The studies by Fu and Richardson offer new insights into the molecular alterations in G1/S arrest caused by iron deficiency, and the mechanisms allowing iron chelators to induce antiproliferative activity and apoptosis.
Iron is essential for DNA synthesis, and iron chelators exert an inhibitory effect on the growth of tumor cells in vitro and in vivo. p21CIP1/WAF1 plays a key role in regulating the cell cycle and inducing apoptosis, and its expression is altered by iron-chelation treatment. In this issue of Blood, Fu and Richardson show that the posttranscriptional downregulation of p21CIP1/WAF1 following iron depletion is due to 2 mechanisms: decreased translocation of p21CIP1/WAF1 mRNA from the nucleus to the translational machinery in the cytosol, and the induction of ubiquitin-independent proteasomal degradation of the p21CIP1/WAF1 protein. The paper is commendable for its careful methodology, and the conclusions are strongly supported by the results of a series of well-designed experiments.
Previous studies have shown that p21CIP1/WAF1 is a positive regulator of G1-phase progression,1 and that it also has antiapoptotic activity.2 Promotion of G1 progression by p21CIP1/WAF1 is due to its ability to stabilize cyclin D1–cdk complexes. Because iron chelation decreases the expression of both cyclin D1 and p21CIP1/WAF1, it will prevent the formation of such complexes and promote G1/S arrest. Likewise, decreased p21CIP1/WAF1 expression is able to induce cancer-cell apoptosis.3
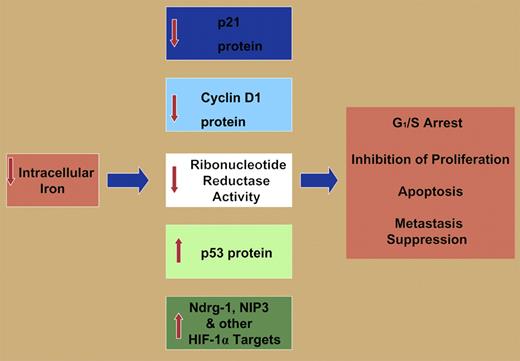
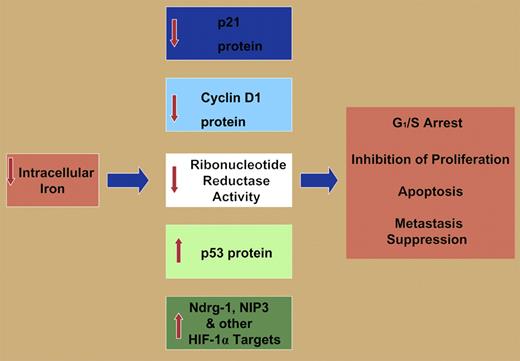
However, the ability of iron chelators to inhibit cell proliferation and to induce apoptosis is apparently due to their effects on multiple molecular targets (see figure), including inhibition of ribonucleotide reductase, the rate-limiting step of DNA synthesis4 decreasing cyclin D1 expression, and the up-regulation of the growth and metastasis suppressor Ndrg-1.5 It may be argued that the existence of multiple targets of iron chelators playing key roles in proliferation and apoptosis may facilitate their ability to function as antitumor agents.
Molecular effectors involved in G1/S arrest, inhibition of proliferation, apoptosis, and metastasis suppression modulated by iron deprivation including: down-regulation of p21CIP1/WAF1 protein and cyclin D1 protein, decreased activity of ribonucleotide reductase, increased p53 protein expression, increased expression of the metastasis suppressor protein Ndrg-1, and the up-regulation of the apoptosis-inducing protein NIP3. Both NIP3 and Ndrg-1 are targets of the hypoxia-inducible factor-1α (HIF-1α) transcription factor. Other HIF-1α targets are probably also involved in the iron-depletion–induced inhibition of proliferation and induction of apoptosis. See the complete figure in the article beginning on page 752.
Molecular effectors involved in G1/S arrest, inhibition of proliferation, apoptosis, and metastasis suppression modulated by iron deprivation including: down-regulation of p21CIP1/WAF1 protein and cyclin D1 protein, decreased activity of ribonucleotide reductase, increased p53 protein expression, increased expression of the metastasis suppressor protein Ndrg-1, and the up-regulation of the apoptosis-inducing protein NIP3. Both NIP3 and Ndrg-1 are targets of the hypoxia-inducible factor-1α (HIF-1α) transcription factor. Other HIF-1α targets are probably also involved in the iron-depletion–induced inhibition of proliferation and induction of apoptosis. See the complete figure in the article beginning on page 752.
Before considering iron chelators for tumor therapy in the clinical setting, several obstacles should be overcome. First, in the absence of iron overload, the use of iron chelators involves serious toxicity including neurologic complications and inhibition of normal, rapidly proliferating cells such as the hemopoietic tissues. Hence, innovative strategies should be developed to target chelators to relevant tissues without damaging other organs. Second, in order to be effective, chelators should be able to penetrate cells easily and have prolonged presence in the circulation. Several new orally effective chelators may fulfill these demands. Although the suggestion to exploit the regulation of cell cycle by iron depletion for therapeutic purposes may, at present, appear premature, it is important to understand the molecular mechanisms involved, and the article by Fu and Richardson offers essential new insights into these mechanisms.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■