Abstract
The hepatic peptide hormone hepcidin is considered the central regulator of iron metabolism. Characterizing the circulating levels of this peptide is critical to understanding its role in the development of clinically relevant syndromes, such as anemia of inflammation/chronic disease, and may provide insight into potential clinical interventions. While quantitative methods have been published for the determination of urinary hepcidin and serum prohepcidin, no definitive methods have been published for the determination of hepcidin in serum. In this report, we describe a quantitative method for the determination of both human and mouse hepcidin in serum and plasma. The method employs protein precipitation and solid-phase extraction followed by liquid chromatographic separation and tandem mass spectrometry detection. The method has a quantitative range of 0.25 ng/mL to 500 ng/mL serum for mouse hepcidin and 1 ng/mL to 500 ng/mL serum for human hepcidin. The method uses small sample volumes (50 μL for mice and 100 μL for humans) and 96-well formats for rapid sample processing. The method was used to establish baseline serum and plasma concentrations of hepcidin in normal C57Bl/6 mice and healthy human volunteers.
Introduction
Hepcidin, a 25-amino acid peptide synthesized in the liver, was initially isolated from human urine extracts and human blood ultrafiltrate.1,2 Hepcidin negatively regulates the absorption of iron from the intestine,3 iron release from hepatic stores,4 and the recycling of iron by macrophages.5,6 Consistent with its role in iron homeostasis, it has also been demonstrated that hepcidin production is regulated by hypoxia and anemia.7,8 Several studies have also provided evidence supporting the role of hepcidin as a causative agent in clinically relevant syndromes such as hemochromatosis and anemia of chronic disease/inflammation.9-17 Characterizing the circulating levels of hepcidin and factors that regulate its expression is critical to better defining the role of this peptide in both normal physiology and the development of clinically relevant aberrations of iron homeostasis. In addition to the diagnostic potential, insights from such analyses may provide opportunity for the development of novel therapeutic interventions based on agonizing or antagonizing this pathway.
To better understand the relationship between hepcidin and other iron regulatory proteins (ie, transferrin, ferritin, hemojuvelin, ferroportin) in healthy and diseased (anemic) patient populations, a quantitative method needs to be developed for determining hepcidin serum concentrations. While an understanding of tissue expression and cellular localization of hepcidin has been gleaned through the application of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western-blot methods,18 the quantitative relationship to peripheral concentrations is lacking. Urine and serum/plasma are target matrices that can be used clinically to study the levels of hepcidin in different disease states. Currently, the relationship of hepcidin to disorders of iron metabolism has been established via the measurement of urinary hepcidin concentrations using an immuno-dot,19 SDS-PAGE and Western blot,20 and surface-enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI-TOF-MS)21 assay format. Recently, 2 reports described the use of SELDI-TOF-MS for the semiquantitative determination of hepcidin in human serum.22,23 The SELDI-TOF-MS methods reported limits of detection of approximately 45 ng/mL22 and 61 ng/mL.23 While neither method reported absolute quantitative measurements of serum hepcidin, relationships were established between relative serum hepcidin levels and serum ferritin levels in samples from hemodialysis patients. Unfortunately, this approach may not have the level of analytic sensitivity necessary to determine serum hepcidin concentrations in healthy subjects or patients suffering from hepcidin deficiencies. Another report measured hepcidin levels in serum using a Western-blot assay.24 The method used a rabbit antibody directed against a 13-amino acid sequence in active human hepcidin. The authors indicated that while a correlation between serum ferritin and serum hepcidin was confirmed, the relationship did not correlate as well as a previous study that compared urinary hepcidin and serum ferritin levels.20 The poor correlation observed could be attributed to the method itself, which did not measure active hepcidin but rather an immunoreactive band at approximately 9 kDa, which is a larger antigen than expected for active human hepcidin (2.789 kDa).
Due to the lack of availability of a serum hepcidin assay, some investigators have employed an enzyme-linked immunosorbent assay (ELISA) method that measures prohepcidin, the 84-amino acid precursor of the active hepcidin peptide, with the inference that serum hepcidin would be directly correlated, or at least related, to these concentrations.25,26 In healthy subjects, mean serum prohepcidin concentrations were determined to be in the range of 106.2 ng/mL to 196 ng/mL. However, these levels were not significantly different from various patient groups that had mean serum prohepcidin concentrations of 148.4 ng/mL (chronic renal insufficiency), 70.2 ng/mL (hereditary hemochromatosis), and 115.0 ng/mL serum (renal anemia), respectively. In another study using the prohepcidin ELISA, no significant change was observed in the human serum prohepcidin level in response to a lipopolysaccharide (LPS) injection, whereas the urinary hepcidin level increased significantly from 1 to 3 hours following injection of LPS.27 Thus, serum prohepcidin levels did not correlate with urinary hepcidin levels in this study and cast doubt on the utility of prohepcidin measurements as a diagnostic surrogate for iron regulatory disorders or as a correlative determinate of circulating hepcidin levels.
While the importance of hepcidin in iron homeostasis is well established, its exact role as a causative or diagnostic indicator in various clinical disorders of iron regulation requires additional investigation. These efforts would benefit from the application of a robust and quantitative serum/plasma hepcidin method. In this report, we describe the development of a quantitative method for the determination of hepcidin in mouse and human plasma and serum using liquid chromatography tandem mass spectrometry.
Patients, materials, and methods
All collections were performed in-house under the approval of Lilly Research Laboratories' internal policy committee.
Synthetic human hepcidin, mouse hepcidin, and internal standard
Synthetic human hepcidin (DTHFPICIFCCGCCHRSKCGMCCKT, 2789.4 g/mol) was obtained from Peptide International Incorporated (Louisville, KY). A 100-μg human hepcidin/mL distilled water stock solution was prepared for use in plasma and serum standards and verification samples. Crude mouse hepcidin 1 (amino acids 1-25) was synthesized by Midwest Bio-Tech (Fishers, IN). The crude mouse peptide was dissolved in 6 M guanidine and extracted using a Waters C18 Sep-Pak 10 g cartridge (Waters Corporation, Milford, MA). The extracted mouse hepcidin peptide was speed-vac dried and dissolved in a reconstitution buffer (6 M guanidine, 1 M Tris-HCl, 100 mM DTT, pH 8.0) at 50°C for 1 hour. The dissolved mouse hepcidin peptide was diluted 20-fold into a solution containing 2 M urea and 3 mM L-cysteine and air oxidized over 3 days. The peptide was isolated using a Waters C18 Sep-Pak cartridge and subsequently purified on a Vydac C18 column (Grace Vydac, Hesperia, CA) using a shallow acetonitrile gradient in water with 0.1% TFA. The purified active mouse hepcidin 1 (DTNFPICIFCCKCCNNSQCGICCKT, 2752.02 g/mol) was concentrated by speed-vac. A 100-μg mouse hepcidin/mL distilled water stock solution was prepared to use in plasma and serum standards and verification samples. The internal standard, calcitonin gene-related peptide (CGRP; Sigma-Aldrich, St Louis, MO), was diluted into distilled water to a concentration of 200 μg/mL. A working internal standard solution of 50 ng/mL water was prepared prior to sample analysis (Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Plasma and serum collection from humans and mice
Two issues were considered when blood collection strategies were designed for the determination of hepcidin levels: (1) if hepcidin could differentially partition into plasma or serum during the processing of blood; and (2) the variability of hepcidin levels from individual humans or animals. To determine if hepcidin differentially partitions during the processing of blood, sera and plasma were collected from each of 5 male and 5 female healthy human volunteers. Plasma and sera were also collected, via cardiac puncture, from 5 male and 5 female normal C57Bl/6 mice (Charles River Laboratories, Wilmington, MA) maintained on the certified rodent diet 5002 (PMI Nutrition International, Henderson, CO). To determine the variability of hepcidin within an individual, a second collection of serum and plasma was obtained from 5 different healthy male volunteers. The study was designed to look at day-to-day changes in hepcidin levels by analyzing 5 consecutive daily morning collections from each of the 5 healthy volunteers. For all serum sample collections, the blood was collected into 10-mL vacutainer serum tubes (BD, Franklin Lakes, NJ), allowed to stand for 15 minutes, then centrifuged at 3500g for 25 minutes. To obtain plasma, blood was collected into 10-mL sodium heparin vacutainer tubes (BD) and centrifuged at 3500g for 25 minutes. The serum and plasma were transferred from the vacutainer tubes into polypropylene tubes and stored at −70°C until the time of analysis.
Method design to determine relative standard deviation (RSD) and relative error (RE)
We screened several lots of plasma and sera in an effort to find sources with no detectable hepcidin levels. We found one lot of pooled human serum (SeraCare Life Sciences, Milford, MA) that showed no detectable levels of hepcidin, but we lacked a sufficient supply to conduct all validation experiments. In the absence of an adequate supply of hepcidin-depleted plasma or serum for either mice or humans, we used control rabbit serum (Bioreclaimation, Hicksville, NY) as a surrogate matrix for determinations. Rabbit serum was chosen on the presumption that rabbit hepcidin has a different molecular weight than either mouse or human hepcidin and would not interfere with the assay. Analysis of several rabbit serum lots showed no matrix interference with either mouse or human hepcidin. Control rabbit serum was spiked with either mouse or human hepcidin at 500 ng/mL by the addition of 5 μL of the 100-μg hepcidin/mL water solutions into 995 μL of control serum. The 500-ng hepcidin/mL serum standards were diluted in control rabbit serum to yield 100, 50, 10, 5.0, 2.0, 1.0, 0.50, and 0.25 ng/mL standards. All standard curves were regressed using a linear 1/X2 regression function.
Verification samples to determine precision and accuracy were prepared for mouse and human hepcidin at 500, 100, 50, 10, 5.0, 2.0, 1.0, 0.50, and 0.25 ng/mL control rabbit serum. Precision (coefficient of variation) was expressed as percentage relative standard deviation (% RSD; standard deviation/mean × 100). Accuracy was expressed as percentage relative error (% RE; [mean value/theoretical value] − 1 × 100). Percentage RSD and % RE were determined by analyzing 6 replicate verification samples at each concentration on each of 3 days. Additional verification samples for freeze-thaw and dilutional linearity were prepared in which mouse hepcidin was spiked (standard addition) into pooled mouse serum (Harlan, Indianapolis, IN, and Lampire Biological Laboratories, Pipersville, PA) and pooled mouse plasma (Harlan and Lampire Biological Laboratories). Additional verification of freeze-thaw and dilutional linearity of human hepcidin was performed by comparative analysis of human serum and standard addition of human hepcidin into pooled plasma (SeraCare Life Sciences) or serum of healthy human subjects. All freeze-thaw samples were analyzed before the first freeze-thaw cycle and after the third freeze-thaw cycle to determine stability.
Serum and plasma sample preparation
All sample preparation steps were performed at room temperature. A 50-μL (mouse assay) or 100-μL (human assay) aliquot of plasma or serum was manually transferred to a 96-well polypropylene microtiter plate (ABgene, Rochester, NY) in which each 300-μL well is cone shaped. A 100-μL aliquot of acetonitrile (Burdick and Jackson, Muskegon, MI) was added to each well and mixed one time. A 50-μL aliquot of the internal standard solution (50 ng CGRP/mL distilled water) was added to each well, and the microtiter plate was then centrifuged at 7.0g for 10 minutes at 5°C in a Jouan GR4-22 centrifuge (Jouan, Winchester, VA). After centrifugation, a Quadra-96 Model 320 Liquid Handling Station (Tomtec, Hamden, CT) was used to transfer and mix the protein precipitation supernatant (110 μL for the mouse assay and 125 μL for the human assay) into a Waters Oasis HLB 30-μm μElution solid-phase extraction (SPE) plate containing 550 μL distilled water. Each well of the extraction plate was washed 2 times with 400 μL distilled water. Elution was performed with the addition of 180 μL 0.05/20/80 (trifluororoacetic acid/water/acetonitrile, vol/vol/vol) to each well of the plate. The eluant was collected into a 96-well polypropylene microtiter plate and evaporated to dryness at 50°C using a Jones SPE Dual Dry evaporator (Jones Chromatography, Lakewood, CO). The plate required approximately 40 minutes to dry. The contents of each well of the microtiter plate were reconstituted with 100 μL of 0.02% aqueous acetic acid and injected using the conditions described.
Liquid chromatography/mass spectrometry
Reconstituted samples were injected (20 μL) using a HTS PAL auto injector (Leap Technologies, Carrboro, NC) onto a 35 × 2-mm CAPCELL PAK UG C18 column packed with 5-μm particles with 300Å pore size (Phenomenex, Torrance, CA). The column was maintained at 30°C with a Hot-sleeve TCM-2000 column heater (Analytical Sales, Pompton Plains, NJ). A pair of Shimadzu LC10-AD vp (Shimadzu Scientific, Columbia, MD) pumps were used to deliver a programmed gradient (time, % B: 0, 10; 1, 10; 4, 90; 4.2, 90; 4.21, 10; 5.5, stop) of mobile phase A (0.125/1/500, acetic acid/hexafluoroisopropanol/water, vol/vol/vol) and mobile phase B (0.125/1/50/350/100, acetic acid/hexafluoroisopropanol/water/methanol/acetonitrile, vol/vol/vol/vol/vol) at a rate of 0.200 mL/min. Methanol and acetonitrile solvents were obtained from Burdick and Jackson. Hexafluoroisopropanol and trifluoroacetic acid were obtained from Sigma-Aldrich. Acetic acid was obtained from Mallinckrodt Baker (Phillipsburg, NJ). The column eluant was connected to an ionspray source on a Finnigan TSQ Quantum Ultra (Thermo Electron, Waltham, MA) electrospray mass spectrometer with a spray voltage of 4200 V and a capillary temperature of 325°C. The most abundant charge state of each analyte was subjected to fragmentation via collisional-induced dissociation (CID; collision energy of 22) using compressed zero-grade Argon (99.998%; Linde Gas, Wilmington, DE).
Peak detection/integration and statistical treatment
Data acquisition of injected samples was performed using XCalibur software (version 1.4, Thermo Electron). Data integrations were performed using Genesis peak area integration settings in LCQuan (version 1.4, Thermo Electron).
Results
The intent of this investigation is to provide a quantitative method for the determination of mouse and human hepcidin in either plasma or serum. To avoid screening additional lots of serum and plasma pools to identify a source in which hepcidin levels were below the quantifiable range of the assay, we elected to use rabbit serum as a surrogate matrix. Because of this, we were obligated to provide additional verifications that are normally not incurred with the use of an identical matrix. Several examples of the use of surrogate matrices for quantitation of exogenous and endogenous molecules have been reported.28-33 We chose rabbit serum because initial observations indicated no interferences with either human or mouse hepcidin detection. Due to the difficulty of synthesizing human and mouse hepcidin, we were not able to use a stable-labeled internal standard that would have been preferred for development of this mass spectrometry-based assay. We used CGRP for this assay because in previous liquid chromatography mass spectrometry (LC/MS) assays conducted in our facilities, it provided efficient extraction and detection from serum. Although the CGRP was not an ideal internal standard choice, and may not be sufficient for all matrix types, the data obtained during the development and verification of this method demonstrated its utility for normalizing variations in quantitative transfers.
Extraction of hepcidin from plasma and serum matrices
Extraction of hepcidin from plasma was initially evaluated using acetonitrile-precipitated plasma. While recoveries of hepcidin using acetonitrile precipitation were greater than 70%, the data showed matrix interferences that caused poor sensitivity. We therefore investigated solid-phase extraction to isolate hepcidin. Two different solid-phase resins were evaluated for hepcidin recovery. A tC18 μElution SPE plate (Waters Corporation) was tested for extraction of hepcidin but provided decreased recovery compared with the HLB μElution plate (Waters Corporation). The μElution plates were selected for development because they required less than 200-μL elution volumes for recovery of bound analytes. The low elution volume allowed the eluant to be collected directly into the conical wells of 96-well microtiter plates, thus eliminating the need for sample transfer. The low elution volume also allowed for rapid eluant evaporation. Initial attempts to isolate hepcidin from plasma and serum showed that protein precipitation prior to loading onto the SPE cartridge provided the best recovery with no matrix interferences. Overall recovery was determined by comparing the peak area from an injection of a neat solution to the peak area of an extracted serum sample. The overall recovery of the analytes from rabbit serum was calculated to be 59.2%, 78.7%, and 92.4% for human hepcidin, mouse hepcidin, and the internal standard, respectively.
Interassay statistics and hepcidin stability
The interassay relative standard deviation and relative error for human and mouse hepcidin in rabbit serum are shown in Table 1. The standard curves used to regress the spiked samples showed an average R2 value of 0.99 for human hepcidin and 0.98 for mouse hepcidin. Both relative standard deviation and relative error were determined on each of 3 separate days. The human hepcidin samples showed an interassay relative error range of −9.3% to 1.1% and an interassay relative standard deviation range of 11.0% to 15.3% over the curve range of 1 ng/mL to 500 ng/mL serum. The relative standard deviation and relative error for mouse hepcidin showed an interassay relative error range of −8.3% to 12.0% and an interassay relative standard deviation range of 1.2% to 15.9% over the curve range of 0.25 ng/mL to 500 ng/mL serum.
To evaluate freeze-thaw stability and to demonstrate the lack of matrix effects, a standard addition was performed on both pooled human and pooled mouse sera. The pooled sera were measured prior to and after standard addition so that detectable levels of endogenous hepcidin could be determined. Data for the standard addition and freeze-thaw experiments for human and mouse hepcidin are shown in Table 2. Replicate analysis of the pooled human sera before standard addition did not show any detectable levels of hepcidin. The standard addition of 5 ng and 50 ng human hepcidin to 1 mL pooled human sera showed average hepcidin levels of 4.6 and 59.4 ng/mL. The data thus indicated relative errors of −7.3% and 18.9% of the expected levels after standard addition. These data also indicated that no matrix effects were observed with the analysis of human serum when rabbit serum was used for regression. Analysis of replicate freeze-thaw samples showed relative errors of −7.7% and 15.6% for the 5 ng and 50 ng human hepcidin/mL human serum samples, respectively. The freeze-thaw data suggested no effects of human hepcidin determination after 3 freeze-thaw cycles. The 7-week freezer stability data indicated that both the 5 ng and 50 ng human hepcidin/mL human serum samples are within ± 25% of the expected concentration.
Replicate analysis of the pooled mouse sera before standard addition showed an average endogenous level of 3.15 ng mouse hepcidin/mL. The addition of 20 ng and 200 ng mouse hepcidin/mL to the pooled mouse sera showed average relative errors of −8.9% and −21.7% when compared with the nonspiked pooled mouse serum. Recovery determinations for the standard-addition samples of 20 ng and 200 ng mouse hepcidin/mL serum were made with the assumption of 3.15 ng endogenous mouse hepcidin/mL. Therefore, because of endogenous levels of 3.15 ng/mL, it was expected that the 20 ng/mL and 200 ng/mL standard-addition samples would have nominal values of 23.15 and 203.15 ng/mL, respectively. The freeze-thaw replicates show that the nonspiked pooled mouse serum and the 20 ng/mL and 200 ng/mL serum standard-addition samples showed relative errors of −1.1%, −14.1%, and −26.5%, respectively. The nonpooled and standard-addition samples thus indicated that no effects were observed for the determination of mouse hepcidin after 3 freeze-thaw cycles. The 8-week freezer stability data indicated that nonspiked and 20 ng and 200 ng mouse hepcidin/mL mouse serum samples are within ± 25% of the expected concentration.
To determine the effect of diluting samples that could potentially be outside the quantifiable range, we performed 1:100 dilutions of human and mouse sera that were spiked at 5000 ng hepcidin/mL. Replicates of the 5000 ng/mL dilution samples were diluted 1:100 in control rabbit serum and analyzed. The data for the replicate analysis of the dilutional linearity samples are shown in Table 2. The 1:100 dilutions of the 5000 ng/mL standard-addition samples showed relative errors of 1.7% and −17.8% for human and mouse hepcidin, respectively. The dilutional linearity data indicated that no matrix effects were observed following a 1:100 dilution in the surrogate matrix.
Standard additions of human and mouse hepcidin into human and mouse plasma indicated relative standard deviations and errors within the ranges reported for serum (data not shown), thus indicating no measurable difference in hepcidin response between plasma and serum.
Analysis of hepcidin in human plasma and serum from healthy volunteers
To determine baseline concentrations and variability of hepcidin in healthy human subjects, we analyzed the serum and plasma from 10 individual subjects (Figure 1). The data showed median plasma and serum hepcidin levels of 4.9 ng/mL and 3.6 ng/mL, respectively, in healthy male volunteers. One of the human male subjects did not have any detectable hepcidin levels. The relative standard deviation for the male volunteers was 114.4% and 137.1% in serum and plasma, respectively. Female volunteers showed median plasma and serum hepcidin levels of 2.8 ng/mL and 3.6 ng/mL, respectively. The relative standard deviation for the female volunteers was 166.2% and 171.1% in serum and plasma, respectively.
Plasma and serum hepcidin concentrations in healthy human volunteers. Hepcidin concentrations in normal male plasma (●), normal male serum (○), normal female plasma (●), and normal female serum (○). The data shown represent concentrations obtained from each individual sample. The median (- - -) is shown for each group.
Plasma and serum hepcidin concentrations in healthy human volunteers. Hepcidin concentrations in normal male plasma (●), normal male serum (○), normal female plasma (●), and normal female serum (○). The data shown represent concentrations obtained from each individual sample. The median (- - -) is shown for each group.
A second study of human plasma and serum samples involved the collection of blood each morning for 5 consecutive days from the same 5 healthy male volunteers. For simplification, only the serum hepcidin data for the serial collections are shown (Figure 2). The median serum hepcidin levels ranged from 2.6 ng/mL to 16.4 ng/mL over the 5-day collection. The average serum hepcidin levels ranged from 2.6 ng/mL to 18.6 ng/mL. The entire sample set (25 plasma and 25 serum samples) showed an overall average of 12.8 ng/mL compared with 9.0 ng/mL observed in the previous collection (Figure 1). The serum hepcidin levels from the 5-day group also yielded a relative standard deviation range of 26.8% to 66.2%. Individual serum hepcidin values ranged from less than 1.0 ng/mL to 31.3 ng/mL. The individual difference between the 5-day plasma and serum hepcidin levels ranged from 3.9% to 15.9%, whereas the overall average was less than 8%.
Serum hepcidin levels in 5 healthy human volunteers collected on each of 5 consecutive days. Hepcidin concentrations in normal male serum are plotted. The respective data are shown below the plot. For comparative purposes, plasma samples were drawn at the same time and only the plasma (average) is indicated below the serum hepcidin average. The data shown represent concentrations obtained from each individual sample.
Serum hepcidin levels in 5 healthy human volunteers collected on each of 5 consecutive days. Hepcidin concentrations in normal male serum are plotted. The respective data are shown below the plot. For comparative purposes, plasma samples were drawn at the same time and only the plasma (average) is indicated below the serum hepcidin average. The data shown represent concentrations obtained from each individual sample.
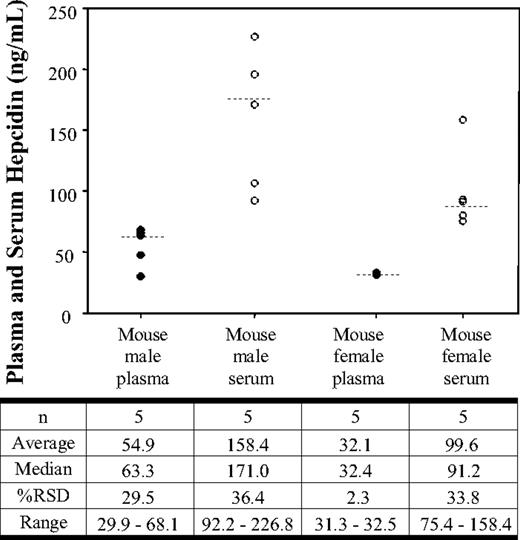
Analysis of hepcidin in mouse plasma and serum samples from normal untreated mice
The serum and plasma hepcidin levels in normal untreated C57BL/6 mice are shown in Figure 3. Analysis of the data illustrates that the median plasma and serum hepcidin levels were 63.3 and 171.0 ng/mL, respectively. The relative standard deviation for the male mice was 29.5% and 36.4% in plasma and serum, respectively. Female mice showed median plasma and serum hepcidin levels of 32.4 ng/mL and 91.2 ng/mL, respectively. The relative standard deviation for the female mice was 2.3% for plasma hepcidin and 33.8% for serum hepcidin. The male mice showed an average plasma concentration of 54.9 ng/mL (± 16.2 ng/mL) and an average serum concentration of 158.4 ng/mL (± 57.7 ng/mL). The female mice showed an average plasma concentration of 32.1 ng/mL (± 0.7 ng/mL) and an average serum concentration of 99.6 ng/mL (± 33.7 ng/mL).
Serum and plasma hepcidin concentrations in normal C57Bl/6 mice. Hepcidin concentrations in normal male mouse plasma (●), normal male mouse serum (○), normal female mouse plasma (●), and normal female mouse serum (○). The data shown represent concentrations obtained from each individual sample. The median (- - -) is shown for each group.
Serum and plasma hepcidin concentrations in normal C57Bl/6 mice. Hepcidin concentrations in normal male mouse plasma (●), normal male mouse serum (○), normal female mouse plasma (●), and normal female mouse serum (○). The data shown represent concentrations obtained from each individual sample. The median (- - -) is shown for each group.
Discussion
This is the first published report demonstrating the quantitative determination of active human and mouse hepcidin in serum using LC/MS/MS. The method was developed to specifically detect oxidized hepcidin in which all 4 proposed disulfides are present. The validation statistics indicate that the method provides an accurate and precise measure of hepcidin levels in plasma or sera of mice or humans. The method was applied to characterize baseline hepcidin levels in plasma and serum of human subjects as well as the daily variation in hepcidin concentrations. In addition, we characterized baseline hepcidin levels in mice to provide information on the potential utility of this species as a preclinical model for the role of hepcidin in iron regulation and its relationship to the human situation.
To evaluate hepcidin concentrations from plasma and serum, we evaluated 35 plasma and serum samples from the same 15 healthy human subjects and normal mice. The data from human samples (male and female) show a mean hepcidin difference of only 7.8% between the plasma and serum measurement within each individual, indicating no bias based on the matrix analyzed. In a similar evaluation, serum and plasma were also collected from each of 5 male and 5 female animals. Unlike the situation in human samples, the data indicated that mouse hepcidin concentrations are approximately 3-fold higher when determined out of serum when compared with plasma. We did not observe this difference when mouse hepcidin was directly spiked into mouse serum or plasma, suggesting that the processing of blood to plasma or serum may be the primary cause of differential hepcidin responses in these matrices. Further experiments are needed to understand the mechanism underlying this difference.
In the small set of samples analyzed, no major differences were observed in serum hepcidin concentrations from healthy male and female humans. The mean hepcidin serum concentrations in males and females were 8.3 and 11.2 ng/mL, respectively (Figure 1). While these initial results suggest more similarity than difference, the high variability observed between the groups indicates that samples from a larger population of healthy human subjects would be necessary to make a distinction in hepcidin levels between male and females. Compared with humans, normal C57Bl/6 mice appear to have much higher average serum hepcidin concentrations (131 ± 54 ng/mL). Additionally, there appeared to be a trend for male mice to have somewhat higher (approximately 150%) hepcidin concentrations than female mice. The concentrations measured in C57Bl/6 mouse serum were also much higher than the hepcidin levels (3.15 ± 0.48 ng/mL) determined in the pooled mouse sera used for our validation (Table 2). This observation suggests that baseline mouse hepcidin levels may vary substantially between strains in relation to husbandry conditions (ie, feed) or other factors.
The current data suggest that there is significant variation in baseline levels of human hepcidin between different individuals. To investigate this further, we were interested in understanding the day-to-day variability of hepcidin levels within an individual. To do this we collected blood from 5 separate individuals at the same time on each of 5 consecutive days. The data show that, within an individual, the measured hepcidin concentrations can vary as much 2- to 5-fold from day to day (Figure 2). Whether the day-to-day differences reflect a normal rhythm of hepcidin, the influence of food, mild/unrecognized inflammation, or other environmental factors is not clear. Hepcidin concentrations may also show diurnal variations that have not been captured during the course of this initial study.23 Regardless, the results suggest that there is much less variability in hepcidin levels within an individual than between individuals (Figures 1,2). In healthy human subjects, it appears that mean serum hepcidin concentrations (approximately 8-11 ng/mL) are approximately 10-fold lower than reported levels of serum prohepcidin (approximately 100 to 200 ng/mL).25,26 The high intersubject variability in hepcidin levels in healthy volunteers is also consistent with these previous studies that measured serum prohepcidin.25,26 The biologic significance of the relationship between serum/tissue prohepcidin and hepcidin in normal physiology and diseased states has not yet been established. With the availability of the current method to measure hepcidin levels, we may be able to better understand the interplay between these 2 molecules and other proteins involved in iron regulation. This report focused on free serum hepcidin measurements, although we are aware of the potential for hepcidin to be bound to carrier proteins, undergo deamidiation, or undergo methionine oxidation. The use of acetonitrile precipitation as part of the extraction process could potentially liberate carrier-bound hepcidin, thus yielding a total hepcidin measurement.
In summary, we have developed an LC/MS/MS method for the determination of both human and mouse hepcidin levels in serum and plasma. The method is capable of quantitation of mouse hepcidin from 0.25 to 500 ng/mL, whereas the range of detection for human hepcidin is 1 to 500 ng/mL. The method uses the specificity and sensitivity afforded by tandem mass spectrometry and could most likely be leveraged to differentiate levels of truncated forms of hepcidin (ie, 20- and 25-hepcidin). The method throughput allows 1 analyst to process and analyze one 96-well plate (96 injections) within 10 hours. Subsequent evaluations will focus on the analysis of serum and possibly urine from patients with disorders in iron regulation and animal models designed to provide an understanding of the mechanistic role of hepcidin on iron regulation. These data may prove to be useful in the design of therapeutic interventions based on the hepcidin pathway.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.T.M. designed and performed research, analyzed data, and wrote the paper; D.R.W. planned studies, helped design peptide purification and refolding conditions, analyzed data, and revised the manuscript; P.L. planned studies and performed peptide refolding and purification; and V.J.W. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anthony T. Murphy, Lilly Research Laboratories, Lilly Corporate Center, Indianapolis, IN 46285; e-mail: atm@lilly.com.