For the first time, human granzyme H is shown to directly induce death in tumor target cells, changing its status from orphan to killer.
Cytotoxic lymphocytes are innate (natural killer [NK] cells) and adaptive (T cells) effector cells that play an important role in host defense against infection and malignant transformation. These lymphocytes utilize several mechanisms to induce cell death, but tumor and virus-infected cells are killed primarily via the perforin/granzyme pathway.1 Upon target recognition, cytotoxic granule contents (including perforin and granzymes) are released, and perforin facilitates entry and/or trafficking of granzymes into target cells. Granzymes then cleave intracellular protein substrates, leading to mitochondrial damage and target-cell death.1,2
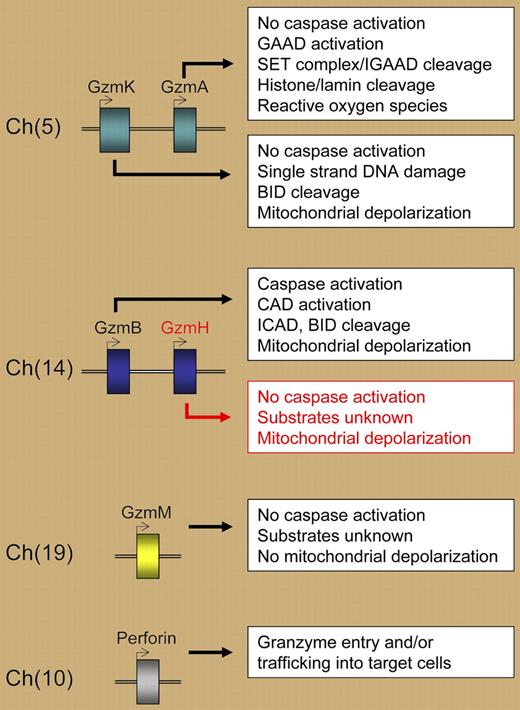
Granzymes are a conserved family of serine proteases situated in 3 chromosomal clusters (see figure). In humans, granzymes are located on chromosomes 5 (A and K), 14 (B and H), and 19 (M). In mice, the granzyme B locus includes additional granzymes downstream of granzyme B: granzymes C, F, N, G, D, and E. Initially, granzymes A and B were considered the primary granule exocytosis pathway killers, as they were shown to cleave distinct intracellular substrates leading to different forms of cell death. The other granzyme family members were collectively referred to as “orphan” granzymes—serine proteases without an identified function.3 Subsequently, this orphan status was revoked for granzyme C in the mouse, and granzymes K and M in humans—all of which have been shown to induce cell death. While it is known that granzyme H protein is abundant in CD56+CD3− NK cells but not in activated T cells, our overall understanding of granzyme H biology is limited.4
In this issue of Blood, Fellows and colleagues demonstrate for the first time that human granzyme H induces death in tumor target cells in a reconstituted in vitro system. The death induced by granzyme H displays many features of apoptosis, including DNA degradation, chromatin condensation, mitochondrial depolarization, and generation of reactive oxygen species. Granzyme H–induced death is distinct from that of granzyme B; it has a slower time course and does not involve caspase activation, cleavage of BID or ICAD, or the release of cytochrome C. Thus, granzyme H is now added to the arsenal of lethal granzymes contained in human NK-cell cytotoxic granules, and this study expands our knowledge of the repertoire of molecules that NK cells may use to kill virus-infected or tumor cells (see figure).
Human granzymes and perforin, their chromosome locations, and selected properties of granzyme-induced cell death. GAAD indicates granzyme A–activated DNase; IGAAD, inhibitor of GAAD; CAD, caspase-activated DNase; ICAD, inhibitor of CAD; and BID, BH3-interacting domain death agonist.
Human granzymes and perforin, their chromosome locations, and selected properties of granzyme-induced cell death. GAAD indicates granzyme A–activated DNase; IGAAD, inhibitor of GAAD; CAD, caspase-activated DNase; ICAD, inhibitor of CAD; and BID, BH3-interacting domain death agonist.
Key questions remain about the mechanism responsible for granzyme H–mediated death. What are the intracellular substrates cleaved by granzyme H to trigger cell death? What is the broader biological significance of granzyme H–induced cell death when NK cytotoxic granules also contain granzymes A, B, K, and M? Genetic knockout models in mice with targeted disruption of granzymes A, B, and M have revealed the relative importance of these serine proteases to host defense against infection and tumors. Because human granzyme H has a homology closest to murine granzyme C, and features of granzyme H–induced death are reminiscent of granzyme C,5 murine models of granzyme C deficiency may be informative about the importance of granzyme H for NK-cell killing. Additionally, identification of granzyme H mutations, or altered expression in human disease, would definitively place granzyme H on the list of key cytotoxic granule effector molecules.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■