We investigated whether atorvastatin (AT) was capable of protecting animals from acute graft-versus-host disease (aGVHD) across major histocompatibility complex (MHC) mismatch barriers. AT treatment of the donor induced a Th-2 cytokine profile in the adoptively transferred T cells and reduced their in vivo expansion, which translated into significantly reduced aGVHD lethality. Host treatment down-regulated costimulatory molecules and MHC class II expression on recipient antigen-presenting cells (APCs) and enhanced the protective statin effect, without impacting graft-versus-leukemia (GVL) activity. The AT effect was partially reversed in STAT6−/− donors and abrogated by L-mevalonate, indicating the relevance of STAT6 signaling and the L-mevalonate pathway for AT-mediated aGVHD protection. AT reduced prenylation levels of GTPases, abolished T-bet expression, and increased c-MAF and GATA-3 protein in vivo. Thus, AT has significant protective impact on aGVHD lethality by Th-2 polarization and inhibition of an uncontrolled Th-1 response while maintaining GVL activity, which is of great clinical relevance given the modest toxicity profile of AT.
Introduction
Acute graft-versus-host disease (aGVHD) is the major complication after allogeneic bone marrow transplantation (BMT) characterized by immunodysregulation and tissue injury including the liver, intestinal tract, and skin.1 Acute GVHD is initiated when donor-derived T cells encounter host derived antigen-presenting cells (APCs) in lymphoid tissues and become activated.2 This process requires costimulation via CD80 and CD86, which are up-regulated on APCs during the early phase of aGVHD. Activation is enhanced by local tissue damage due to the conditioning regimen. Local proinflammatory cytokines such as tumor necrosis factor (TNF), interleukin 1 (IL-1), and interferon γ (IFN-γ), promote T-helper 1 (Th-1) differentiation of donor-derived T cells and enhance their proliferation and reactivity against host tissues.3 Recent reports support the concept that in both mice and humans, massive cytokine release related to the Th-1 phenotype predicts the incidence and severity of aGVHD, whereas patients with high IL-10 production are less at risk for GVHD development.4,5
There is accumulating evidence that treatment with 3-hydroxy-3-methyl-CoA (HMG-CoA) reductase inhibitors (statins) results in a protective Th-2 bias in animal models of Th-1–mediated autoimmune disease and other inflammatory conditions.6,7 Statin-mediated immunomodulatory effects on APCs and T cells are due to interference with the synthesis of L-mevalonate and its downstream isoprenoid metabolites.8,9 Among isoprenylated proteins the Ras superfamily GTPases Ras, Rho, and Rab are crucial in biochemical signaling cascades controlling proinflammatory gene expression and T-cell phenotype.10 In order to be active, GTPases must associate with the T-cell membrane, which is dependent on their covalent attachment to isoprenoids.11 Therefore, statins interfere with the function of Ras and Ras-related GTPases by impairing their targeting to the T-cell membrane.8 Further mechanisms whereby statins may exert their effects on the immune system include the allosteric modification of leukocyte function antigen-1 by a subset of statins12 and the inhibition of activation and maturation of macrophages and microglia cells.7,13
The beneficial effects of statins in preclinical models of autoimmunity6,7,9 have produced considerable interest in using these agents in patients with multiple sclerosis,14 rheumatoid arthritis,15 and chronic GVHD.16 In light of this, we evaluated the effects of different statins on aGVHD, engraftment, and graft-versus-leukemia (GVL) activity in a previously described murine BMT model.17 Here, we elucidate their mechanism of action when T-cell activation is induced by alloantigens.
Materials and methods
Mice
C57BL/6 (H-2Kb, Thy-1.2), FVB (H-2Kq) and Balb/c (H-2Kd, Thy-1.2), Balb/c STAT6−/− (C.129S2-Stat6tm1Gru/J) C57BL/6 perforin-deficient C57BL/6 Prf1tm1Sdz/tm1Sdz (Prf1−/−), and FasL mutant B6Smn.C3 Tnfsf6gld/gld mice were purchased from Jackson Laboratory (Bar Harbor, ME) or Charles River Laboratory (Wilmington, MA). Mice were used between 6 and 12 weeks of age. Only gender-matched combinations were used for transplant experiments. The luciferase-expressing (luc+) transgenic FVB/N L2G85 was previously described.18 All animal protocols were approved by the University Committee on Use and Care of Laboratory Animals at Stanford University.
In vivo drug treatments
Atorvastatin ([AT]; Lipitor, prescription formulation; Pfizer, San Francisco, CA) or Fluvastatin ([FLU]; Lescol, prescription formulation; Novartis, Emeryville, CA) was brought into suspension in phosphate-buffered saline (PBS; 0.4 mg/mL or 0.04 mg/mL) and a 0.5-mL volume of this suspension (equivalent to 10 mg/kg or 1 mg/kg, respectively) was administered to mice orally, once daily, using 20-mm feeding needles (Popper and Sons, New Hyde Park, NY). L-mevalonate (Sigma-Aldrich, St Louis, MO) was dissolved in saline (1 mg/kg) and injected intraperitoneally once daily. Mice treated with PBS served as controls. All treatments were initiated at 10 days before transplantation in the recipient or donor and then discontinued as detailed in the respective experiment.
aGVHD model
Acute GVHD was induced as described previously.17 Briefly, recipients were given 5 × 106 T cell–depleted bone marrow (TCD-BM) cells after lethal irradiation with 800 cGy. To induce aGVHD, the following doses of CD4+/CD8+ T cells were given: 1.2 × 106 (FVB/N→Balb/c), 5 × 105 (C57BL/6→Balb/c), or 2 × 106 (Balb/c→C57BL/6) on day 0. For transplantation of luc+ T-cell subsets, single-cell suspensions from cervical lymph nodes (cLNs), axillary lymph nodes (aLNs), inguinal lymph nodes (iLNs), mesenteric lymph nodes (mLNs), and spleens of FVB-L2G85 mice were enriched to over 90% purity with CD4/CD8-conjugated magnetic beads using the AutoMACS system (Miltenyi Biotech, Auburn, CA). Mice were given antibiotic water (sulfomethoxazole-trimethoprim; Schein Pharmaceutical, Dartmore, CT).
Tumor models
To investigate GVL activity of transferred donor T cells, we employed A20-luc/yfp B-cell leukemia that has been previously demonstrated to migrate primarily to the bone marrow with secondary infiltration of spleen and other lymphoid organs when injected intravenously at the time of BMT, and the BCL1 model tumor model where tumor cells initially infiltrate the spleen and then metastasize to the liver and lung.19,20 For both the A20 and the BCL1 tumor model, the firefly luciferase gene was employed to visualize viable tumor cells as previously described.19 In the first tumor model, animals were injected with A20-luc/yfp B-cell leukemia cells 2 days prior to administration of the T cells to allow the tumor to home and establish. In the second model, 3 × 103 BCL1 tumor cells were given intravenously on day −5 prior to BMT. Tumor engraftment was verified by bioluminescence imaging (BLI) prior to total body irradiation. Animals were then treated with lethal irradiation (8 Gy) and underwent transplantation within 24 hours. Tumor growth was analyzed by BLI and quantification of yfp- or gfp-expressing tumor cells was determined by fluorescence-activated cell sorting (FACS) analysis from the respective organs.
In vivo and in vitro bioluminescence imaging
In vivo BLI was performed as previously described.21 Briefly, mice were injected intraperitoneally with luciferin (10 μg/g body weight). After 10 minutes, mice were imaged using an IVIS200 charge-coupled device (CCD) imaging system (Xenogen, Alameda, CA) for 5 minutes. In vitro expansion of luciferase transgenic T cells was quantified by adding 6 μg/mL luciferine per well. After 5 minutes, the 96-well plate was imaged using an IVIS200 charge-coupled device (CCD) imaging system (Xenogen, Alameda, CA) for 2 minutes. Expansion is quantified in photons/second/cm2. Imaging data were analyzed and quantified with Living Image Software (Xenogen) and IgorProCarbon (WaveMetrics, Lake Oswego, OR).
Proliferation assays
CD4+ and CD8+ T-cell splenocytes and lymph node cells from luciferase transgenic animals were purified by positive selection. For CFSE labeling 107/mL cells were resuspended in plain PBS and stained with Vybrant CFDA SE (carboxyfluorescein diacetate, succinimidyl ester) Tracer kit (Molecular Probes, Eugene, OR) at a final concentration of 5 μM for exactly 6 minutes at 37°C. Immediately after staining, cells were washed twice in 5 volumes of ice-cold RPMI plus 10% fetal bovine serum (Gibco, Life Sciences, Grand Island, NY), resuspended in PBS, and counted before use in an in vitro assay or intravenous injection. A quantity of 2 × 106 cells/well was cultured in flat-bottomed, 6-well plates and stimulated with 2 μg/mL or 5 μg/mL each of αCD3 (clone 145-2C11; BD Biosciences, San Jose, CA) and αCD28 (clone 37.51; BD Biosciences). Culture medium consisted of RPMI 1640, supplemented with l-glutamine (2 mM), penicillin (100 U/mL), streptomycin (0.1 mg/mL), 2-mercaptoethanol (5 × 10−5 M), and 10% fetal calf serum. After 48 hours of culture, T cells were harvested and analyzed by FACS for CFSE dilution and bioluminescence signal or injected intravenously as indicated for the respective experiments.
Flow cytometry and phospho flow analysis
The following antibodies were used for flow cytometric analysis: unconjugated anti-CD16/32 (2.4G2), CD4 (RM4-5), CD8α (53-6.7), CD25 (PC61), CD11c (M1/70), CD45R/B220 (RA3-6B2), H-2Kq (KH114), H-2Kd (34-2-12), Thy-1.1 (H1S51), Thy-1.2 (53-2.1), Foxp3 (FJK-16s), CD80 (16-10A1), CD86 (GL 1), CD40 (3/23), anti-MHC-II (AF6-120.1). The antibodies were purchased from BD Pharmingen (San Diego, CA) and eBiosciences (San Diego, CA). Anti–c-MAF (M-153), anti–T-bet (clone 4B10), and anti–GATA-3 (clone HG3-31) antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Staining was performed in the presence of purified anti-CD16/32 at saturation to block nonspecific staining. Propidium iodide (Sigma, St Louis, MO) was added prior to analysis to exclude dead cells. All analytical flow cytometry was done on a dual laser LSRScan (BD Immunocytometry Systems, San Diego, CA). For intracellular cytokine staining cells were washed after surface marker staining and permeabilized with Fix/Perm Buffer solution for 12 hours and subsequently washed with permeabilization buffer (both from eBioscience, San Diego, CA) after restimulation for 4 hours with PMA (20 ng/mL; Sigma) and ionomycin (1 μM; Calbiochem, Mannheim, Germany) in the presence of monensin (Golgistop; BD Biosciences). After the cells were washed, the fluorescent conjugate intracellular mAbs against IL-4, IL-10, IFN-γ, or TNF-α (eBioscience) were added and incubated for 30 minutes in the dark. The cells are washed again and analyzed by FACS.
For phospho flow analysis the following antibodies were used: PE anti-Stat1 (clone 4a), PE anti-Stat3 (clone 49), PE anti-Stat4 (clone 38/pStat4), PE anti-Stat5 (clone 47), and Alexa 488 anti-Stat6 (clone 18). Prior to staining, cells were lysed and fixed with 1x PhosphoFlow Lyse/Fix Buffer for 15 minutes at 37°C, then permeabilized with PhosFlow Perm Buffer III on ice for 1 hour (all reagents from BD Phosflow, San Jose, CA) as previously described.22
Western blot analysis
T cells that were isolated after in vitro or in vivo exposure to AT or PBS were counted and resuspended in an appropriate volume (10 μL/million cells) of NP-40 buffer (150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM Na2PO4, 1 mM glycerol phosphate, 1 mM Na3V04, 1 g/mL leupeptin, 1 mM PMSF, Roche protease inhibitor tablet, 20 mM Tris, pH 7.5). After a 30-minute extraction on ice, samples were centrifuged at 15 000g for 15 minutes at 4°C to obtain a clarified supernatant. Proteinase inhibitors were included in all buffers. The concentration of proteins in each sample was determined using a BCA assay kit (Pierce Chemical, Rockford, IL). Each protein sample was suspended in 2 vol of 2x SDS Sample Buffer (Bio-Rad Laboratories, Hercules, CA) and subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) using precast Tris-HCl Ready-Gels (Bio-Rad Laboratories). Proteins were transferred to PVDF membranes (GE Healthcare, Piscataway, NJ) and immunoblotting was performed using conventional methods (Santa Cruz Biotechnology). Pan Ras (clone 18) and Rac1 (clone 102) antibodies were obtained from BD Transduction Labs (San Jose, CA). RhoA (clone 26C4), Rap1 (clone sc-65), T-bet (clone sc-21 749), and GATA-3 (clone sc-268) antibodies were obtained from Santa Cruz Biotechnology. Anti-phospho STAT1 (Y701, clone 4a), STAT4 (pY693, clone 38/pSTAT4), and STAT6 (pY641, clone 71-773.58.11) antibodies were obtained from BD Pharmingen. Goat anti–mouse IgG antibody was obtained from Jackson Immuno-Research Laboratories (West Grove, PA).
GVHD histopathology scoring
Tissues were embedded in optimal cutting temperature medium (OCT) and cryopreserved at −80°C. Fresh frozen sections of 5-μm thickness were mounted on positively charged precleaned microscope slides (Superfrost/Plus; Fisher Scientific, Hampton, NH) and stored at −80°C. Hematoxylin/eosin (H/E) staining of paraffin-embedded tissue sections was performed according to standard protocols and evaluated by an experienced pathologist (N.K.) according to a previously published histopathology scoring system.23 Evaluation of the stained tissue sections was performed on a Nikon microscope (Eclipse, TE 300; Melville, NY). Standard magnifications were 200×/0.45 NA and 400×/0.60 NA. Microscopic photographs were obtained using a Spot digital camera (Diagnostic Instruments, Sterling Heights, MI).
51Cr release cytotoxicity assay
Tumor targets (A20 or Yac1 cells) were labeled with 51Cr (DuPont-NEN, Boston, MA) by incubating 1 × 106 cells in 300 μCi (11.1 MBq) of 51Cr at 37°C for 2 hours in 5% CO2. The labeled cells were washed 3 times with PBS, resuspended in cRPMI, and plated in 96-well plates at a concentration of 1 × 104 cells/mL in triplicate. Donor animals used for isolation of CD8+ effector cells were pretreated with PBS or AT by oral garvage and a single injection of LPS (30 μg/animal subcutaneously 7 days prior to CD8 T cell isolation). CD8+ effector cells were added at specified ratios (5:1 or 20:1) and incubated at 37°C for 5 hours in 5% CO2. At the completion of each assay, supernatant was collected and counted using a gamma counter (Cobra/AII; Packard Bioscience, Meriden, CT). The percent-specific 51Cr lysis was calculated with the following equation: percent-specific lysis = 100 × (test release) − (spontaneous release) / (maximal release) − (spontaneous release).
ELISA-based cytokine analysis
Serum was collected from Balb/c recipients on different time points after transplantation. Cell culture supernatants were taken at the time of peak production for each cytokine: IL-10 (96 hours), IFN-γ (72 hours), TNF (72 hours), and IL-4 (120 hours). Enzyme-linked immunosorbent assay (ELISA) was performed according to the manufacturer's instructions (R&D Systems, Minneapolis, MN). Briefly, samples were diluted 1:2 to 1:5, and the cytokine was captured by the specific primary mAb precoated on the microplate and detected by horseradish peroxidase–labeled secondary mAbs. Plates were read at 450 nm using a microplate reader (model Spectra Max 190; Bio-Rad Laboratories). Recombinant cytokines were used as standards. Samples and standards were run in duplicate, and the sensitivity of the assays was 16 pg/mL to 20 pg/mL for each cytokine, depending on the sample dilution.
Statistical analysis
Differences in animal survival (Kaplan-Meier survival curves) were analyzed by log-rank test. Differences in proliferation of conventional luc transgenic T cells, supernatant cytokines, mean fluorescence of costimulatory molecule expression, serum cytokine levels, GVHD histopathology scores, and chimerism studies were analyzed using the 2-tailed Student t test and a P value of less than .05 was considered statistically significant.
Results
Donor pretreatment with AT confers aGVHD protection through reduction in T-helper cell 1 cytokine production and alloreactive T-cell expansion
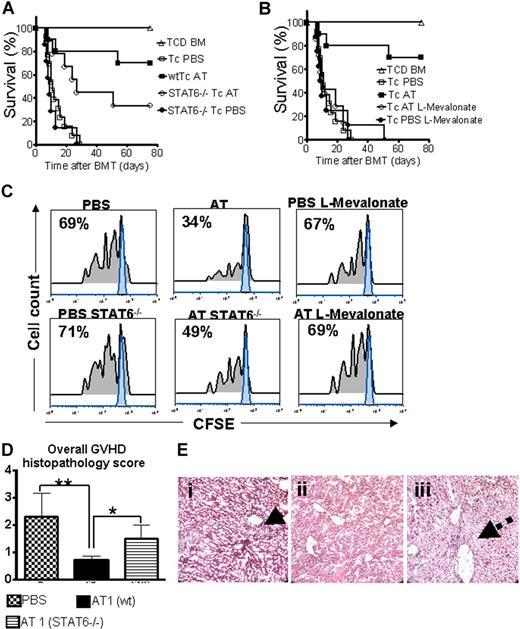
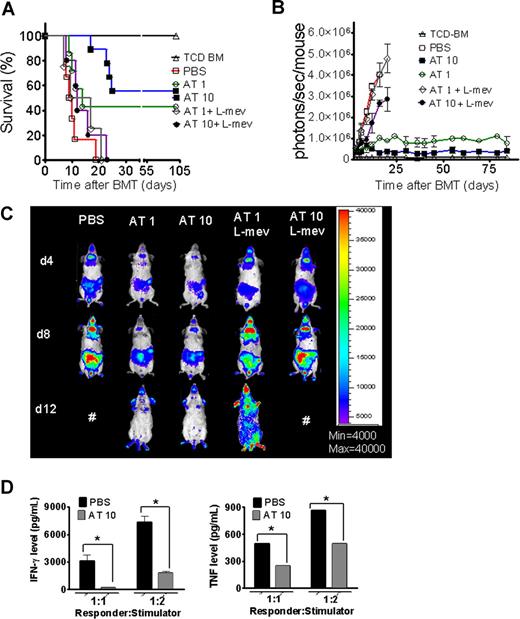
A hallmark of aGVHD is the expansion of alloreactive T cells in the proinflammatory environment, induced by the conditioning regimen and dysregulated immune mechanisms.1,24 In order to evaluate the impact of AT on donor T-cell expansion and aGVHD induction, luc+FVB/N donors were fed with AT at a dose of either 1 mg/kg (AT1) or 10 mg/kg (AT10) or PBS for 10 days prior to T-cell harvest and injection into the irradiated recipient.
Animals receiving TCD-BM and T cells derived from PBS-fed animals displayed aGVHD signs and died within 20 days after BMT (Figure 1A) whereas animals receiving T cells from donors fed with AT survived significantly longer. The protective AT effect could be antagonized by treating the donor additionally with L-mevalonate (Figure 1A). Donor pretreatment reduced expansion of luciferase transgenic (luc+) T cells after BMT as quantified by photons over the total body area (Figure 1B,C). T cells derived from PBS-treated donors produced more IFN-γ and TNF than T cells from AT10-treated animals in allogeneic mixed leukocyte reactions (MLRs) (Figure 1D). IL-10 production was higher when T cells from AT-pretreated donors as compared with PBS-pretreated donors were used as responder cells at the 1:2 (responder/stimulator) ratio (P < .05) and a trend toward a higher IL-10 production in the 1:1 ratio (NS). Increased IL-4 production was found when T cells from AT- as compared with PBS-treated animals (P < .05) were used in allogeneic MLRs (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Donor pretreatment with atorvastatin improves survival after major MHC mismatch BMT by reducing alloreactive T-cell expansion in vivo. (A) Balb/c mice were given 5 × 106 TCD-BM cells and 1.2 × 106 CD4+/CD8+ (4:1) T cells (both H-2Kq) after lethal irradiation with 800 cGy. Donor animals were fed with either PBS or AT with or without L-mevalonate by oral garvage for 10 days prior to transplantation. Survival of mice receiving TCD-BM (▵, n = 15), with T cells from donors treated with PBS (□, n = 15), atorvastatin 1 mg/kg (○, AT1, n = 15), atorvastatin 10 mg/kg (■, AT10, n = 15), AT1 plus L-mevalonate (◇, n = 10), AT10 plus L-mevalonate (●, n = 10). Percentage survival of Balb/c recipients is significantly higher when T cells are derived from donors treated with atorvastatin 1 mg/kg or 10 mg/kg as compared with PBS (○ versus □, P < .001; ■ versus □, P < .001). Survival data from 2 independent experiments including 8 and 7 recipients, respectively, are combined. (B) Expansion of luciferase-labeled T cells was quantified in emitted photons over total body area at serial time points after BMT. BLI signal intensity of mice receiving TCD-BM (▵, n = 15) with T cells from donors treated with PBS (□, n = 15), atorvastatin 1 mg/kg (○, AT1, n = 15), atorvastatin 10 mg/kg (■, AT10, n = 15), AT1 plus L-mevalonate (◇, n = 10), or AT10 plus L-mevalonate (●, n = 10). Signal intensity is significantly higher in animals receiving T cells from PBS-treated as compared with AT1- or AT10-treated animals (□ vs ○, P = .007; □ vs ■, P = .003). (C) Single time points depicting the expansion of luciferase transgenic (luc+) donor T cells in representative Balb/c mice receiving T cells from donors treated as indicated on top of each column for days 4, 8, and 12 after BMT. (D) Cytokine levels were measured in the supernatant of cocultures combining irradiated (30 Gy) APCs (Stimulator, H-2Kd) with T cells (Responder, H-2Kq) of each cell type, 2 × 105 cells/well in flat-bottomed, 96-well plates derived from donors that were fed for 10 days with PBS or atorvastatin 10 mg/kg (AT) as indicated in the respective histogram (*P < .05). ELISA was performed as described in detail in “ELISA-based cytokine analysis.”
Donor pretreatment with atorvastatin improves survival after major MHC mismatch BMT by reducing alloreactive T-cell expansion in vivo. (A) Balb/c mice were given 5 × 106 TCD-BM cells and 1.2 × 106 CD4+/CD8+ (4:1) T cells (both H-2Kq) after lethal irradiation with 800 cGy. Donor animals were fed with either PBS or AT with or without L-mevalonate by oral garvage for 10 days prior to transplantation. Survival of mice receiving TCD-BM (▵, n = 15), with T cells from donors treated with PBS (□, n = 15), atorvastatin 1 mg/kg (○, AT1, n = 15), atorvastatin 10 mg/kg (■, AT10, n = 15), AT1 plus L-mevalonate (◇, n = 10), AT10 plus L-mevalonate (●, n = 10). Percentage survival of Balb/c recipients is significantly higher when T cells are derived from donors treated with atorvastatin 1 mg/kg or 10 mg/kg as compared with PBS (○ versus □, P < .001; ■ versus □, P < .001). Survival data from 2 independent experiments including 8 and 7 recipients, respectively, are combined. (B) Expansion of luciferase-labeled T cells was quantified in emitted photons over total body area at serial time points after BMT. BLI signal intensity of mice receiving TCD-BM (▵, n = 15) with T cells from donors treated with PBS (□, n = 15), atorvastatin 1 mg/kg (○, AT1, n = 15), atorvastatin 10 mg/kg (■, AT10, n = 15), AT1 plus L-mevalonate (◇, n = 10), or AT10 plus L-mevalonate (●, n = 10). Signal intensity is significantly higher in animals receiving T cells from PBS-treated as compared with AT1- or AT10-treated animals (□ vs ○, P = .007; □ vs ■, P = .003). (C) Single time points depicting the expansion of luciferase transgenic (luc+) donor T cells in representative Balb/c mice receiving T cells from donors treated as indicated on top of each column for days 4, 8, and 12 after BMT. (D) Cytokine levels were measured in the supernatant of cocultures combining irradiated (30 Gy) APCs (Stimulator, H-2Kd) with T cells (Responder, H-2Kq) of each cell type, 2 × 105 cells/well in flat-bottomed, 96-well plates derived from donors that were fed for 10 days with PBS or atorvastatin 10 mg/kg (AT) as indicated in the respective histogram (*P < .05). ELISA was performed as described in detail in “ELISA-based cytokine analysis.”
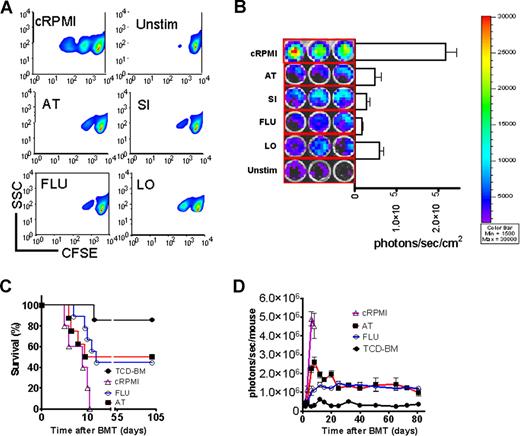
In vitro statin exposure reduces alloreactive T-cell expansion in vivo and confers aGVHD protection
To probe the in vitro effect on T-cell expansion, T cells were stimulated in the presence of the indicated statins. T-cell expansion was potently inhibited as measured by CFSE dilution (Figure 2A) or signal intensity of proliferating luc+ T cells (Figure 2B). Transfer of preactivated T cells rapidly induced progressive aGVHD (Figure 2C). In vitro exposure to FLU or AT improved survival significantly (Figure 2C). In vivo expansion of the statin-exposed luc+ T cells was significantly reduced as compared with T cells cultured in the absence of statins (Figure 2D). Although T-cell proliferation was reduced, trafficking to secondary lymphoid organs was not impacted by in vitro exposure of the transferred T cells to AT or FLU as investigated by immunofluorescence microscopy (Figure S1), which may be relevant for clearance of pathogens or tumor cells in these locations. Control groups receiving either allogeneic TCD-BM or syngeneic TCD-BM and syngeneic luc+ T cells did not show any BLI signal when the threshold of 4000 to 40 000 photons/second per mouse was applied (data not shown).
In vitro statin treatment reduces T-cell expansion in response to alloantigen in vitro and in vivo. (A) In vitro expansion of CFSE- (A) or luciferase-labeled (B) CD4+/CD8+ T cells (4:1, FVB/N, 2 × 105 T cells/ flat-bottomed, 96-well plate, 200 μL per well) in the presence of CD3/CD28 stimulation (2 μg/mL or 5 μg/mL each) for 48 hours in complete media (cRPMI) and the indicated statins (10 μM). Afterward, cells were washed and analyzed by FACS for CFSE dilution. cRPMI indicates no statin; AT, atorvastatin; SI, simvastatin; FLU, fluvastatin; LO, lovastatin; Unstim, no CD3/CD28 mAb stimulation. T cells expand upon in vitro stimulation (upper panel). AT, SI, FLU, and LO reduce T-cell expansion significantly as measured by levels of CFSE dilution. (B) The same experimental in vitro conditions as described in panel A were employed. Expansion of luciferase transgenic T cells was quantified in photons/second/cm2. All statins reduce in vitro T-cell expansion significantly as compared with cRPMI (P < .01). (C) Survival of mice receiving TCD-BM (●, n = 10) with T cells exposed to CD3/CD28 stimulation as described in panel A in cRPMI alone (▵, n = 10) or with FLU (○, n = 10) or AT (■, n = 10). Survival of Balb/c recipients was significantly higher when T cells were exposed to AT or FLU as compared with cRPMI (■ vs ▵, P = .003; and ○ vs ▵, P = .004). All Balb/c recipients (H-2d) underwent transplantation as described in “aGVHD model.” Data from 2 independent experiments are combined. (D) Expansion of luciferase-labeled (luc+) T cells was quantified in emitted photons over total body area at serial time points after BMT in mice receiving TCD-BM (●, n = 10) with T cells exposed to cRPMI (▵, n = 10), fluvastatin (FLU, ○, n = 10), or atorvastatin (AT, ■, n = 10). Signal intensity is significantly higher in animals receiving T cells exposed to cRPMI only as compared with FLU- and AT-treated animals (▵ vs ○, P < .001; and ▵ vs ■, P < .001). Data from 2 independent experiments are combined.
In vitro statin treatment reduces T-cell expansion in response to alloantigen in vitro and in vivo. (A) In vitro expansion of CFSE- (A) or luciferase-labeled (B) CD4+/CD8+ T cells (4:1, FVB/N, 2 × 105 T cells/ flat-bottomed, 96-well plate, 200 μL per well) in the presence of CD3/CD28 stimulation (2 μg/mL or 5 μg/mL each) for 48 hours in complete media (cRPMI) and the indicated statins (10 μM). Afterward, cells were washed and analyzed by FACS for CFSE dilution. cRPMI indicates no statin; AT, atorvastatin; SI, simvastatin; FLU, fluvastatin; LO, lovastatin; Unstim, no CD3/CD28 mAb stimulation. T cells expand upon in vitro stimulation (upper panel). AT, SI, FLU, and LO reduce T-cell expansion significantly as measured by levels of CFSE dilution. (B) The same experimental in vitro conditions as described in panel A were employed. Expansion of luciferase transgenic T cells was quantified in photons/second/cm2. All statins reduce in vitro T-cell expansion significantly as compared with cRPMI (P < .01). (C) Survival of mice receiving TCD-BM (●, n = 10) with T cells exposed to CD3/CD28 stimulation as described in panel A in cRPMI alone (▵, n = 10) or with FLU (○, n = 10) or AT (■, n = 10). Survival of Balb/c recipients was significantly higher when T cells were exposed to AT or FLU as compared with cRPMI (■ vs ▵, P = .003; and ○ vs ▵, P = .004). All Balb/c recipients (H-2d) underwent transplantation as described in “aGVHD model.” Data from 2 independent experiments are combined. (D) Expansion of luciferase-labeled (luc+) T cells was quantified in emitted photons over total body area at serial time points after BMT in mice receiving TCD-BM (●, n = 10) with T cells exposed to cRPMI (▵, n = 10), fluvastatin (FLU, ○, n = 10), or atorvastatin (AT, ■, n = 10). Signal intensity is significantly higher in animals receiving T cells exposed to cRPMI only as compared with FLU- and AT-treated animals (▵ vs ○, P < .001; and ▵ vs ■, P < .001). Data from 2 independent experiments are combined.
AT and FLU reduce costimulatory molecule and MHC class II expression on recipient APCs
Previous studies have demonstrated that AT inhibits MHC class II expression on microglia by blocking multiple CIITA promoters.7 We determined the in vivo impact of AT and FLU on recipient APCs which have are crucial in aGVHD induction2 by feeding the recipients for 10 days prior to BMT with AT or FLU. Recipient type APCs derived from liver, spleen, and lymph nodes (LNs) were analyzed at different time points after BMT. Surface expression of CD80, CD86, CD40, and MHC class II was decreased in animals treated with the indicated statins as compared with PBS-treated recipients on day 0 prior to transplantation (Figure 3A) and day 2 after BMT (Figure 3B). Treatment with AT or FLU also increased the amount of CD4+IL-4+ and CD4+IL-10+ cells (Figure 3C). Interestingly, the frequency of CD4+IL-4−IL-10+ cells was found to be increased from 7% to 18% upon AT treatment (Figure 3C). The CD4+IL-4−IL-10+ phenotype is “Tr1-like,” and resembles a cell population that was demonstrated to promote transplantation tolerance.25 When comparing the geometric mean derived from 3 independent experiments, the frequency of CD4+IL-4−IL-10+ cells was significantly higher in the AT treatment group as compared with the PBS group (P < .05).
AT and FLU recipient treatment reduced expression of costimulatory molecules and MHC class II on antigen-presenting cells in liver and lymphoid organs. (A) Representative flow cytometric analysis of the cell surface expression of CD80, CD86, CD40, and MHC class II on CD11c+ APCs isolated from Balb/c recipients after 10 days of feeding with phosphate-buffered saline (PBS, heavy black line), atorvastatin (AT, red line), or fluvastatin (FLU, blue line) prior to transplantation (day 0). The thin black line represents the isotype control. (B) Recipient-type APCs were isolated on day 2 after transplantation from the indicated organs. The mean fluorescence intensity of the H-2KdCD11c gated population is quantified for the indicated surface molecules (PBS vs AT, *P < .05). (C) IL-4 and IL-10 expression within the CD4+ T-cell population derived from the spleen prior to transplantation is shown. AT and FLU pretreatment as described in panel A increases the frequency of CD4+IL-4+IL-10+ T cells as well as CD4+IL-4−IL-10+ Tr-1 regulatory cells. One representative FACS analysis of 3 independent experiments is shown. (D) Balb/c mice were given 5 × 106 TCD-BM cells and 1.2 × 106 CD4+/CD8+ (4:1) T cells (both H-2q) after lethal irradiation with 800 cGy. Recipient animals were fed with either PBS or the indicated statin by oral gavage for 10 days prior to transplantation. Survival of mice receiving TCD-BM (●, n = 10) plus T cells, and being pretreated with PBS (▵, n = 10), fluvastatin (○, n = 10), or atorvastatin (■, n = 10). Survival of Balb/c recipients is significantly higher when recipients are treated with FLU or AT as compared with PBS (○ vs ▵, P = .004; ■ vs ▵, P = .006). (E) Expansion of luciferase-labeled (luc+) T cells was quantified in emitted photons over total body area at serial time points after BMT in mice receiving TCD-BM (●, n = 10) and being pretreated with PBS (▵, n = 10), fluvastatin (FLU, ○, n = 10), or atorvastatin (AT, ■, n = 10). Signal intensity is significantly higher in animals pretreated with PBS only as compared with FLU- and AT-treated animals (▵ vs ○, P = .003; ▵ vs ■, P < .003). Data from 2 independent experiments are combined.
AT and FLU recipient treatment reduced expression of costimulatory molecules and MHC class II on antigen-presenting cells in liver and lymphoid organs. (A) Representative flow cytometric analysis of the cell surface expression of CD80, CD86, CD40, and MHC class II on CD11c+ APCs isolated from Balb/c recipients after 10 days of feeding with phosphate-buffered saline (PBS, heavy black line), atorvastatin (AT, red line), or fluvastatin (FLU, blue line) prior to transplantation (day 0). The thin black line represents the isotype control. (B) Recipient-type APCs were isolated on day 2 after transplantation from the indicated organs. The mean fluorescence intensity of the H-2KdCD11c gated population is quantified for the indicated surface molecules (PBS vs AT, *P < .05). (C) IL-4 and IL-10 expression within the CD4+ T-cell population derived from the spleen prior to transplantation is shown. AT and FLU pretreatment as described in panel A increases the frequency of CD4+IL-4+IL-10+ T cells as well as CD4+IL-4−IL-10+ Tr-1 regulatory cells. One representative FACS analysis of 3 independent experiments is shown. (D) Balb/c mice were given 5 × 106 TCD-BM cells and 1.2 × 106 CD4+/CD8+ (4:1) T cells (both H-2q) after lethal irradiation with 800 cGy. Recipient animals were fed with either PBS or the indicated statin by oral gavage for 10 days prior to transplantation. Survival of mice receiving TCD-BM (●, n = 10) plus T cells, and being pretreated with PBS (▵, n = 10), fluvastatin (○, n = 10), or atorvastatin (■, n = 10). Survival of Balb/c recipients is significantly higher when recipients are treated with FLU or AT as compared with PBS (○ vs ▵, P = .004; ■ vs ▵, P = .006). (E) Expansion of luciferase-labeled (luc+) T cells was quantified in emitted photons over total body area at serial time points after BMT in mice receiving TCD-BM (●, n = 10) and being pretreated with PBS (▵, n = 10), fluvastatin (FLU, ○, n = 10), or atorvastatin (AT, ■, n = 10). Signal intensity is significantly higher in animals pretreated with PBS only as compared with FLU- and AT-treated animals (▵ vs ○, P = .003; ▵ vs ■, P < .003). Data from 2 independent experiments are combined.
The impact of FLU and AT on recipient APCs translated into partial aGVHD protection with a long-term survival of 50% and 40%, respectively, compared with 0% survival in animals not treated with these drugs (Figure 3D). Serial longitudinal BLI measurements demonstrated reduced T-cell expansion when the recipients were pretreated with AT or FLU (Figure 3E).
Sustained Th-2 commitment of donor T cells after BMT predicts aGVHD protection
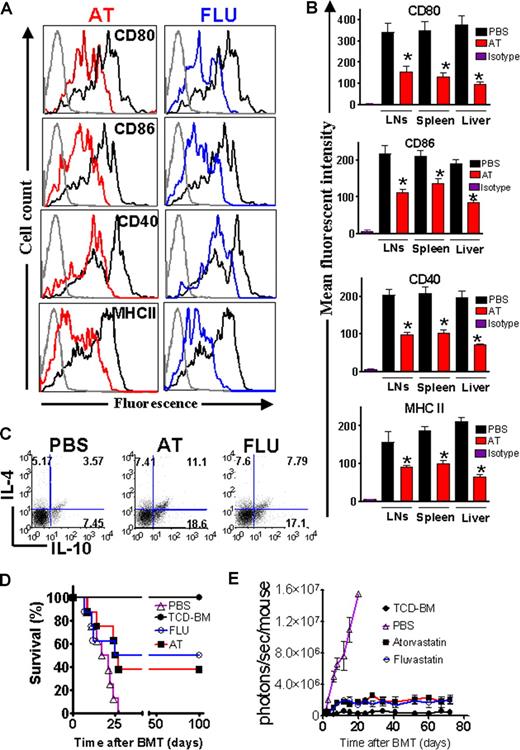
To investigate whether the observed statin effect on donor T cells and recipient APCs would synergize in a combined approach, both recipient and donor received AT (1 mg/kg) or PBS treatment. Survival of Balb/c recipients when both recipient and donor were fed with AT was 65%, as compared with 43% when only the T-cell/BM donors were fed with AT and 40% when only the recipients were fed (Figure 4A). When comparing each group with the PBS control group, the survival rate was improved in the Donor + Recipient AT feeding group (AT(D+R); P = .003), the donor-only AT feeding treatment group (AT(D); P = .007), and the recipient-only AT feeding group (AT(R); P = .036) (Figure 4A). The improved survival rate was correlated with reduced proinflammatory serum cytokine levels of TNF and IFN-γ in animals receiving donor and recipient AT treatment as compared with PBS (Figure 4B) after BMT.
Combined donor and recipient feeding has an additive protective effect and protection is predicted by the kinetics of Th-2 biased donor T cells in secondary lymphoid organs and the liver. (A) Survival rate of mice receiving TCD-BM alone (●, n = 10). Survival rate of recipients of TCD-BM with T cells when donor and recipient were treated with PBS (▵, n = 10) or AT (AT(D + R), ○, n = 10). Recipients of TCD-BM with T cells when the donor (□, n = 10) or the recipient (▾, n = 10) was treated with AT. Survival percentage of Balb/c recipients when both recipient and donor were fed with AT (65%), when only the T-cell/BM donors were fed with AT (43%) and when only the recipient was fed with AT (40%). (B) Serum was collected from recipients on the indicated days after BMT and IFN-γ and TNF levels were determined by ELISA (*P < .05, **P < .01). (C) Adoptively transferred CD4+H-2Kq+ donor T cells were isolated from the indicated organs and analyzed for their intracellular cytokine profile on days 3, 15, and 25. The y-axis depicts the percentage of IL-4–, IL-10–, TNF-, or IFN-γ–positive CD4+ T cells within the total CD4+H-2Kq+ donor population. Data presented are derived from 2 independent experiments (3 mice per time point, values are mean plus or minus standard deviation [SD]).
Combined donor and recipient feeding has an additive protective effect and protection is predicted by the kinetics of Th-2 biased donor T cells in secondary lymphoid organs and the liver. (A) Survival rate of mice receiving TCD-BM alone (●, n = 10). Survival rate of recipients of TCD-BM with T cells when donor and recipient were treated with PBS (▵, n = 10) or AT (AT(D + R), ○, n = 10). Recipients of TCD-BM with T cells when the donor (□, n = 10) or the recipient (▾, n = 10) was treated with AT. Survival percentage of Balb/c recipients when both recipient and donor were fed with AT (65%), when only the T-cell/BM donors were fed with AT (43%) and when only the recipient was fed with AT (40%). (B) Serum was collected from recipients on the indicated days after BMT and IFN-γ and TNF levels were determined by ELISA (*P < .05, **P < .01). (C) Adoptively transferred CD4+H-2Kq+ donor T cells were isolated from the indicated organs and analyzed for their intracellular cytokine profile on days 3, 15, and 25. The y-axis depicts the percentage of IL-4–, IL-10–, TNF-, or IFN-γ–positive CD4+ T cells within the total CD4+H-2Kq+ donor population. Data presented are derived from 2 independent experiments (3 mice per time point, values are mean plus or minus standard deviation [SD]).
To evaluate the fate of donor-derived AT–induced Th-2 T cells in the allogeneic recipient, we analyzed the frequency of donor CD4 T cells with respect to intracellular Th-2 and Th-1 cytokines after BMT. Higher frequencies of CD4+IL-4+ and CD4+IL10+ T cells were found in the AT group as compared with the PBS group (Figure 4C). Intracellular TNF- and IFN-γ–positive H-2KqCD4+ T cells were reduced in the LNs and livers of AT as compared with PBS-pretreated animals (Figure 4C). Frequencies of H-2KqCD8+IL-4+ and H-2KqCD8+IL10+ T cells were comparable in the AT group and the PBS group (Figure S2A). Intracellular IFN-γ–positive H-2KqCD8+ T cells were transiently reduced on day 3 in the LNs and livers of AT as compared with PBS-pretreated animals (Figure S2A). Expansion of H-2KqCD8+ T cells was reduced in the AT group as compared with the PBS group (Figure S2B). This reduction may be indirect through the CD4 Th-2 cell population that was induced by the AT treatment.
Statin-mediated aGVHD protection is partially STAT6 dependent
Activated signal transducer and activator of transcription 6 (STAT6) is required for IL-4–dependent Th-2 lineage commitment.26,27 To study the AT effect on aGVHD development in the absence of STAT6 signaling, wild-type (wt) or Stat6tm1Gru/J donors were employed. STAT6 deficiency of the donor led to reduced AT-mediated protection but did not completely abrogate the statin effect on aGVHD lethality with an overall survival of 33% as compared with 70% (Figure 5A). Coadministration of L-mevalonate abrogated the protective AT effect (Figure 5B). Improved survival in the AT-treated groups correlated with reduced expansion of CFSE-labeled donor T cells in vivo as shown for a representative animal from each group (Figure 5C). Consistent with the survival data, expansion of STAT6−/− T cells was higher as compared with wt T cells when both were exposed to AT in vivo (Figure 5C). Histopathologic aGVHD scoring 10 days after BMT identified the wt AT group as having the lowest disease score, whereas treatment of STAT6−/− donors with AT1 resulted in an intermediate score (Figure 5D). When comparing aGVHD target organs we observed the most notable differences between the groups in liver histopathology. T cells from AT-treated STAT6−/− donors led to intermediate liver damage with a low degree of lymphocytic infiltration surrounding the bile ducts (Figure 5E).
STAT6 deficiency allows only for partial protection by AT while L-mevalonate reverses the protective statin effect. C57BL/6 mice (H-2b, Th-1.1) were given 5 × 106 TCD-BM cells and 2 × 106 CD4+/CD8+ (4:1) T cells (both H-2d, Thy-1.2) after lethal irradiation with 800 cGy. Donor animals were fed with either PBS or AT for 10 days prior to transplantation. (A) Survival of mice receiving TCD-BM (▵, n = 15) with T cells from PBS-treated donors (□, n = 15), atorvastatin-treated (AT, 10 mg/kg) wt donors (■, n = 15), and STAT6-deficient donors treated with PBS (●, n = 15) or AT (○, n = 15). Percentage survival of Balb/c recipients is significantly higher when T cells are derived from wt donors as compared with STAT6-deficient donors when both are treated with AT (■ vs ○, P = .02). Data from 2 independent experiments are combined. (B) Survival of mice receiving TCD-BM (▵, n = 15) with T cells from PBS-treated donors (□, n = 15), atorvastatin-treated (AT, 10 mg/kg) donors (■, n = 15), AT with L-mevalonate pretreatment (○, n = 15), and PBS with L-mevalonate (●, n = 15). Percentage survival of Balb/c recipients is significantly higher when T cells are derived from AT as compared with AT with L-mevalonate–treated donors (■ vs ○, P = .004). Data from 2 independent experiments are combined. (C) In vivo expansion of CFSE-labeled Balb/c (H-2Kd, Thy-1.2+) CD4 T cells in C57BL/6 recipients (H-2Kd Thy-1.1+) on day 3 after BMT is depicted. Percentages of T cells having undergone cell division are indicated in the left upper corner of each histogram. Proliferation of T cells in the group pretreated with AT is significantly reduced as compared with PBS, STAT6−/− with AT, and AT with L-mevalonate (P < .05). (D) At 10 days after transplantation, mice from the indicated group were killed and sections of small bowel, large bowel, and liver were stained with H&E. Slides were analyzed by a pathologist blinded to the treatment groups (N.K.) for evidence of pathologic damage. Histopathologic scoring was performed as described in “GVHD histopathology scoring” with a range from 0 (absence of GVHD signs) to 4 (maximal GVHD damage). Data are pooled from 3 experiments, representing 6 animals per group. (E) Histopathologic evaluation of representative liver samples collected 10 days after transplantation is shown for the PBS group (i) and the groups where donor T cells are derived from AT-treated wt (ii) or STAT6−/− (iii) donors. Maximal liver tissue damage and strong lymphocytic infiltration surrounding the bile duct region as indicated by the solid arrow (i), minimal tissue damage (ii), intermediate liver damage, and low degree of lymphocytic infiltration surrounding the bile duct (iii) (dashed arrow). Magnification is ×400.
STAT6 deficiency allows only for partial protection by AT while L-mevalonate reverses the protective statin effect. C57BL/6 mice (H-2b, Th-1.1) were given 5 × 106 TCD-BM cells and 2 × 106 CD4+/CD8+ (4:1) T cells (both H-2d, Thy-1.2) after lethal irradiation with 800 cGy. Donor animals were fed with either PBS or AT for 10 days prior to transplantation. (A) Survival of mice receiving TCD-BM (▵, n = 15) with T cells from PBS-treated donors (□, n = 15), atorvastatin-treated (AT, 10 mg/kg) wt donors (■, n = 15), and STAT6-deficient donors treated with PBS (●, n = 15) or AT (○, n = 15). Percentage survival of Balb/c recipients is significantly higher when T cells are derived from wt donors as compared with STAT6-deficient donors when both are treated with AT (■ vs ○, P = .02). Data from 2 independent experiments are combined. (B) Survival of mice receiving TCD-BM (▵, n = 15) with T cells from PBS-treated donors (□, n = 15), atorvastatin-treated (AT, 10 mg/kg) donors (■, n = 15), AT with L-mevalonate pretreatment (○, n = 15), and PBS with L-mevalonate (●, n = 15). Percentage survival of Balb/c recipients is significantly higher when T cells are derived from AT as compared with AT with L-mevalonate–treated donors (■ vs ○, P = .004). Data from 2 independent experiments are combined. (C) In vivo expansion of CFSE-labeled Balb/c (H-2Kd, Thy-1.2+) CD4 T cells in C57BL/6 recipients (H-2Kd Thy-1.1+) on day 3 after BMT is depicted. Percentages of T cells having undergone cell division are indicated in the left upper corner of each histogram. Proliferation of T cells in the group pretreated with AT is significantly reduced as compared with PBS, STAT6−/− with AT, and AT with L-mevalonate (P < .05). (D) At 10 days after transplantation, mice from the indicated group were killed and sections of small bowel, large bowel, and liver were stained with H&E. Slides were analyzed by a pathologist blinded to the treatment groups (N.K.) for evidence of pathologic damage. Histopathologic scoring was performed as described in “GVHD histopathology scoring” with a range from 0 (absence of GVHD signs) to 4 (maximal GVHD damage). Data are pooled from 3 experiments, representing 6 animals per group. (E) Histopathologic evaluation of representative liver samples collected 10 days after transplantation is shown for the PBS group (i) and the groups where donor T cells are derived from AT-treated wt (ii) or STAT6−/− (iii) donors. Maximal liver tissue damage and strong lymphocytic infiltration surrounding the bile duct region as indicated by the solid arrow (i), minimal tissue damage (ii), intermediate liver damage, and low degree of lymphocytic infiltration surrounding the bile duct (iii) (dashed arrow). Magnification is ×400.
Biochemical effects of AT-mediated Th-2 lineage commitment
There is currently no information on the effect of statins on the prenylation status of Ras and Rho GTPases when T cells encounter APCs mismatched for class I and II MHC. We thus evaluated Ras and Rho, as well as STAT4 and STAT6 phosphorylation and T-bet, c-MAF, and GATA-3 expression in this situation. Addition of AT induced a Th-2 cytokine shift in the alloantigen-exposed T cells with lower intracellular IFN-γ and TNF levels, while IL-4 and IL-10 levels were increased (Figure 6A). We found that RAS, RAP-1, and Rho-B prenylation was reduced in T cells exposed to AT as compared with those exposed to alloantigen alone (Figure 6B). To analyze donor-type CD4 T cells after transplantation, we used Western blotting and phospho-flow analysis28 (Figure 6C,D). Furthermore, we found that AT decreased STAT4 phosphorylation (Figure 6D) and T-bet expression (Figure 6E) significantly.
Differential impact of AT on RAS, RAP-1, and Rho-B prenylation, T-bet, GATA3, and c-MAF expression. (A) CD4+ T cells (H-2Kq) were exposed to irradiated allogeneic APCs (H-2Kd) of each cell type, 2 × 106 T cells/flat-bottomed, 6-well plate for 48 hours in the presence or absence of AT (10 μM), harvested and analyzed by intracellular cytokine staining with anti–IL-10, –IL-4, –TNF-α, or –IFN-γ. Mean fluorescence for the representative cytokines of CD4+H-2Kq+ gated cells is presented. (B) Cells cultured under the conditions described in panel A were then washed and responder T cells (H-2Kq) were re-isolated by MACS with anti–H-2Kq-FITC and anti-FITC beads. Protein samples from in vitro–treated T cells were subject to Western blot analysis. AT is effective at inhibiting the prenylation of Ras, Rap-1, and Rho-B in the allogeneic activation culture. Increased amounts of nonprenylated proteins are found when AT is present (solid arrow). (C) T cells were harvested from AT-treated donors on day 0 (left panel) or on day 3 (right panel) after BMT from the recipients' secondary lymphoid organs and were analyzed by Western blot for the indicated total or phosphorylated proteins. (D) Mean fluorescence for the representative pSTATs is analyzed by phospho-flow analysis of CD4+H-2Kq+ gated cells, presented for the respective day prior to or after BMT. MFI for pSTAT4 was 11.4 (± 0.7) versus 47.4 (± 3.4) for the AT versus the PBS group, respectively, prior to transfer into the irradiated host (P < .05). MFI for pSTAT4 was 97.2 (± 2.1) versus 541.4 (± 11.3) for the AT versus the PBS group, respectively, on day 3 after BMT (P < .01). (E) Donor type T cells derived from AT-treated donors are presented for the respective day prior to or after BMT. Mean fluorescence for c-MAF, T-bet, and GATA-3 within CD4+H-2Kq+ gated cells is presented. MFI for T-bet was 12.4 (± 0.8) versus 98.4 (± 6.3) for the AT versus the PBS group, respectively, prior to transfer into the irradiated host (P < .01). MFI for T-bet was 137.2 (± 4.1) versus 21.4 (± 5.2) for the PBS versus the AT group, respectively, on day 3 after BMT (P < .01).
Differential impact of AT on RAS, RAP-1, and Rho-B prenylation, T-bet, GATA3, and c-MAF expression. (A) CD4+ T cells (H-2Kq) were exposed to irradiated allogeneic APCs (H-2Kd) of each cell type, 2 × 106 T cells/flat-bottomed, 6-well plate for 48 hours in the presence or absence of AT (10 μM), harvested and analyzed by intracellular cytokine staining with anti–IL-10, –IL-4, –TNF-α, or –IFN-γ. Mean fluorescence for the representative cytokines of CD4+H-2Kq+ gated cells is presented. (B) Cells cultured under the conditions described in panel A were then washed and responder T cells (H-2Kq) were re-isolated by MACS with anti–H-2Kq-FITC and anti-FITC beads. Protein samples from in vitro–treated T cells were subject to Western blot analysis. AT is effective at inhibiting the prenylation of Ras, Rap-1, and Rho-B in the allogeneic activation culture. Increased amounts of nonprenylated proteins are found when AT is present (solid arrow). (C) T cells were harvested from AT-treated donors on day 0 (left panel) or on day 3 (right panel) after BMT from the recipients' secondary lymphoid organs and were analyzed by Western blot for the indicated total or phosphorylated proteins. (D) Mean fluorescence for the representative pSTATs is analyzed by phospho-flow analysis of CD4+H-2Kq+ gated cells, presented for the respective day prior to or after BMT. MFI for pSTAT4 was 11.4 (± 0.7) versus 47.4 (± 3.4) for the AT versus the PBS group, respectively, prior to transfer into the irradiated host (P < .05). MFI for pSTAT4 was 97.2 (± 2.1) versus 541.4 (± 11.3) for the AT versus the PBS group, respectively, on day 3 after BMT (P < .01). (E) Donor type T cells derived from AT-treated donors are presented for the respective day prior to or after BMT. Mean fluorescence for c-MAF, T-bet, and GATA-3 within CD4+H-2Kq+ gated cells is presented. MFI for T-bet was 12.4 (± 0.8) versus 98.4 (± 6.3) for the AT versus the PBS group, respectively, prior to transfer into the irradiated host (P < .01). MFI for T-bet was 137.2 (± 4.1) versus 21.4 (± 5.2) for the PBS versus the AT group, respectively, on day 3 after BMT (P < .01).
AT treatment led to a slight increase of pSTAT6 and a lower level of pSTAT1 and pSTAT4 in the donor CD4 T cells prior to their injection into the irradiated recipient (Figure 6D). These differences increased significantly on day 3 after transplantation, with a higher expression of pSTAT1, pSTAT3, and pSTAT4 in PBS- as compared with AT-pretreated donor-type CD4 T cells (Figure 6D). Conversely, AT-pretreated T cells had a higher pSTAT6 level (Figure 6D), which is compatible with the observed Th-2 induction by AT. Expression of T-bet, which has been shown to be a key intracellular factor for Th-1 commitment,29 was reduced by AT pretreatment (Figure 6E). c-MAF and GATA-3, which are relevant for Th-2 commitment, were increased in T cells derived from AT-pretreated donors on day 3 after BMT (Figure 6E). These data demonstrate the profound effect of AT at various molecular levels on CD4 T cells with respect to Th-1/Th-2 polarization.
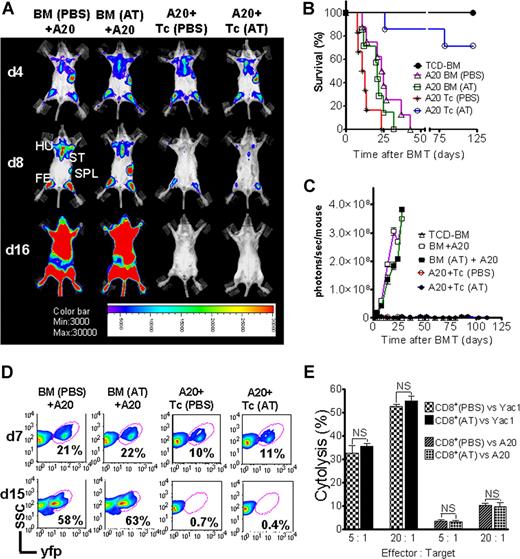
AT treatment is permissive for GVL activity and donor engraftment
Activation and cytolytic effector function of donor-derived cytotoxic T lymphocytes (CTLs) is critical for the elimination of the underlying malignant disease after BMT. To study if GVL activity was maintained in the presence of Th-2 polarization, induced by preemptive AT treatment, we employed A20-luc/yfp cells (H-2Kd).17,20 Where indicated, T cells were given with accompanying previous AT or PBS treatment of donor and recipient. Serial BLI images showed leukemic BM infiltration on day 4 after transplantation in all animals demonstrating similar leukemic engraftment (Figure 7A). Animals that received TCD-BM and T cells plus PBS achieved tumor control (Figure 7A), yet died from aGVHD (Figure 7B). AT-pretreated recipients receiving T cells from AT-treated donors rejected the A20 cells, as determined by loss of tumor signal (Figure 7C) indicative of intact in vivo effector function of CTLs against the leukemia cells. To evaluate if the observed signal loss was indeed associated with tumor clearance, spleen and BM were investigated by FACS analysis and histology. FACS analysis of BM on day 7 after transplantation indicated a distinct yfp+cell population in all groups, with lower frequencies in animals receiving T cells, representing the early GVL effect (Figure 7D). Consistent with the BLI data, animals receiving T cells and PBS or AT treatment demonstrated complete tumor clearance by day 15 (Figure 7D). To probe in vitro cytolytic activity of CD8+ T cells against different tumor target cells, CD8+ T cells were isolated from donor animals, pretreated with AT or PBS, and employed for in vitro killing assays against A20 or Yac1 cell lines. No difference in cytolytic activity was found independent of AT treatment and as shown for in vitro cytolysis of Yac1 T-cell lymphoma cells (Figure 7E).
GVL effector function of cytolytic T cells is preserved in the presence of atorvastatin treatment of donor and recipient. Tumor growth and GVL effect are visualized by bioluminescence imaging (A), recipient survival (B), emitted photons over time (C), and FACS analysis (D). All Balb/c recipients (H-2Kd) were given 105 A20 leukemia cells (H-2Kd) on day 0 and BMT was performed as described in “Tumor models.” (A) Representative animals of each group display similar luc+A20 tumor cell localization on day 4 (top row) after BMT. Tumor elimination in animals receiving T cells on days 8 (middle row) and 16 (bottom row). Mice underwent transplantation with TCD-BM after PBS (first column) or AT (second column) treatment, TCD-BM plus T cells exposed to PBS (third column) or AT (fourth column). Tumor infiltration is found in the following locations: ST, sternum; HU, humerus; FE, femur; SPL, spleen. (B) Survival of BALB/c mice receiving TCD-BM alone (●, n = 10), TCD-BM plus A20 leukemia cells after pretreatment of donor and recipient with PBS (▵, n = 10) or AT (□, n = 10), TCD-BM plus A20 leukemia cells plus T cells after pretreatment of donor and recipient with PBS (*, n = 10) or AT (○, n = 10). Survival: ○ versus *, P < .001. Data from 2 independent experiments are combined. (C) Expansion of luc+A20 tumor cell as measured in photons over total body area (photons/second/mouse). Animals rejecting the A20 leukemia cells demonstrate a durable signal loss as depicted until day 112 after BMT. Data from 2 independent experiments are combined. (D) Bone marrow samples with yfp+ A20 cells of recipients on days 7 and 15 after transplantation with TCD-BM plus A20 leukemia cells after pretreatment of donor and recipient with PBS or AT, TCD-BM plus A20 leukemia cells plus T cells after pretreatment of donor and recipient with PBS or AT as indicated for the respective column. (E) In vitro cytolytic effector function of CD8+ T cells (FVB/N) derived from animals treated with PBS or AT. Target cells were A20 or Yac1 tumor cells and chromium release assay was performed as described in “51Cr release cytotoxicity assay.”
GVL effector function of cytolytic T cells is preserved in the presence of atorvastatin treatment of donor and recipient. Tumor growth and GVL effect are visualized by bioluminescence imaging (A), recipient survival (B), emitted photons over time (C), and FACS analysis (D). All Balb/c recipients (H-2Kd) were given 105 A20 leukemia cells (H-2Kd) on day 0 and BMT was performed as described in “Tumor models.” (A) Representative animals of each group display similar luc+A20 tumor cell localization on day 4 (top row) after BMT. Tumor elimination in animals receiving T cells on days 8 (middle row) and 16 (bottom row). Mice underwent transplantation with TCD-BM after PBS (first column) or AT (second column) treatment, TCD-BM plus T cells exposed to PBS (third column) or AT (fourth column). Tumor infiltration is found in the following locations: ST, sternum; HU, humerus; FE, femur; SPL, spleen. (B) Survival of BALB/c mice receiving TCD-BM alone (●, n = 10), TCD-BM plus A20 leukemia cells after pretreatment of donor and recipient with PBS (▵, n = 10) or AT (□, n = 10), TCD-BM plus A20 leukemia cells plus T cells after pretreatment of donor and recipient with PBS (*, n = 10) or AT (○, n = 10). Survival: ○ versus *, P < .001. Data from 2 independent experiments are combined. (C) Expansion of luc+A20 tumor cell as measured in photons over total body area (photons/second/mouse). Animals rejecting the A20 leukemia cells demonstrate a durable signal loss as depicted until day 112 after BMT. Data from 2 independent experiments are combined. (D) Bone marrow samples with yfp+ A20 cells of recipients on days 7 and 15 after transplantation with TCD-BM plus A20 leukemia cells after pretreatment of donor and recipient with PBS or AT, TCD-BM plus A20 leukemia cells plus T cells after pretreatment of donor and recipient with PBS or AT as indicated for the respective column. (E) In vitro cytolytic effector function of CD8+ T cells (FVB/N) derived from animals treated with PBS or AT. Target cells were A20 or Yac1 tumor cells and chromium release assay was performed as described in “51Cr release cytotoxicity assay.”
In order to investigate the mechanism by which GVL activity against the A20 leukemia cells was mediated in vivo, we employed C57BL/6 donor mice that had a defective Fas-ligand gene (Tnfsf6gld/gld) or were deficient in perforin (Prf1−/−) as donors of BM and T cells. Elimination of luc/yfp+ tumor cells was impaired when Prf1−/− donors were used, and slightly delayed when the donor was FasL defective as compared with wt donors when evaluated by BLI (Figure S3A). However animals receiving FasL-defective T cells eventually cleared the yfp+ A20 cells as shown by FACS analysis on the investigated time points (Figure S3B). Recipients of FasL-defective T cells developed delayed GVHD morbidity which is consistent with previous reports indicating that FasL is important for GVHD development and that in its absence GVHD kinetics are reduced.30 Survival was ameliorated in the AT group as compared with the PBS group when donor T cells were FasL defective (Figure S3C).
The transfer of T cells from PBS-pretreated Prf1−/− mice resulted in 100% GVHD lethality before day 25 after BMT (Figure S3C). Transplantation of Tnfsf6gld/gld T cells from PBS-treated donors induced less severe GVHD with early death of 40% of animals and delayed the death of others (Figure S3C). When we administered AT to inhibit GVHD lethality, the effector mechanisms responsible for the in vivo clearance of A20-luc/yfp leukemia cells could be examined. Animals that received AT and Prf1−/− T cells lacked GVL activity and all animals died early from leukemia (Figure S3C). Animals receiving AT and Tnfsf6gld/gld T cells had initial engraftment of A20 leukemia, which they managed to control between days 10 and 22 after transplantation, but died later (Figure S3C). AT pretreatment did not interfere with perforin-mediated cytolysis or Fas–Fas-ligand interaction–mediated killing. This indicates that the in vivo effects of AT were restricted to T-cell expansion, but did not influence CTL effector mechanisms.
To determine if these observations were restricted to a single tumor model we investigated minimal residual disease conditions by injecting a second tumor cell line, BCL1, 5 days prior to irradiation and transplantation. Recipients of TCD-BM developed overt relapse of the luc+ BCL1 tumor whereas animals that received T cells from PBS- or AT-treated donors managed to control the tumor during the observation period (Figure S3D), with the AT group having an improved survival (Figure S3E) as compared with the PBS group (P = .01).
Discussion
Several studies have demonstrated that statin treatment can induce a protective effect in different cancer models31 as well as allograft rejection and autoimmunity7,32 by pleiotropic mechanisms. The major finding reported in this study is that AT can exert protection across major allogeneic mismatch barriers in different strain combinations by reducing lethal aGVHD while preserving GVL activity and donor engraftment. Th-1/Th-2 polarization of T-helper cell subsets impacts aGVHD development and determines end organ damage.33 Injection of IL-2 plus IL-4 into donor mice resulted in the generation of CD4+IL-4+ and CD4+IL-10+ cells that, upon transfer into sublethally irradiated mice, inhibited aGVHD lethality.34 In humans, elevated IL-10 production by peripheral blood mononuclear cells before BMT was associated with a subsequent low incidence of aGVHD and transplant-related mortality,35,36 whereas low IL-10 promoter activity predicts an increased risk for GVHD.5
In light of these findings and since AT was shown to induce high levels of IL-4 and IL-10 upon T-cell priming,7,8 we pretreated the donor with AT. AT was used in 2 dosages that had been previously demonstrated to exert immunomodulatory effects in the experimental autoimmune encephalomyelitis model.7,8 In humans, 80 mg AT is the highest recommended dose for treatment of hypercholesterolemia.37 Pharmacokinetic data in rodents indicate that larger AT doses are required to achieve similarly effective concentrations.38,39 A dose-finding study of simvastatin combined with standard chemotherapy in patients with myeloma and lymphoma demonstrated that the maximally tolerated dose was 15 mg/kg per day.40 Based on these data we used 2 AT doses: 1 mg/kg and 10 mg/kg.
We found reduced intracellular Th-1 cytokine production in vitro and in vivo when analyzing T cells at different time points. The observation that serum TNF and IFN-γ were reduced upon AT in vivo treatment suggests that the proinflammatory cytokine storm that leads to aGVHD progression1 is affected by the Th-2 polarization. However, aGVHD is not solely Th-1 mediated and develops also in the absence of IFN-γ production.41,42 This may explain our observation that although there was an impressive reduction of GVHD lethality, some animals developed signs of GVHD yet survived.
Our observation that the effect of AT could be reversed by the addition of L-mevalonate, the product of HMG-CoA reductase, indicates that the protective effect was through the mevalonate pathway. That notion is compatible with data in autoimmune models7,8 defining isoprenoids as the essential regulators of Th-1/Th-2 fate and thus offering an approach to modulate pathogenic T-cell responses by AT. In line with that concept we observed that the AT effects were controlled via reduction of isoprenoid intermediates that mediate the membrane association of GTPases involved in T-cell activation.8 Mechanistically, we found that the statin effect on alloantigen-exposed T cells is through reduction of the amount of prenylated Rac-1, RAS, and RhoB, which are critical for Th-1 lineage commitment. Interestingly, recent data on human T cells demonstrated that farnesyltransferase inhibitors (FTIs) that interfere with the farnesylation process of GTPases inhibited cytokine production of T cells in response to activating stimuli at the posttranscriptional level.43
In parallel to the immunomodulation observed by Th-2 induction, the de novo generation of a “Tr1 like” T-cell population that was IL-10+ was observed following in vivo AT administration. There was no impact of different statins on Foxp3+ naturally occurring Treg cells.
Based on the finding that the hepatic immune system is involved in GVHD development,44 and owing to the fact that orally administered statins exert their major lipid lowering effect in the liver, the organ most active in cholesterol synthesis regulation in mammals,45 we investigated the immunomodulatory AT effect on hepatic APCs. Interestingly, we found a strong effect of AT on costimulatory molecule and MHC class II expression on liver APCs. This observation may account for the impressive reduction of T-cell expansion in recipient animals preconditioned with AT and suggests that in vivo statin treatment reduces the alloreactivity of host APCs in secondary lymphoid organs and the liver as a GVHD target organ.
To more precisely dissect the pathways relevant for AT-mediated aGVHD protection, STAT6−/− donors were employed. T cells from STAT6−/− animals display impaired IL-4 responses and almost completely lack Th-2 responses.27 Consistent with the relevance of STAT6 for Th-2 induction, we observed that AT-mediated aGVHD protection was significantly reduced when STAT6−/− donors were employed. The fact that the AT effect reversion was partial is compatible with our observation that AT not only induces Th-2 commitment but also induces a Tr1-like cell population and has a pronounced effect on the costimulatory capabilities of APCs, which are independent of STAT6 deficiency.
The observation that AT treatment resulted in sustained suppression of donor T-cell proliferation might be potentially unfavorable in allogeneic BMT, in which donor T cells are critically important for eliminating residual leukemic cells and enhancing hematopoietic engraftment. We found that AT pretreatment did not cause general impairment of donor T-cell function since T-cell treatment allowed for BM engraftment and eradication of established tumors in 2 distinct model systems. Similar to the Th-1 and Th-2 phenotype in CD4 subsets, T1(Tc1) and T2(Tc2) subsets of CD8+ T cells could be induced by a Th-1 or Th-2 cytokine milieu, respectively.46 Importantly, previous studies have shown that Tc1 and Tc2 T cells exhibit comparable levels of perforin-mediated cytolysis.46 We found that perforin-mediated early GVL effector function was crucial for tumor elimination. Therefore, our observation that AT-mediated Th-2 bias induction was still permissive for GVL activity can be explained by the profound effect on T-cell proliferation but not on cytolytic effector function that allows Tc2 CD8+ T cells to exert perforin-dependent elimination of leukemic cells.
In conclusion, we show that AT can exert aGVHD protection without interfering with GVL activity or donor engraftment by modulating the immune response that ensues after BMT. Since AT acts on both host APCs and donor T cells by different pathways, statin treatment of the donor and/or the recipient prior to allogeneic hematopoietic cell transplantation may be a promising approach in an attempt to reduce aGVHD severity while harnessing the beneficial GVL effect in clinical trials.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Ruby M. Wong for expert assistance with statistical analysis and to the members of the Steinman and the Negrin laboratories for helpful discussion.
This study was supported by grants from the National Institute of Health (NIH; RO1 CA0800065 to R.S.N.) and in part through the National Cancer Institute's Small Animal Imaging Resource Program (SAIRP grant number R24CA92862) and the NCI In Vivo Cellular Molecular Imaging Center (ICMIC) grant P50 CA114747. R.Z. is supported by the Dr Mildred-Scheel-Stiftung, Bonn, Germany. S.Y. is supported by a postdoctoral fellowship from the National Multiple Sclerosis Society.
National Institutes of Health
Authorship
Contribution: R.Z. and S.Y. designed and performed experiments, analyzed data, and wrote the paper; J.B. performed experiments and analyzed data; N.K. performed histopathology scoring; L.S. and R.S.N. helped design experiments, provided overall guidance, and helped write the paper.
R.Z. and S.Y. contributed equally. L.S. and R.S.N. are co-senior authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert S. Negrin, Center for Clinical Science Research, 269 W Campus Dr, Rm 2205, Division of Blood and Marrow Transplantation, Stanford University School of Medicine, Stanford, CA 94305; e-mail:negrs@stanford.edu.

![Figure 4. Combined donor and recipient feeding has an additive protective effect and protection is predicted by the kinetics of Th-2 biased donor T cells in secondary lymphoid organs and the liver. (A) Survival rate of mice receiving TCD-BM alone (●, n = 10). Survival rate of recipients of TCD-BM with T cells when donor and recipient were treated with PBS (▵, n = 10) or AT (AT(D + R), ○, n = 10). Recipients of TCD-BM with T cells when the donor (□, n = 10) or the recipient (▾, n = 10) was treated with AT. Survival percentage of Balb/c recipients when both recipient and donor were fed with AT (65%), when only the T-cell/BM donors were fed with AT (43%) and when only the recipient was fed with AT (40%). (B) Serum was collected from recipients on the indicated days after BMT and IFN-γ and TNF levels were determined by ELISA (*P < .05, **P < .01). (C) Adoptively transferred CD4+H-2Kq+ donor T cells were isolated from the indicated organs and analyzed for their intracellular cytokine profile on days 3, 15, and 25. The y-axis depicts the percentage of IL-4–, IL-10–, TNF-, or IFN-γ–positive CD4+ T cells within the total CD4+H-2Kq+ donor population. Data presented are derived from 2 independent experiments (3 mice per time point, values are mean plus or minus standard deviation [SD]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/13/10.1182_blood-2007-08-106005/3/m_zh80010810940004.jpeg?Expires=1769153041&Signature=bKcNjEVJXHoHkcvyjPqK0pEJz8MtIHPGO6lYIGYDOKXTo0bISvjPR3Hb-lgYTMVqg7VHUj83QUs4wo5T~CSmUQNVqj9GKqkCrdtowX4wHMJhqZU~jt2Vyz7dPB3C~SFU~xpX08q1~2yd4b4Rb9sP8OFj3rt7FFZOH8-tNikUmSX~bXikIUNi8bs2H-CBw8eADkJw3Z5aRwdNhZOocgXYb5hvjBmfivSJPHCbaDREF35ngcY~jOE0frWKwGSRqhfqUxVw7PkwbW-5~TgTc23gggKTyzNomzEBWh7nDX8FGcvCohzw68C6CTtma2lRgWRW44DO-K6TpjrcwKSOO-QKsg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)