3− precursors, but is not required to expand natural Foxp3+ T regs
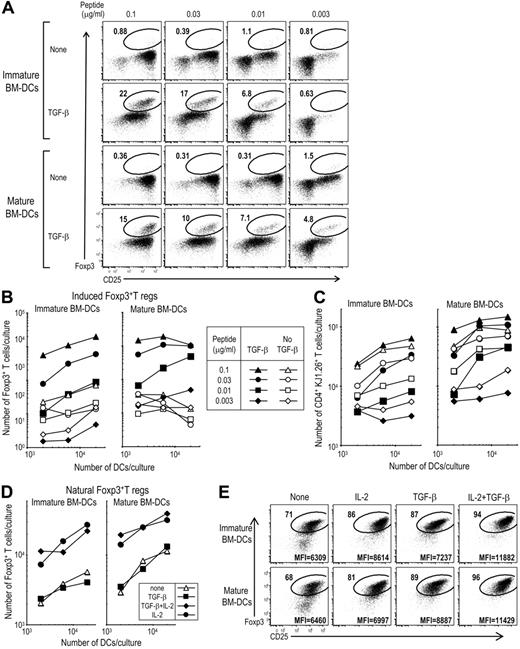
To study APC requirements for the differentiation of T regs from Foxp3− precursors, we used CD4+ T cells from OVA-specific, CD4 TCR transgenic, DO11.10 RAG−/− mice, which lack CD25+ Foxp3+ cells6 as we confirmed (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). These CD4+ T cells were cultured along with immature or mature (LPS-stimulated) bone marrow–derived DCs (BM-DCs) in the presence or absence of 2 ng/mL TGF-β and at different doses of the appropriate OVA peptide. At 5 days, we observed the TGF-β–dependent induction of Foxp3+ T cells by both immature and mature DCs (Figure 1A) and an expansion in total numbers of Foxp3+ cells (Figure 1B, compare closed to open symbols). Two to 10 ng/mL TGF-β were comparably active for inducing Foxp3+ T cells from Foxp3− precursors (data not shown). In the absence of antigen, immature or mature DCs and T cells did not induce Foxp3 (Figure S2). The number of CD4+ KJ1.26+ T cells expanded to greater levels in the presence of TGF-β with mature DCs than with immature DCs (Figure 1C). These data confirm the observations of Luo et al27 that DCs induce Foxp3+ T regs and also indicate that both immature and mature forms of DCs can be active.
BM-DCs plus TGF-β induce differentiation of Foxp3+T regs from DO11.10 RAG−/− Foxp3−CD25+CD4+ T cells. (A) Day-6 BM-DCs were stimulated with or without 50 ng/mL LPS for 16 hours. After washing, 6 × 103 immature (without LPS) or mature DCs (with LPS) were cultured with Foxp3−CD25−CD4+ T cells (2 × 104) from DO11.10 RAG−/− mice for 5 days with peptide in the presence or absence of TGF-β (2 ng/mL). Cells were stained with mAbs to CD4, KJ1.26 clonotype, and CD25-Abs. After fixation, cells were stained with anti-Foxp3 mAb. Plots were gated on CD4+ KJ1.26+ cells. (B) As in panel A, but the indicated numbers of DCs were cultured with Foxp3−CD25−CD4+ T cells (2 × 104) from DO11 RAG−/− mice for 5 days with the indicated doses of peptide in the presence or absence of TGF-β (2 ng/mL). The absolute numbers of Foxp3+CD4+KJ1.26 clonotype+ T cells per culture at 5 days are shown. (C) As in panel A, but absolute numbers of CD4+KJ1.26 clonotype+ T cells per culture at 5 days are shown. (D) As in panel A, but freshly isolated CD25+CD4+T cells from DO11.10 RAG+/+ mice (2 × 104, > 90% Foxp3+) were cultured with immature or mature BM-DCs plus 0.1 μg/mL peptide in the presence or absence of TGF-β (2 ng/mL) or IL-2 (100 U/mL). (E) As in panel D, but freshly isolated CD25+CD4+T cells from DO11.10 RAG+/+ mice were cultured with immature or mature BM-DCs (2 × 104). Geometric mean fluorescence intensity (MFI) for Foxp3 within the circle is shown in the bottom of the plots. Data are representative from 2 to 3 independent experiments.
BM-DCs plus TGF-β induce differentiation of Foxp3+T regs from DO11.10 RAG−/− Foxp3−CD25+CD4+ T cells. (A) Day-6 BM-DCs were stimulated with or without 50 ng/mL LPS for 16 hours. After washing, 6 × 103 immature (without LPS) or mature DCs (with LPS) were cultured with Foxp3−CD25−CD4+ T cells (2 × 104) from DO11.10 RAG−/− mice for 5 days with peptide in the presence or absence of TGF-β (2 ng/mL). Cells were stained with mAbs to CD4, KJ1.26 clonotype, and CD25-Abs. After fixation, cells were stained with anti-Foxp3 mAb. Plots were gated on CD4+ KJ1.26+ cells. (B) As in panel A, but the indicated numbers of DCs were cultured with Foxp3−CD25−CD4+ T cells (2 × 104) from DO11 RAG−/− mice for 5 days with the indicated doses of peptide in the presence or absence of TGF-β (2 ng/mL). The absolute numbers of Foxp3+CD4+KJ1.26 clonotype+ T cells per culture at 5 days are shown. (C) As in panel A, but absolute numbers of CD4+KJ1.26 clonotype+ T cells per culture at 5 days are shown. (D) As in panel A, but freshly isolated CD25+CD4+T cells from DO11.10 RAG+/+ mice (2 × 104, > 90% Foxp3+) were cultured with immature or mature BM-DCs plus 0.1 μg/mL peptide in the presence or absence of TGF-β (2 ng/mL) or IL-2 (100 U/mL). (E) As in panel D, but freshly isolated CD25+CD4+T cells from DO11.10 RAG+/+ mice were cultured with immature or mature BM-DCs (2 × 104). Geometric mean fluorescence intensity (MFI) for Foxp3 within the circle is shown in the bottom of the plots. Data are representative from 2 to 3 independent experiments.
To compare the role of TGF-β on induced and naturally occurring T regs, natural Foxp3+CD25+CD4+ T regs were FACS purified from DO11.10 RAG+/+ mice (> 90% Foxp3+) and were cultured with immature or mature BM-DCs and peptide in the presence or absence of TGF-β and also IL-2, side by side with the experiment in Figure 1A-C. In contrast to the induction of Foxp3 from Foxp3− precursors (Figure 1B), we observed no expansion of natural Foxp3+ T regs in the presence of TGF-β by both immature and mature DCs (Figure 1D closed boxes). Natural Foxp3+ T regs proliferated in the presence of IL-2, especially with mature BM-DCs (Figure 1D) as in our previous report.32 Natural Foxp3+ T regs cultured in the presence of mature or immature DCs plus or minus IL-2 or TGF-β expressed similar amounts of Foxp3 to the induced Foxp3+ T regs (MFI = 6600-7300 in Figure 1A), although natural T regs cultured with both IL-2 and TGF-β expressed higher Foxp3 (Figure 1E).
These data indicate that both mature and immature DCs require TGF-β to induce Foxp3+ T regs from Foxp3− precursors, but TGF-β is not required for DCs to expand natural Foxp3+ T regs with supplemental IL-2.
Lower numbers of spleen DCs induce higher numbers of Foxp3+T regs and with lower doses of antigen than splenic APCs
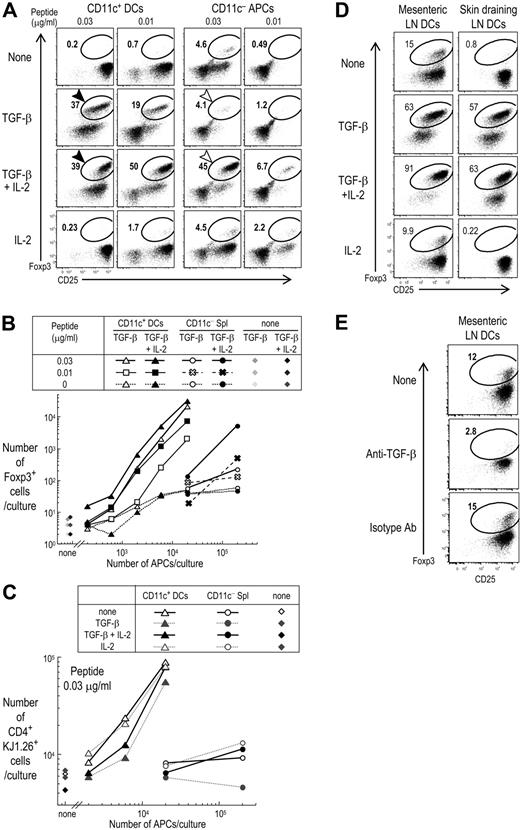
To assess DCs from lymphoid organs, we studied spleen DCs, which are immature in the steady state and contribute to deletional tolerance in the periphery.34,–36 Foxp3−CD25−CD4+ T cells from DO11.10 RAG−/− mice were cultured with spleen CD11c+ DC-enriched or DC-depleted CD11c− APCs, with or without exogenous TGF-β or IL-2, and OVA peptide. At day 5, when we assessed the percentages of Foxp3+ cells in the culture (Figure 2A), the spleen DCs could differentiate Foxp3+ cells in the presence of TGF-β and low doses of OVA peptide, even in the absence of IL-2. The yield of Foxp3+ cells corresponded to 40% to 50% of the KJ1.26 clonotype+ CD4+ T cells in the culture (Figure 2A, black arrows, and Figure S3). In contrast, both IL-2 and TGF-β had to be added when CD11c− spleen cells were the APCs (Figure 2A, open arrows). It was also apparent that higher doses of peptide antigen were required by CD11c− APCs (Figures 2A,B and S3), and the number of Foxp3+ cells induced by CD11c− spleen cells with higher doses of peptide antigen was much lower than with CD11c+ DCs (Figure S3B). Some Foxp3+ cells seemed to be induced by CD11c− APCs at 0.03 μg/mL peptide in the absence of TGF-β with or without IL-2, but the total numbers of induced Foxp3+ cells was low (< 700). When dose response curves were constructed, CD11c+ DCs were at least 100 times more effective in inducing Foxp3+ T regs with preprocessed peptide as antigen and exogenous TGF-β, but in the absence of IL-2 (Figure 2B). Although the addition of both TGF-β and IL-2 allowed CD11c− spleen cells to induce a significant percentage of Foxp3+ cells, the total yield of Foxp3+ cells was still much higher in cultures with spleen CD11c+ DCs than with CD11c− APCs (Figure 2B). To obtain the same numbers of Foxp3+ cells in the presence of both TGF-β and IL-2, 30 times fewer spleen CD11c+ DCs than CD11c− spleen cells were required (Figure 2B). These results were repeatable (Figure S4). Also, the total yield of clonotype-positive cells was much higher relative to the use of CD11c− cells (Figure 2C). Interestingly, if the peptide dose was increased to 0.3 μg/mL or more, the induction of Foxp3 was reduced to 20% when spleen CD11c+ DCs were the APCs, and there was stronger proliferation of Foxp3−CD25−CD4+ T cells (Figure S3A, and data not shown). Taken together, spleen CD11c+ DCs are more effective than CD11c− spleen cells to differentiate Foxp3+ T regs in the presence of TGF-β and antigen.
Foxp3+CD25+CD4+ T cells are induced from Foxp3−CD25−CD4+ T cells more effectively by spleen CD11c+ DCs than CD11c− APCs, and DCs from mesenteric lymph nodes differentiate Foxp3+T reg in the absence of exogenous TGF-β. (A) Foxp3−CD25−CD4+ T cells (2 × 104) from DO11.10 RAG−/− mice were cultured for 5 days with CD11c+ DCs (2 × 104) or CD11c− cells (2 × 105) from spleen and indicated doses of OVA peptide in the presence or absence of TGF-β (2 ng/mL) and/or IL-2 (100 U/mL). Cells were stained with mAbs to CD4, clonotype (KJ1.26), CD25, and Foxp3. Plots were gated on CD4+ KJ1.26+ cells. (B) As in panel A, but absolute numbers of Foxp3+CD4+KJ1.26 clonotype+ T cells per culture at 5 days were shown. (C) As in panel A, but the absolute numbers of CD4+KJ1.26 clonotype+ T cells per culture at 5 days were shown. (D) As in panel A, but Foxp3−CD25−CD4+ T cells from DO11.10 RAG−/− mice were cultured for 5 days at 0.03 μg/mL peptide with CD11c+ DCs (2 × 104) from mesenteric or skin draining lymph nodes in the presence or absence of TGF-β (2 ng/mL) and/or IL-2 (100 U/mL). (E) As in panel D, but anti–TGF-β mAb (10 μg/mL) or isotype control was added into the culture. Data are representative of 2 to 4 independent experiments.
Foxp3+CD25+CD4+ T cells are induced from Foxp3−CD25−CD4+ T cells more effectively by spleen CD11c+ DCs than CD11c− APCs, and DCs from mesenteric lymph nodes differentiate Foxp3+T reg in the absence of exogenous TGF-β. (A) Foxp3−CD25−CD4+ T cells (2 × 104) from DO11.10 RAG−/− mice were cultured for 5 days with CD11c+ DCs (2 × 104) or CD11c− cells (2 × 105) from spleen and indicated doses of OVA peptide in the presence or absence of TGF-β (2 ng/mL) and/or IL-2 (100 U/mL). Cells were stained with mAbs to CD4, clonotype (KJ1.26), CD25, and Foxp3. Plots were gated on CD4+ KJ1.26+ cells. (B) As in panel A, but absolute numbers of Foxp3+CD4+KJ1.26 clonotype+ T cells per culture at 5 days were shown. (C) As in panel A, but the absolute numbers of CD4+KJ1.26 clonotype+ T cells per culture at 5 days were shown. (D) As in panel A, but Foxp3−CD25−CD4+ T cells from DO11.10 RAG−/− mice were cultured for 5 days at 0.03 μg/mL peptide with CD11c+ DCs (2 × 104) from mesenteric or skin draining lymph nodes in the presence or absence of TGF-β (2 ng/mL) and/or IL-2 (100 U/mL). (E) As in panel D, but anti–TGF-β mAb (10 μg/mL) or isotype control was added into the culture. Data are representative of 2 to 4 independent experiments.
TGF-β is a cytokine that is highly expressed in mucosal tissues.37 We wondered if DCs from mesenteric lymph nodes (LNs) would induce T regs without having to add TGF-β. When we cultured CD4+ T cells from OVA transgenic DO11.10 RAG−/− mice with DCs from mesenteric LNs without any exogenous TGF-β, Foxp3 was induced on 13.8% ± 4.2% of CD4+ T cells (4 experiments) (Figure 2D). If the cell yield of Foxp3+ cells per culture was investigated, 2 × 104 Foxp3−CD25−CD4+ T cells gave rise to about 4000 to 8000 Foxp3+ cells per culture by DCs from mesenteric LNs without exogenous TGF-β (data not shown). In contrast, the induction of Foxp3 without exogenous TGF-β was lower with DCs from skin draining superficial LNs or spleen DCs, although we observed some variability (4.7% ± 3.1% in 4 experiments). The induction of Foxp3 by DCs from mesenteric LNs in the presence of IL-2 was 10.4% (± 2.5%; 4 experiments), and there was no significant difference from the culture without any IL-2 (P > .06). Interestingly, if CD4+ T cells from OVA transgenic DO11.10 RAG−/− mice were cultured with DCs from mesenteric LNs along with TGF-β and IL-2, further induction of Foxp3 was observed on about 80% to 90% of CD4+T cells (Figure 2D). To test if the induction of Foxp3 by DCs from mesenteric LNs used endogenous TGF-β, we performed blocking experiments. The presence of a blocking TGF-β mAb completely blocked Foxp3 induction by DCs from mesenteric LNs (Figure 2E). This indicates that DCs in some tissues can induce Foxp3+ T reg in vitro using endogenous TGF-β.
Endogenous IL-2 is required for CD80/86+ DCs to induce Foxp3+ T regs
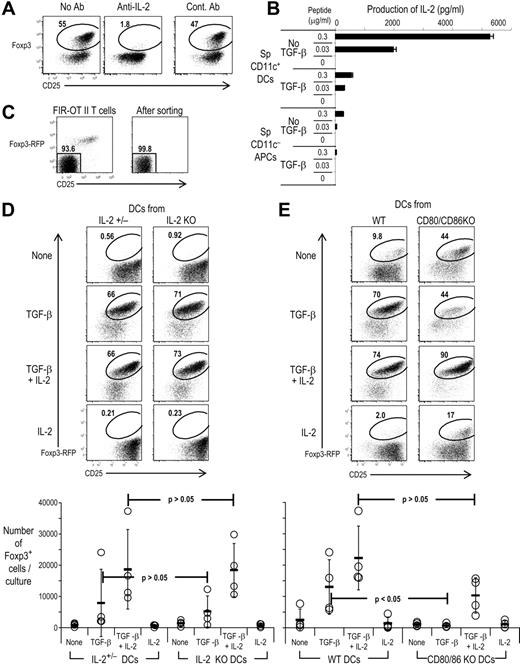
The observation that spleen CD11c+ DCs did not require exogenous IL-2 to induce T regs suggested that some of the cells in the culture were producing IL-2. IL-2 is important for natural Foxp3+ T regs to survive and maintain their function.38,,–41 Recently, it has been reported that IL-2 is important for differentiating Foxp3+CD25+CD4+ T regs from CD25−CD4+ wild-type T cells using anti-CD3 and anti-CD28 stimulation.42,43 The antigen-dependent induction of Foxp3 by spleen DCs, peptide, and TGF-β was completely blocked by anti–IL-2 mAb but not control mAb (Figure 3A), indicating that endogenous IL-2 is necessary for DCs to induce Foxp3+ T regs. When we checked the concentration of IL-2 in the supernatants, TGF-β suppressed IL-2 production, but significant amounts of IL-2 were detectable even in the presence of TGF-β with spleen CD11c+ DCs, in contrast to spleen CD11c− APCs (Figure 3B). Not only the production of IL-2, but also IFN-γ, IL-6, IL-17, IL-1β, IL-4. IL-5, IL-10, IL-12 p70, IL-13, and TNF-α in the supernatants with DCs were all reduced by TGF-β (Figure S5, and data not shown). Thus, the use of DCs as APCs allows IL-2 to be produced in the amounts needed by differentiating T regs, even in the presence of TGF-β.
Endogenous IL-2 from T cells stimulated by CD80/CD86+/+ DCs is required for the differentiation of Foxp3+CD25+CD4+ T cells. (A) As in Figure 2, but Foxp3−CD25−CD4+ T cells from DO11.10 RAG−/− mice were cultured with spleen CD11c+ DCs with peptide plus TGF-β in the presence or absence of blocking anti–IL-2 Ab or control Abs (20 μg/mL). (B) Foxp3−CD25−CD4+ T cells from DO11.10 RAG−/− mice were cultured with spleen CD11c+ DCs (2 × 104) or CD11c− APCs (2 × 105) with indicated dose of peptide in the presence or absence of TGF-β. After day 5, culture supernatants were collected, and the concentration of IL-2 measured by Luminex. (C) CD4+ T cells from Foxp3-IRES-RFP knock-in OVA OTII CD4 transgenic mice (FIR-OTII) were stained with anti-CD4 and CD25 Abs, and Foxp3−CD25−CD4+ T cells were purified by flow cytometry (left). The purity of Foxp3−CD25−CD4+ T cells after sorting was higher than 99.5% (right; gated on CD4+ T cells. (D) The Foxp3−CD25−CD4+ T cells (2 × 104) from FIR-OTII mice were cultured with spleen CD11c+ DCs (2 × 104) for 5 days in the presence of peptide (0.03 μg/mL) with or without TGF-β (2 ng/mL) and/or IL-2 (100 U/mL). Cells were gated on CD4+ T cells. The absolute numbers of Foxp3+CD4+T cells per culture from 4 different experiments with spleen DCs or immature BM-DCs are shown. P value is provided by Student t test. (E) As in panel D, but spleen CD11c+ DCs or immature BM-DCs were prepared from CD80/CD86−/− or wild-type (WT) mice in the presence of peptide with or without TGF-β (2 ng/mL) and/or IL-2 (100 U/mL). Cells were gated on CD4+ T cells. The absolute numbers of Foxp3+CD4+ T cells per culture from 4 different experiments with spleen DCs or immature BM-DCs are shown. P value is provided by Student t test. Data are representative of 2 to 4 independent experiments.
Endogenous IL-2 from T cells stimulated by CD80/CD86+/+ DCs is required for the differentiation of Foxp3+CD25+CD4+ T cells. (A) As in Figure 2, but Foxp3−CD25−CD4+ T cells from DO11.10 RAG−/− mice were cultured with spleen CD11c+ DCs with peptide plus TGF-β in the presence or absence of blocking anti–IL-2 Ab or control Abs (20 μg/mL). (B) Foxp3−CD25−CD4+ T cells from DO11.10 RAG−/− mice were cultured with spleen CD11c+ DCs (2 × 104) or CD11c− APCs (2 × 105) with indicated dose of peptide in the presence or absence of TGF-β. After day 5, culture supernatants were collected, and the concentration of IL-2 measured by Luminex. (C) CD4+ T cells from Foxp3-IRES-RFP knock-in OVA OTII CD4 transgenic mice (FIR-OTII) were stained with anti-CD4 and CD25 Abs, and Foxp3−CD25−CD4+ T cells were purified by flow cytometry (left). The purity of Foxp3−CD25−CD4+ T cells after sorting was higher than 99.5% (right; gated on CD4+ T cells. (D) The Foxp3−CD25−CD4+ T cells (2 × 104) from FIR-OTII mice were cultured with spleen CD11c+ DCs (2 × 104) for 5 days in the presence of peptide (0.03 μg/mL) with or without TGF-β (2 ng/mL) and/or IL-2 (100 U/mL). Cells were gated on CD4+ T cells. The absolute numbers of Foxp3+CD4+T cells per culture from 4 different experiments with spleen DCs or immature BM-DCs are shown. P value is provided by Student t test. (E) As in panel D, but spleen CD11c+ DCs or immature BM-DCs were prepared from CD80/CD86−/− or wild-type (WT) mice in the presence of peptide with or without TGF-β (2 ng/mL) and/or IL-2 (100 U/mL). Cells were gated on CD4+ T cells. The absolute numbers of Foxp3+CD4+ T cells per culture from 4 different experiments with spleen DCs or immature BM-DCs are shown. P value is provided by Student t test. Data are representative of 2 to 4 independent experiments.
DCs stimulated with bacterial products produce IL-2.44 To investigate the potential contribution of this IL-2, we prepared DCs from IL-2−/− mice. Since the IL-2−/− mice were on the B6 background, we used T cells from OVA transgenic OT II B6 mice that had also been crossed with Foxp3-IRES-RFP knock-in (FIR-OTII) mice31 (Figure 3C left). This RFP knock-in allowed us to remove fluorescent Foxp3+ cells from the CD25− CD4+ T-cell fraction of the RAG sufficient mice, permitting the isolation for highly purified Foxp3−CD25− cells by FACS (Figure 3C right). Spleen DCs or immature BM-DCs were again able to induce the formation of T regs in the presence of exogenous TGF-β and antigen, but DCs from both IL-2−/− and control mice (IL-2+/− or wild type) were similar for inducing Foxp3+ T regs (Figure 3D). When we checked the concentration of IL-2 in the supernatants, similar amounts of IL-2 were detectable even in the presence of TGF-β with both IL-2−/− DCs and IL-2+/+ DCs (data not shown). Therefore, while endogenous IL-2 is required for the induction of Foxp3+ T regs with TGF-β, the source is primarily T cells.
B7 molecules on DCs amplify the production of IL-2 from T cells.45 To assess the role of B7 costimulatory molecules during the induction of Foxp3, DCs from CD80/CD86 double-knockout mice (CD80/CD86−) were cultured with Foxp3−CD25− FIR-OT II T cells. In contrast to wild-type DCs, CD80/CD86−/− DCs induced Foxp3 at a much lower efficiency in the presence of exogenous TGF-β (Figure 3E). When we checked the production of IL-2, wild-type DCs gave rise to significant amounts of IL-2 (> 500 pg/mL) even in the presence of TGF-β as in Figure 3B, but CD80/CD86−/− DCs in the presence of TGF-β induced only 24.9 pg/mL of IL-2. To examine if exogenous IL-2 restored the capacity of CD80/CD86−/− DCs to induce Foxp3, Foxp3−CD25− FIR-OT II T cells were cultured with CD80/CD86−/− DCs in the presence of both TGF-β and IL-2. In the presence of both TGF-β and IL-2, CD80/CD86−/− DCs induced Foxp3 on about 90% of T cells as well as an expansion in total numbers of Foxp3+ T regs (Figure 3E). This indicates that T-cell costimulation through CD80/CD86 is not required for the induction of Foxp3 directly, but instead, CD80/CD86 on DCs is important for T cells to produce the required IL-2.
Foxp3+T regs induced by DCs plus TGF-β proliferate extensively
It has been reported that the induction of Foxp3+ T regs can take place in Foxp3−CD4+ HA transgenic T cells by the targeting of antigens in vivo to DCs, but that the induced T regs underwent little if no cell division.46 Surprisingly, when CD4+ T cells from DO11.10 RAG−/− mice were CFSE labeled prior to stimulation with spleen CD11c+ DCs and TGF-β, cell division proved to be active in the newly differentiated Foxp3+T cells at day 3 and also day 6 (Figure 4A). By day 6, most of the Foxp3+ cells had divided 6 times. Next, we compared CFSE dilution with spleen CD11c+ DCs and DC-depleted spleen APCs at various doses of peptide as in Figure 2 (Figure 4B). At day 6, DC-depleted spleen APCs induced Foxp3 on divided cells at 0.3 μg/mL peptide, but the proliferation was reduced relative to that induced by spleen CD11c+ DCs (Figure 4B). Thus, DCs in the presence of TGF-β bring about both proliferation and differentiation of peripheral T regs in vitro, even with relatively low doses of antigenic peptide.
DC-induced, differentiating, peripheral Foxp3+CD25+CD4+ T cells undergo substantial proliferative activity. (A) As in Figure 2, but Foxp3−CD25−CD4+ T cells from DO11.10 RAG−/− mice (2 × 104) were CFSE labeled and cultured with CD11c+ DCs (2 × 104) from spleen with or without OVA peptide (0.03 μg/mL) in the presence or absence of TGF-β. At day 3 or day 6, cells were stained with anti-CD4 and KJ1.26 clonotype Abs. After fixation, cells were stained with anti-Foxp3 or isotype control mAb. Foxp3 expression and CFSE dilution were shown gated on CFSE+CD4+KJ1.26 clonotype+ T cells.  indicates proliferating cells expressed Foxp3. (B) As in panel A, but CFSE-labeled DO11.10 RAG−/− Foxp3−CD25−CD4+ T cells were cultured with spleen CD11c+ DCs (2 × 104) or DC-depleted spleen cells (CD11c− APC, 2 × 105) at the indicated dose of peptide in the presence of TGF-β (2 ng/mL). Foxp3 expression and CFSE dilution were analyzed at day 6. Data are representative of 2 independent experiments.
indicates proliferating cells expressed Foxp3. (B) As in panel A, but CFSE-labeled DO11.10 RAG−/− Foxp3−CD25−CD4+ T cells were cultured with spleen CD11c+ DCs (2 × 104) or DC-depleted spleen cells (CD11c− APC, 2 × 105) at the indicated dose of peptide in the presence of TGF-β (2 ng/mL). Foxp3 expression and CFSE dilution were analyzed at day 6. Data are representative of 2 independent experiments.
DC-induced, differentiating, peripheral Foxp3+CD25+CD4+ T cells undergo substantial proliferative activity. (A) As in Figure 2, but Foxp3−CD25−CD4+ T cells from DO11.10 RAG−/− mice (2 × 104) were CFSE labeled and cultured with CD11c+ DCs (2 × 104) from spleen with or without OVA peptide (0.03 μg/mL) in the presence or absence of TGF-β. At day 3 or day 6, cells were stained with anti-CD4 and KJ1.26 clonotype Abs. After fixation, cells were stained with anti-Foxp3 or isotype control mAb. Foxp3 expression and CFSE dilution were shown gated on CFSE+CD4+KJ1.26 clonotype+ T cells.  indicates proliferating cells expressed Foxp3. (B) As in panel A, but CFSE-labeled DO11.10 RAG−/− Foxp3−CD25−CD4+ T cells were cultured with spleen CD11c+ DCs (2 × 104) or DC-depleted spleen cells (CD11c− APC, 2 × 105) at the indicated dose of peptide in the presence of TGF-β (2 ng/mL). Foxp3 expression and CFSE dilution were analyzed at day 6. Data are representative of 2 independent experiments.
indicates proliferating cells expressed Foxp3. (B) As in panel A, but CFSE-labeled DO11.10 RAG−/− Foxp3−CD25−CD4+ T cells were cultured with spleen CD11c+ DCs (2 × 104) or DC-depleted spleen cells (CD11c− APC, 2 × 105) at the indicated dose of peptide in the presence of TGF-β (2 ng/mL). Foxp3 expression and CFSE dilution were analyzed at day 6. Data are representative of 2 independent experiments.
Foxp3+T regs induced by DCs plus TGF-β express CD62L and CD103
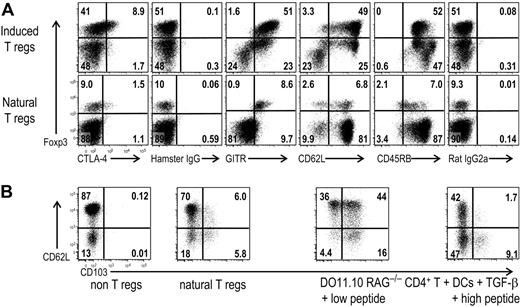
Next we compared the phenotype of the induced Foxp3+ T regs by DCs plus TGF-β with naturally occurring T regs (Figure 5). Naturally occurring CD25+CD4+T regs constitutively express CTLA-447,–49 and GITR (glucocorticoid-induced TNF receptor family-related gene).50,51 A proportion of the CD25+CD4+ T regs also expressed the CD62L lymph node homing selectin.52,53 Recently, it has been reported that natural Foxp3+ T regs have low levels of CD127 or IL-7Rα.54,55 When we examined the phenotype of the DC-induced Foxp3+ T regs, the latter did not express CD127 (data not shown). However, a portion of the induced Foxp3+ T regs expressed CTLA-4 to the same extent as naturally occurring CD25+CD4+ T regs (Figure 5A). The Foxp3+ T regs induced by DCs plus TGF-β also expressed GITR (Figure 5A), and most of them expressed CD62L (Figure 5A). CD45RBlow is another marker of CD25+ CD4+ regulatory T cells.48 We observed that CD45RB expression was higher on induced Foxp3+ T regs than on the naturally occurring CD25+CD4+ T regs, even though it was still lower than Foxp3− cells in the same culture (Figure 5A).
DC-induced Foxp3+T regs have a comparable phenotype to natural T regs. (A) As in Figure 2, but Foxp3−CD25−CD4+ T cells from DO11.10 RAG−/− mice were cultured with CD11c+ spleen DCs and 0.03 μg/mL peptide in the presence of TGF-β. These cultured cells (top) or freshly isolated spleen cells from DO11.10 RAG+/+ mice (bottom) were stained with mAbs to CD4, clonotype (KJ1.26), and the indicated Abs or isotype controls. Plots were gated on CD4+ KJ1.26+ cells for the induced T regs (top) and on CD4+ cells for freshly isolated spleen cells (bottom). (B) Freshly isolated spleen cells from DO11.10 RAG+/+ mice (non–T regs, natural T regs) or the cultured cells as in panel A but with low peptide (0.03 μg/mL) or high peptide (0.3 μg/mL) were stained with CD4, clonotype (KJ1.26), CD62L, CD103, and Foxp3 Abs. Plots for non-T regs were gated on Foxp3−CD25−CD4+ T cells, and plots for natural T regs were gated on Foxp3+CD25+CD4+ T cells. Plots were gated on Foxp3+CD4+KJ1.26+ cells for cultured cells. Data are representative of 2 to 4 independent experiments.
DC-induced Foxp3+T regs have a comparable phenotype to natural T regs. (A) As in Figure 2, but Foxp3−CD25−CD4+ T cells from DO11.10 RAG−/− mice were cultured with CD11c+ spleen DCs and 0.03 μg/mL peptide in the presence of TGF-β. These cultured cells (top) or freshly isolated spleen cells from DO11.10 RAG+/+ mice (bottom) were stained with mAbs to CD4, clonotype (KJ1.26), and the indicated Abs or isotype controls. Plots were gated on CD4+ KJ1.26+ cells for the induced T regs (top) and on CD4+ cells for freshly isolated spleen cells (bottom). (B) Freshly isolated spleen cells from DO11.10 RAG+/+ mice (non–T regs, natural T regs) or the cultured cells as in panel A but with low peptide (0.03 μg/mL) or high peptide (0.3 μg/mL) were stained with CD4, clonotype (KJ1.26), CD62L, CD103, and Foxp3 Abs. Plots for non-T regs were gated on Foxp3−CD25−CD4+ T cells, and plots for natural T regs were gated on Foxp3+CD25+CD4+ T cells. Plots were gated on Foxp3+CD4+KJ1.26+ cells for cultured cells. Data are representative of 2 to 4 independent experiments.
The CD103 αEβ7 integrin is known to be expressed by a fraction of natural Foxp3+T regs.56,57 It is reported that the β7 integrin gene promoter is responsive to TGF-β,58 and the induced Foxp3+ T regs express CD103.21 CD103 is necessary for the retention of T regs at the site of Leishmania major infection.59 CD103 also plays an important role in mucosal immune regulation because T reg cell–based suppression of colitis was lost in CD103−/− recipients.60 When we examined the expression of CD103 on freshly isolated spleen cells, Foxp3−CD4+ T cells did not express CD103 (Figure 5B), whereas a small fraction of natural Foxp3+ T regs expressed CD103 (Figure 5B). Interestingly, after a week of culture with DCs plus TGF-β, lower concentrations of peptide (0.03 μg/mL) resulted in higher percentages of CD103+-induced Foxp3+ T regs as well as CD62L+ T regs, whereas higher concentration of peptide reduced the induction of CD103+ Foxp3+ cells (Figure 5B).
These results suggest that induced Foxp3+ T regs are similar in phenotype to natural Foxp3+ T regs except for some differences in CD45RB and CD103. The amount of antigen can affect the expression of CD103 on the induced Foxp3+ T regs, which in turn may affect their migration capacity to inflammatory sites.
DC-induced Foxp3+ T regs suppress T-cell responses in vitro
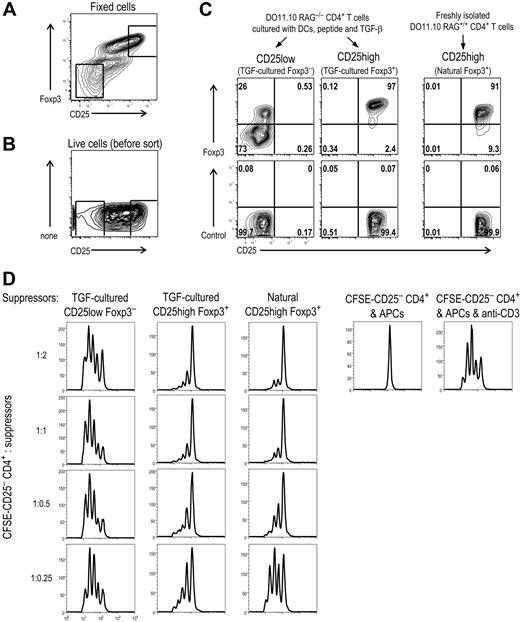
To test the suppressive function of the induced Foxp3+ T regs in vitro, we needed to separate the T regs from Foxp3− T cells in the culture. First we cultured DO11.10 RAG−/− CD4+ T cells with DCs plus peptide and TGF-β, and an aliquot of the cells was fixed and stained with mAbs to Foxp3, CD25, and CD4. This provided gates to sort the induced Foxp3+ T cells based on their higher expression of CD25 (Figure 6A) when the remaining live cells were stained with anti-CD4 and CD25 Abs (Figure 6B). After sorting, the CD25 high cells were more than 95% Foxp3+, whereas the CD25 low cells were 10% to 25% Foxp3+ (Figure 6C). Natural CD25+CD4+ T regs from DO11.10 RAG+/+ were also purified, and they were more than 90% Foxp3+ (Figure 6C). Graded numbers of these cells were then added to CFSE-labeled CD25−CD4+ responder T cells stimulated with spleen APCs and anti-CD3 Ab (Figure 6D). The response of the CFSE-labeled CD25−CD4+ cells was accelerated by the addition of the CD25 low fraction from the DCs plus TGF-β culture, even in low doses (Figure 6D, left vertical row). In contrast, the proliferation of CD25−CD4+ T cells was suppressed by either the induced CD25 high Foxp3+ or natural CD25 high Foxp3+ T regs at high suppressor-to-effector ratios, 2:1, and this suppression diminished when the Foxp3+ T regs were diluted (Figure 6D middle and right rows). We also did the same suppression assay using FIR-OT II mice. Again, the induced RFP+CD25+CD4+ T cells suppressed the response of CD25−CD4+ T cells stimulated with spleen APCs and anti-CD3 Ab (data not shown). Therefore, Foxp3+ T regs induced by DCs plus TGF-β have suppressive activity in vitro.
DC-induced Foxp3+ T cells are suppressive in vitro. (A) Foxp3−CD25−CD4+ T cells from DO11.10 RAG−/− mice were cultured for 7 days with CD11c+ spleen DCs (2 × 104), peptide (0.03 μg/mL), and TGF-β (2 ng/mL). Cells were stained with anti-CD4, CD25, and Foxp3 Abs. (B) As in panel A, but live cells were stained with anti-CD4 and CD25 Abs for sorting. The square indicates the gate for sorting. (C) Sorted cells from (B) were fixed and further stained with anti-Foxp3 Ab. The purity of sorted natural CD25+CD4+ T regs was also shown. (D) CD25−CD4+ responder T cells (2 × 104) from DO11.10 mice were CFSE-labeled and stimulated with spleen APCs (105) and anti-CD3 mAb. To these, the induced Foxp3+ T regs purified as in panel C were added in graded numbers. After 3 days, CFSE dilution was analyzed with flow cytometry. Dead cells were gated out by TOPRO-3 iodide. Data are representative of 2 independent experiments.
DC-induced Foxp3+ T cells are suppressive in vitro. (A) Foxp3−CD25−CD4+ T cells from DO11.10 RAG−/− mice were cultured for 7 days with CD11c+ spleen DCs (2 × 104), peptide (0.03 μg/mL), and TGF-β (2 ng/mL). Cells were stained with anti-CD4, CD25, and Foxp3 Abs. (B) As in panel A, but live cells were stained with anti-CD4 and CD25 Abs for sorting. The square indicates the gate for sorting. (C) Sorted cells from (B) were fixed and further stained with anti-Foxp3 Ab. The purity of sorted natural CD25+CD4+ T regs was also shown. (D) CD25−CD4+ responder T cells (2 × 104) from DO11.10 mice were CFSE-labeled and stimulated with spleen APCs (105) and anti-CD3 mAb. To these, the induced Foxp3+ T regs purified as in panel C were added in graded numbers. After 3 days, CFSE dilution was analyzed with flow cytometry. Dead cells were gated out by TOPRO-3 iodide. Data are representative of 2 independent experiments.
DC-induced Foxp3+ T regs inhibit tumor rejection by CD25−CD4+ T cells
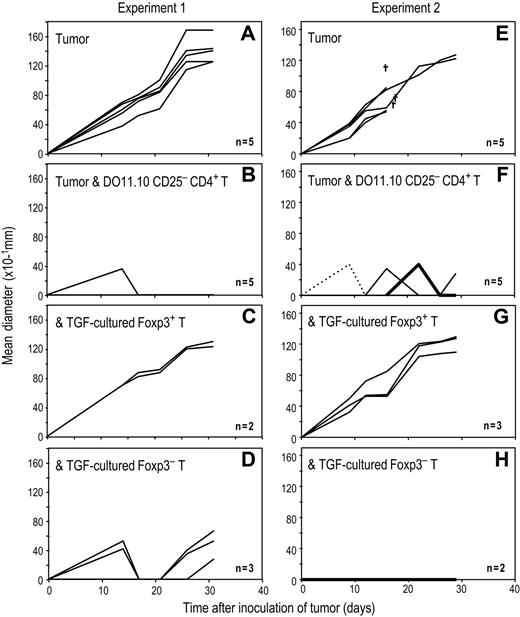
To examine the suppressive function of antigen-specific–induced Foxp3+ T regs in vivo, we used an OVA-expressing A20 tumor.33 In 4 separate experiments, sublethally irradiated BALB/c mice succumbed to an intradermal injection of OVA-A20 cells (Figure 7A,E; data from 2 experiments are shown). However, if these mice were adoptively transferred with freshly isolated DO11.10 RAG−/− CD25−CD4+ T cells, the growth of the OVA-A20 cells was blocked (Figure 7B,F). After inducing Foxp3+ T regs with DCs, peptide and TGF-β from DO11.10 RAG−/−CD4+ T cells, CD25 high Foxp3+ and CD25 low Foxp3− T cells were purified by flow cytometry as in Figure 6A-C. When the induced Foxp3+ T regs (> 95% Foxp3+ as in Figure 6C) were co-transferred with freshly isolated DO11.10 RAG−/− CD25−CD4+ T cells at a 1:1 ratio, all mice developed growing tumors (Figure 7C,G), indicating that the induced Foxp3+ T regs suppressed tumor rejection by DO11.10 RAG−/− CD25−CD4+ T cells. In contrast, when CD25 low, Foxp3− T cells from the same culture (10% to 25% Foxp3+ as in Figure 6C) were co-transferred, most mice still rejected the tumor cells (Figure 7D,H). These results indicate that Foxp3+ T regs induced by DCs are suppressive in vivo.
DC-induced Foxp3+ T cells suppress OVA specific immunity in vivo. (A) BALB/c mice were sublethally irradiated (4.5 Gy). After 6 hours, they were injected intradermally on back with OVA-expressing A20 tumor (4 × 106). (B) As in panel A, but irradiated recipients were injected intradermally with OVA-expressing A20 tumor and were also injected intravenously with freshly isolated CD25−CD4+ T cells (2 × 106) from DO11.10 RAG−/− mice. (C) As in panel B, but irradiated recipients were injected intradermally with OVA-expressing A20 tumor and were also injected intravenously with freshly isolated CD25−CD4+ T cells from DO11.10 RAG−/− mice along with the TGF-β cultured Foxp3+ T regs (2 × 106). The TGF-β cultured Foxp3+ T regs were induced by DCs and TGF-β from RAG−/− DO11.10 mice and purified as in Figure 6A and supplemental Figure 9. (D) As in panel C, but instead of the TGF-cultured Foxp3+ T regs, TGF-cultured Foxp3− T cells (2 × 106) were injected. The TGF-cultured Foxp3− T cells were from the same culture with panel C and purified as in Figure 7A-C. (E-H) The same experiment with panels A-D was repeated.
DC-induced Foxp3+ T cells suppress OVA specific immunity in vivo. (A) BALB/c mice were sublethally irradiated (4.5 Gy). After 6 hours, they were injected intradermally on back with OVA-expressing A20 tumor (4 × 106). (B) As in panel A, but irradiated recipients were injected intradermally with OVA-expressing A20 tumor and were also injected intravenously with freshly isolated CD25−CD4+ T cells (2 × 106) from DO11.10 RAG−/− mice. (C) As in panel B, but irradiated recipients were injected intradermally with OVA-expressing A20 tumor and were also injected intravenously with freshly isolated CD25−CD4+ T cells from DO11.10 RAG−/− mice along with the TGF-β cultured Foxp3+ T regs (2 × 106). The TGF-β cultured Foxp3+ T regs were induced by DCs and TGF-β from RAG−/− DO11.10 mice and purified as in Figure 6A and supplemental Figure 9. (D) As in panel C, but instead of the TGF-cultured Foxp3+ T regs, TGF-cultured Foxp3− T cells (2 × 106) were injected. The TGF-cultured Foxp3− T cells were from the same culture with panel C and purified as in Figure 7A-C. (E-H) The same experiment with panels A-D was repeated.
Discussion
A subpopulation of CD4+ lymphocytes, which express the Foxp3 transcription factor and high-affinity IL-2 receptor (CD25), are produced in the thymus and are termed natural T regulatory cells (T regs). These T regs maintain self-tolerance by actively suppressing autoimmunity.61,–63 The APC requirements for natural T regs have begun to be studied, and it is now evident that mature antigen-presenting DCs are able to expand natural T regs.32,64,–66 The DC-expanded T regs suppress type 1 diabetes in nonobese diabetic (NOD) mice and graft versus host disease (GVHD) in an antigen-specific manner.67,–69
The development and function of T regs is controlled by the X-chromosome–encoded forkhead transcription factor, Foxp3.6,–8 Foxp3 is the most specific current marker for T regs because, unlike CD25, Foxp3 is not up-regulated in activated effector T cells.31,70
In addition to the thymus as a site for the differentiation of T regs, it is known that Foxp3+ T regs can be induced from CD25−CD4+ nonregulatory T cells in the periphery by TGF-β supplementation.17,,–20,27 Here we address an important facet in the mechanism underlying the induction of peripheral T regs from Foxp3− precursors, which is that DCs are specialized APCs relative to other cells in mouse spleen. Specifically, we found that Foxp3+ T regs were induced by 100 times fewer spleen DCs than DC-depleted splenic APCs and required much lower doses of antigenic peptide.
The costimulatory features of DCs are in part responsible for their enhanced potency relative to other spleen cells. For one thing, the DCs are able to induce the required IL-2 from the T cells and thus do not require exogenous IL-2. Importantly, we found that CD80/CD86 double-knockout DCs had a reduced capacity to induce Foxp3 with TGF-β, but the addition of exogenous IL-2 allowed the costimulation-poor DCs to induce Foxp3 on most T cells. It is reported that B7 costimulation is required for CD25−CD4+ T cells to convert into CD25+CD4+ T regs,71 but our results suggested that CD80/CD86 costimulation is required for T cells to induce IL-2 and is not directly required to co-stimulate the expression of Foxp3.
We also found that DCs require much lower doses of antigen to induce Foxp3, 3 to 10 times less than required by DC-depleted spleen APCs. In fact, higher doses of peptide with DCs led to increased amounts of IL-2 (Figure 3B) and increased proliferation of Foxp3−CD4+ T cells. This may explain a prior report that B cells are more active as APCs than DCs for inducing T regs, because high doses of T-cell receptor signaling via anti-CD3 Abs were used.28 It is possible that one difference between CD11c+ DCs and CD11c− APCs is increased TCR stimulation because CD11c− APCs in the presence of TGF-β start to induce Foxp3+ cells with higher amounts of peptide antigen. However, the total numbers of Foxp3+ cells induced by DC-depleted spleen APCs in the presence of TGF-β was lower than by CD11c+ DCs (Supplemental Figure 3). Therefore, we think that DCs are specialized to induce Foxp3+ cells in the presence of TGF-β.
Interestingly, immature BM-DCs and freshly isolated spleen DCs are at least as effective as mature DCs in inducing Foxp3+ T regs from DO11.10 RAG−/− Foxp3− T cells. In contrast, mature DCs are much more effective for inducing Th1 and cytolytic effector cells.72,73 This suggests that DCs, upon capturing antigens in situ as is observed in the steady state, will preferentially induce T regs from CD4+ T cells, as long as TGF-β is provided. This would allow the immune system to sustain tolerance to harmless self and environmental antigens that are captured in the steady state (reviewed in74 ). Our observation that DCs also require lower doses of antigen than non-DCs likewise are consistent with an important physiologic role where the amounts of harmless antigens may be low but still capable of immunization if captured by maturing DCs.
An intriguing finding would be to control the production of active TGF-β from DCs themselves so that the differentiation of T regs would occur upon antigen presentation by these cells, as has been reported to occur in tumors.75 Consistent with this, we found that DCs from the intestinal environment are able to differentiate some Foxp3+ T regs through TGF-β. Possibly the environment or the DCs themselves are providing a source of TGF-β. We would like to suggest that the presentation of antigen by DCs in vivo, coupled with production of TGF-β, will allow for the induction of antigen-specific Foxp3+ T regs in vivo. For example, it is reported that immature BM-DCs produce TGF-β mRNA,76 and TGF-β produced by DCs from tumor-bearing hosts expands CD25+CD4+ T regs that contribute to immune suppression in mice.75 After we submitted the manuscript, it was shown that retinoic acid acts synergistically to induce Foxp3 together with exogenous TGF-β, and a subset of DCs from intestinal environment provides active retinoic acid through their own vitamin A metabolizing enzymes.77,,–80 Therefore, it is possible that endogenous retinoic acid might contribute to the induction of Foxp3 in our experiments.
Taken together, DCs are specialized APCs for the differentiation of functional Foxp3+ T regs from Foxp3− precursors. This parallels the reported efficacy of DCs in inducing other pathways of CD4+ T-cell differentiation including Th1,22,23 Th2,13,24 Th17,25 and IL-10–producing Tr1 cells.10,26 Coupled with their known capacities in antigen capture and processing, as well as localization to the T-cell areas, the ability of DCs to induce T regs would allow for the induction of antigen-specific T regs in vivo. This could provide a new avenue for the suppression of unwanted immune responses in autoimmunity, allergy, and transplantation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Klara Velinzon and Tamara Shengelia for expert cell sorting, Judy Adams for help with graphics, Christopher Fiorese for technical assistance, Anne Marshak-Rothstein and Richard Flavell for A20-OVA cells and FIR mice, and Xunrong Luo and Kristin Tarbell for advice.
This work was supported by NIH grant AI51573 and a program project grant from the Juvenile Diabetes Research Foundation International. DO11.10 RAG−/− mice were obtained through the NIAID Exchange Program.
National Institutes of Health
Authorship
Contribution: S.Y., K.I., and R.M.S. designed research. S.Y. and A.J.B. performed research. R.S. and M.D. contributed new reagents/analytic tools. S.Y., R.S., K.I., and R.M.S. analyzed data. S.Y. and R.M.S. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sayuri Yamazaki, Laboratory of Cellular Physiology and Immunology, Rockefeller University, 1230 York Ave, New York, NY 10021-6399; e-mail:yamazas@rockefeller.edu.