Abstract
Programmed death-1 (PD-1) is a critical mediator of virus-specific CD8+ T-cell exhaustion. Here, we examined the expression of PD-1 on simian immunodeficiency virus (SIV)-specific CD8+ T cells and its possible involvement in regulation of cytokine production, proliferation, and survival of these cells. The majority of SIV-specific CD8+ T cells expressed a PD-1high phenotype, independent of their differentiation status, in all tissues tested. PD-1 expression gradually declined on CD8+ T cells specific for SIV-derived epitopes that had undergone mutational escape, indicating that antigen-specific TCR stimulation is the primary determinant of PD-1 expression. SIV-specific PD-1highCD8+ T cells produced IFN-γ, TNF-α, and IL-2 under cognate peptide stimulation. While CD8+ T cells that proliferated in response to antigen had a PD-1high phenotype, it was determined that there was a reduced proliferative capacity of PD-1high compared with PD-1low SIV-specific CD8+ T cells. PD-1high SIV-specific CD8+ T cells were highly susceptible to cell death leading to loss of such cells after in vitro stimulation. Thus, PD-1 is a negative regulator of SIV-specific CD8+ T cells, operating predominantly through the induction of cell death. Manipulation of the interaction of PD-1 with its ligands could thus potentially restore the CD8+ T-cell responses in SIV infection.
Introduction
Virus-specific CD8+ T cells are the predominant effectors through which the immune system controls viral infections.1,2 While several lines of evidence indicate that human immunodeficiency virus (HIV)-specific CD8+ T cells are involved in the control of HIV replication,3-10 several reports have focused on intrinsic defects in these cells to explain their failure to clear the virus, thereby leading to progression to acquired immunodeficiency syndrome (AIDS) in all infected individuals.11-14 Similarly, simian immunodeficiency virus (SIV)-specific CD8+ T cells contribute substantially to the partial control of viremia in rhesus macaques; depletion of CD8+ T cells results in increased viremia in SIV-infected animals,15,16 while viral escape at targeted epitopes accelerates disease progression and death in vaccinated animals.4 Furthermore, in acute SIV infection, cytotoxic SIV-specific CD8+ T cells appear concurrently with the waning of early viremia.17 As in human infection with HIV, functional defects in SIV-specific CD8+ T cells have been reported, potentially explaining why virtually all SIV-infected rhesus macaques progress to simian AIDS and death despite a readily measurable CD8+ T-cell response.17-20 Therefore, understanding factors that regulate the function(s) of SIV- and HIV-specific CD8+ T cells is critical in the fight against AIDS
Chronic viral infection with ongoing antigenic stimulation often results in exhaustion of virus-specific CD8+ T cells.21,22 Chronically stimulated virus-specific CD8+ T cells express only low levels of receptors for IL-7 and IL-1523 and lose the ability to maintain homeostatic proliferation. In addition, they lose the ability to produce key cytokines that are critical for the maintenance of CD8+ T-cell memory.24 In humans, the function and phenotype of chronically stimulated CD8+ T cells differ among viral infections. HIV-specific CD8+ T cells are less polyfunctional and more sensitive to apoptosis than CD8+ T cells specific for other persistent viral infections, such as cytomegalovirus (CMV).25,26 In SIV infection, total CD4+ and CD8+ T cells are characterized by increased sensitivity to cell death,27,28 a phenomenon that predominantly affects uninfected cells.29 A major target of CD8+ T cells in Mamu-A*01-expressing monkeys is the immunodominant Gag epitope, CM9 (CTPYDINQM; residues 181-189).30-32 While CM9-specific CD8+ T cells are present in SIV-infected monkeys, their ability to produce IFN-γ and TNF-α and to exert cytolytic activity is reduced in chronic SIV infection.19,33 This defect can be partially overcome through vaccination; some vaccines can enhance SIV-specific CD8+ T-cell responses,34,35 leading to greater IFN-γ, TNF-α, and IL-2 production by these cells that then correlates with protection against disease progression.36
In a murine model for chronic viral infection, recovery of lymphocytic choriomeningitis virus (LCMV)-specific CD8+ T cells from exhaustion has been accomplished in vivo by blocking the interaction between PD-1 and its ligand PD-L1.37 Most importantly, recent studies have revealed a critical role for this interaction in regulating the function and survival of HIV-specific CD8+ T cells.38-40 PD-1, a member of the CD28 family is a negative regulator of T-cell activation,41 originally identified as a surface receptor involved in the apoptosis of cancer cells.42 While the expression of PD-1 on virus-specific CD8+ T cells has been linked by some to impaired production of cytokines37,38,40 others have emphasized the predominant role of this receptor in regulating the survival of these cells.39,43-45 The molecular basis of these PD-1-dependent functional defects is as yet unknown. Understanding the role of PD-1 in regulating the functional properties of virus-specific CD8+ T cells could ultimately lead to interventions to restore greater numbers of HIV-specific (or SIV-specific) CD8+ T cells with enhanced functionality in vivo.
The role of PD-1 in SIV-specific CD8+ T-cell responses is currently unknown. Here, we report on the sustained high expression of PD-1 on SIV CM9-specific CD8+ T cells. Expression of PD-1 at high levels on these CD8+ T cells was associated with reduced proliferative capacity and increased susceptibility to death. Thus, as in HIV infection, our data point to a negative role for PD-1 in SIV-specific CD8+ T-cell responses.
Materials and methods
Animals
Colony-bred rhesus macaques (Macaca mulatta), obtained from Covance Research Products (Denver, PA), were housed and handled in accordance with the standards of the American Association for the Accreditation of Laboratory Animal Care. Sixteen macaques were infected intravenously with a SIVmac251 stock as described46,47 ; 4 were vaccinated with rAd-5 vectors encoding SIVmac239 env or gag-pol48 and subsequently challenged with SIVmac239; 5 were vaccinated with NYVAC-SIVgag-pol-env and challenged with SIVmac251; and 2 animals were pretreated with antiretroviral therapy (ART) + IL-2 and challenged with SIVmac251. Mucosal cells were isolated from jejunum, colon, and rectal tissues as previously described.49 Both intraepithelial lymphocytes (IEL) and lamina propia lymphocytes (LPL) were collected. Briefly, samples were incubated in RPMI 1640 medium containing 15 U collagenase (type II; Sigma, St Louis, MO) and DNase (Invitrogen, Frederick, MD), 100 U/mL penicillin, 100 U/mL streptomycin, 5 mL l-glutamine, 5 mL HEPES buffer, and 5% FCS. All incubations were done at 37°C with rapid shaking for 30 minutes. After washing, cells were resuspended in complete RPMI 1640 (100 U/mL penicillin, 100 U/mL streptomycin, 10% FCS) and stored on ice until use. Supernatants were enriched for lymphocytes by isotonic discontinuous Percoll (Sigma) density gradient, 35% to 60% (vol/vol), centrifugation. Epithelial cells form a band above the 35% gradient, and lymphocytes form a band at the interface between the 35% and 60% Percoll layers.
Antibodies
Directly conjugated antibodies were obtained from the following: (1) BD Biosciences (San Jose, CA): CD3-FITC (catalog no. 556611), CD3-Cy7APC (catalog no. 557757), CD8-Pacific Blue (catalog no. 558207), CD8-APC (catalog no. 555369), IFNg-FITC (catalog no. 554700), TNFa-Cy7PE (catalog no. 557647), IL2-PE (catalog no. 554566), CD95-Cy5PE (catalog no. 559773); (2) Beckman Coulter (Hialeah, FL): CD28-ECD (catalog no. 6607111). Biotinylated anti-PD-1 antibody was obtained from R&D (catalog no. BAF 1086; Minneapolis, MN) and streptavidin (PE or Qdot 565), from Molecular Probes (Eugene, OR). Annexin V-Cy5PE was conjugated in our laboratory (http://drmr.com/abcon/index.html). Fluorescent pMamu-A*01 tetrameric complexes were prepared in our laboratory as previously described50 : CM9 (Gag, CTPYDINQM; residues 181-189)-(APC or Qdot 705) and TL8 (Tat, TTPESANL; residues 28-35)-Qdot 605. Quantum dots were obtained from Quantum Dot Corporation (Hayward, CA). Violet amine viability dye (Vivid) was purchased from Invitrogen. A monoclonal antibody (clone CL10) was raised against human PD-1 by immunizing mice with a chimeric protein consisting of residues 1-147 of the mature sequence of PD-1 and the Fc region of mouse IgG. The antibody epitope overlaps with the region implicated in ligand binding by mutagenesis of murine PD-1.51
Measurement of cytokine production
Previously frozen peripheral blood mononuclear cells (PBMCs), splenocytes, or axillary lymph node cells were thawed and resuspended at 2 × 106/mL in RPMI medium (Invitrogen) supplemented with 10% heat-inactivated fetal calf serum, penicillin (100 U/mL), streptomycin (100 μg/mL), glutamine (2 mM), costimulatory monoclonal antibodies (anti-CD28 and anti-CD49d) at 1 μg/mL (Becton Dickinson, San Jose, CA), monensin (0.7 μg/mL; BD Biosciences, Lewisville, TX), and brefeldin A (10 μg/mL; Sigma). Peptide corresponding to the autologous CM9 wild-type virus sequence (purity > 95%; Bio-Synthesis, Lewisville, TX) was used at 2 μg/mL to stimulate cognate SIV-specific CD8+ T cells. Costimulatory antibodies/monensin/brefeldin alone were used as a negative control, with staphylococcus enterotoxin B (SEB; Sigma) at 1 μg/mL as a positive control. Cells were incubated for 6 hours, washed, and stained with anti-PD-1-biotin. Following a staining step with streptavidin-Qd565, cells were washed twice and incubated with tetramer CM9-Qd705 at 37°C for 20 minutes. After washing, cells were surface stained with CD8-Pacific Blue and annexin V-Cy5PE. Cells were then permeabilized (Cytofix/CytoPerm kit; BD Biosciences) and stained with CD3-Cy7APC, IFNg-FITC, TNFa-Cy7PE, and IL-2-PE. CaCl2 (2.5 mM) was included in all steps following the tetramer staining.
Proliferation studies
Proliferation assays were based on carboxyfluorescein diacetate succinimidyl ester (CFSE) dilution. Cells were thoroughly washed with phosphate-buffered saline (PBS), labeled with 0.25 mM CFSE (Molecular Probes), adjusted to 2 × 106/mL, and cultured for 6 days in the absence or presence of 2 μg/mL CM9 peptide and anti-CD28/anti-CD49d costimulatory monoclonal antibodies at 1 μg/mL each. In some experiments, an anti-PD-1 monoclonal antibody (clone CL10) was included at 20 μg/mL. Cells were harvested and stained with anti-PD-1-biotin, streptavidin-PE, and subsequently with CM9-Qd705. Following a washing step, cells were surface stained with CD3-Cy7APC, CD8-Pacific Blue, and annexin V-Cy5PE. For some experiments, cells were stained with anti-PD-1-biotin/streptavidin-PE and CD3-FITC, and CD3+PD-1+ and CD3+PD-1− populations were sorted under sterile conditions by using a fluorescence-activated cell sorting (FACS) Aria system in a BSL-3 facility. Sorted cells were labeled with CFSE (CD3+PD-1+) or FarRed (CD3+PD-1−) (Molecular Probes) and cultured in the absence or presence of CM9 peptide (2 μg/mL) or SEB (1 μg/mL) for 6 days. Cells were then harvested and stained as described for the unsorted ones.
Apoptosis studies
Cells at 2 × 106/mL were cultured for 18 to 22 hours in the absence or presence of CM9 peptide (2 μm/mL), harvested, and stained with anti-PD-1-biotin/streptavidin-Qd565 and subsequently with CM9-Qd705. In some experiments, the combination of CM9-Qd705 and TL8-Qd605 was used. Following a washing step, cells were surface stained with CD3-Cy7APC, CD8-APC, annexin V-Cy5PE, and Vivid. CaCl2 (2.5 mM) was included in all steps following the tetramer staining.
Flow cytometry
Cells were analyzed with a modified LSRII flow cytometer (BD Immunocytometry Systems, San Jose, CA). Between 200 000 and 106 events were acquired for each condition. Antibody-capture beads (BD Biosciences) stained separately with individual monoclonal antibodies used in the test samples were used for electronic compensation. For compensation of annexin V-Cy5PE, CFSE, or FarRed, cells stained with the proper dye at the time of experimental set-up were used. Data analysis was performed using FlowJo version 6.0 (TreeStar, Ashland, OR). Forward scatter area (FSC-A) versus forward scatter height (FSC-H) was used to gate out cell aggregates. Annexin V was used to exclude apoptotic cells from our analysis. The cells were then gated through a FSC-A versus side scatter height (SSC-H) plot to isolate small lymphocytes. Following this, CD3-Cy7APC-positive cells were selected and PD-1 expression was measured in gated total CD8+ T cells and tetramer+ (CM9-Qd705 and TL8-Qd605) cells and in relation to their differentiation level by using CD28-TRPE and CD95-Cy7PE.
Viral RNA sequencing
SIV isolation and cDNA synthesis and sequencing procedure have been recently described.50
Statistics
Statistical analysis was performed using Student t test, and P values of less than .05 were considered significant. The JMP statistical analysis program was used (SAS Institute, Cary, NC).
Results
SIV-specific CD8+ T cells express high levels of PD-1 independently of their maturation status and tissue localization
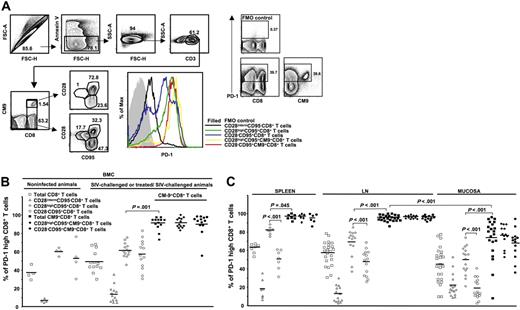
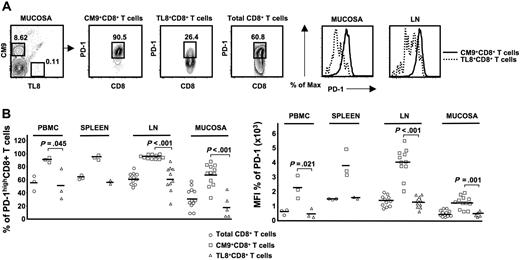
Increased expression of PD-1 has been described on virus-specific CD8+ T cells under conditions of chronic antigen persistence.52 Whether SIV-specific CD8+ T cells are similarly affected is not known. We therefore determined the expression of PD-1 on total, naive, memory, and SIV-specific (CM9)32 CD8+ T cells, being careful to gate out dead or dying (annexin V+) cells from the analysis (Figure 1A). We found that CM9-specific CD8+ T cells express remarkably high levels of PD-1 (Figure 1A). This was true for both the CD28highCD95+ (central) and CD28−CD95+ (effector) memory compartments (Figure 1A) in which the CM9-specific cells were found. The total memory and naive CD8+ T-cell populations all exhibited lower expression of PD-1 than did the CM9-specific cells, with the CD28highCD95+ population expressing higher levels of PD-1 than the CD28−CD95+ population, which, in turn, expressed higher levels than the naive (CD28intermCD95−) population (Figure 1A). Interestingly, the pattern of PD-1 expression on the total CD28−CD95+CD8+ T cells was consistently characterized by 2 distinct populations (Figure 1A), indicating an additional complexity of cell differentiation in this compartment.
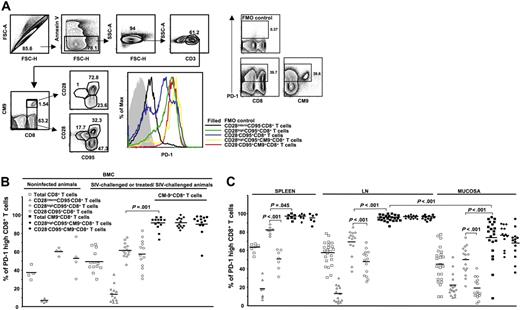
The majority of CM9-specific CD8+ T cells express high levels of PD-1. (A) PD-1 expression was measured by applying a polychromatic flow cytometry assay (left panel). The gating scheme for identification of cell populations is shown. Histograms depict the PD-1 expression on total and CM9-specific CD8+ T cells from the same sample and in relation to their maturation status. The combination of CD28 and CD95 was used for identification of memory populations. The control (all antibodies except aPD-1) staining is also shown. Representative flow cytometry plots depicting the PD-1high and PD-1low CD3+ T-cell populations are shown on the right panel. (B) Pooled data showing the percentage (%) of CD8+ T cells expressing a PD-1high phenotype in PBMC compartment from noninfected (n = 4) and SIV-challenged or treated/SIV-challenged (n = 13) animals. (C) The percentage (%) of PD-1high CD8+ T cells located in spleen, LN (axillary, n = 8; inguinal, n = 8; mesenteric, n = 4; paratracheal, n = 3), and mucosa (rectal, n = 11; jejunum, n = 9; colon, n = 4) is shown. PD-1 expression in memory populations of total and CM9+CD8+ T cells from all tissues tested is also shown. All animals were infected for at least 8 weeks. Horizontal lines depict mean values. The P values were calculated using Student t test.
The majority of CM9-specific CD8+ T cells express high levels of PD-1. (A) PD-1 expression was measured by applying a polychromatic flow cytometry assay (left panel). The gating scheme for identification of cell populations is shown. Histograms depict the PD-1 expression on total and CM9-specific CD8+ T cells from the same sample and in relation to their maturation status. The combination of CD28 and CD95 was used for identification of memory populations. The control (all antibodies except aPD-1) staining is also shown. Representative flow cytometry plots depicting the PD-1high and PD-1low CD3+ T-cell populations are shown on the right panel. (B) Pooled data showing the percentage (%) of CD8+ T cells expressing a PD-1high phenotype in PBMC compartment from noninfected (n = 4) and SIV-challenged or treated/SIV-challenged (n = 13) animals. (C) The percentage (%) of PD-1high CD8+ T cells located in spleen, LN (axillary, n = 8; inguinal, n = 8; mesenteric, n = 4; paratracheal, n = 3), and mucosa (rectal, n = 11; jejunum, n = 9; colon, n = 4) is shown. PD-1 expression in memory populations of total and CM9+CD8+ T cells from all tissues tested is also shown. All animals were infected for at least 8 weeks. Horizontal lines depict mean values. The P values were calculated using Student t test.
Subsequently, cells from a large group of monkeys (n = 31) were analyzed. These included monkeys that were not infected (n = 4), those that were treated before infection with SIV (9 vaccinated/SIV-challenged and 2 ART + IL-2/SIV-challenged), and those that were infected with SIV without prior manipulation (n = 16). Analysis of total CD8+ T cells in the PBMC compartment from infected animals revealed a pattern of PD-1 expression similar to that recently reported for total CD8+ T cells from HIV-infected donors.39 The CD28highCD95+ memory cells expressed the highest levels of PD-1 analyzed either in terms of percentage (59.9% ± 9.1%, n = 13) or mean fluorescence intensity (MFI) (806 ± 84.9), while naive cells express the lowest levels of PD-1 (14.8% ± 8.7%, n = 13, P < .001; MFI, 226.7 ± 21.3, P < .001) (Figure 1B; Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article). No difference between total CD8+ T cells from noninfected and infected animals was found (Figure 1B). Similarly no difference was found when MFI values were analyzed for total CD8+ T cells (Figure S1). CM9-specific CD8+ T cells were found to express higher PD-1 levels than total CD28highCD95+ CD8+ T cells (89.6% ± 2%, n = 13, P < .001; MFI, 1908.5 ± 225.4, P = .001). No difference in CM9-specific CD8+ T-cell memory populations was found (92.01% ± 2.8% versus 89.5% ± 2.9%, n = 13, for CD28highCD95+ and CD28−CD95+ cells, respectively) (Figure 1B). There was also no difference in the MFI of PD-1 on these populations of CM9-specific CD8+ T cells (Figure S1). Similarly, no difference in PD-1 expression was found between CM9-specific CD8+ T cells from SIV-infected animals that did and did not have preinfection treatment regimens (data not shown).
As for PBMCs, the CD28highCD95+ memory population expressed the highest PD-1 levels in the total CD8+ T-cell compartment in all tissues tested (Figure 1C). Again, CM9-specific CD8+ T cells were found to express significantly higher levels of PD-1 compared with the total CD28highCD95+ memory population in spleen (92.3% ± 12.1%, n = 7 versus 81.6% ± 3.6%, n = 7, P = .045), lymph nodes (95.3% ± 2.7%, n = 23 versus 68.5% ± 14.5%, n = 14, P < .001) and mucosa (73.3% ± 22%, n = 21 versus 49.5% ± 14.7%, n = 16, P = .001) (Figure 1C). Interestingly, CM9-specific CD8+ T cells from mucosa were found to express significantly lower levels of PD-1 compared with these cells from other tissues (P < .001) (Figure 1C). This finding also held when the MFI of PD-1 was examined (Figure S1). We did not found a correlation between viral load and PD-1 expression in the small group of PBMCs samples tested. Taken together, our data clearly show that SIV-specific CD8+ T cells are characterized by high expression of PD-1, which is independent of maturation and tissue localization.
Persistently high expression of PD-1 requires continuous antigen-specific stimulation
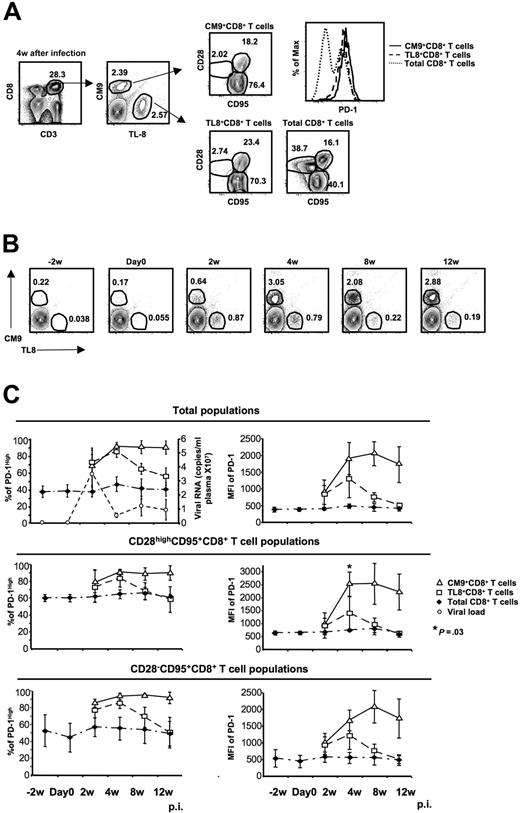
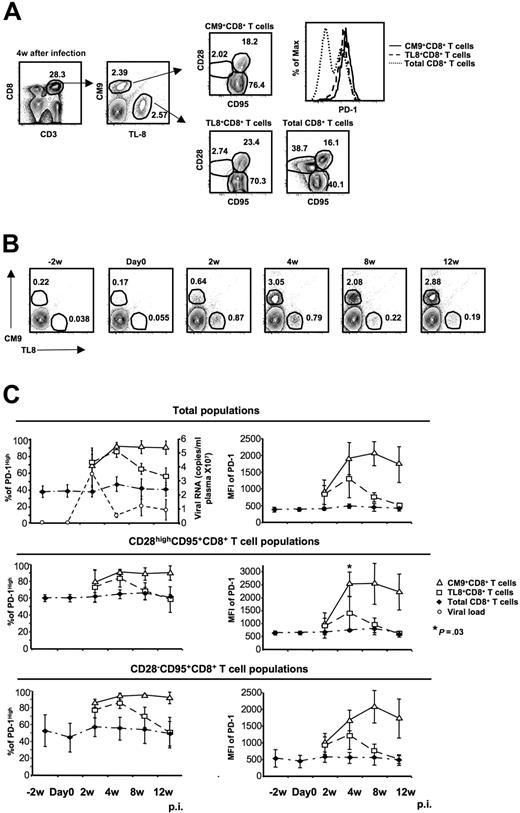
What regulates the expression of PD-1 on virus-specific CD8+ T cells is not known. To test the hypothesis that chronic antigen-specific stimulation determines PD-1 expression levels, we examined SIV-specific CD8+ T cells specific for an epitope in Tat (TL8: TTPESANL; residues 28-35) that undergoes mutation shortly after infection50 and asked whether the kinetics of PD-1 expression on CM9- and TL8-specific CD8+ T-cell populations differ. PBMCs from 4 monkeys collected at different time points before and after infection with SIVmac251 were analyzed simultaneously for expression of CD3, CD8, CD28, CD95, PD-1, and TCRs cognate for CM9 and TL8 (Figure 2A). Representative flow cytometry plots showing the 2 virus-specific CD8+ T-cell populations are shown in Figure 2B. The expression of PD-1 on total CD8+ T cells was found to be quite stable through 12 weeks of infection in all CD8+ memory populations tested (Figure 2C). On CM9-specific CD8+ T cells, PD-1 gradually increased during the first 4 weeks of infection and remained high through week 12 (Figure 2C). In contrast, PD-1 expression on TL8-specific CD8+ T cells increased during the first 4 weeks and thereafter declined, reaching levels similar to those on total CD8+ T cells by 12 weeks after infection (Figure 2C top panels). These findings were true whether percent positivity or MFI of PD-1 was analyzed, and held when the comparisons were confined to the CD28highCD95+ or CD28−CD95+ memory populations of SIV-specific and non-SIV-specific CD8+ T cells (Figure 2C). Sequencing analysis of viral RNA confirmed the existence of escape mutants within the TL8 epitope at 8 weeks after infection, while no mutation was observed for the CM9 epitope (Figure S2), in agreement with previously described data.53
Chronic antigen-specific stimulation is indispensable for persistent high expression of PD-1 on CM9+CD8+ T cells. (A) Representative flow cytometry showing the simultaneous detection of CM9- and TL8-specific CD8+ T-cell populations from the same sample as well as their memory status. Histogram depicts the PD-1 expression on total, CM9-specific, and TL8-specific CD8+ T cells from the same sample. (B) Representative plots showing the CM9- and TL8-specific CD8+ T-cell populations from one animal at different time points before and after infection. The animal was challenged with SIVmac251 on day 0. (C) The expression of PD-1 (as % or MFI) before and after infection on total, CM9-specific, and TL8-specific CD8+ T cells is shown. The plasma viral load for all animals is also shown (top left panel). PD-1 expression was analyzed in total (top panel), CD28highCD95+ (middle panel), and CD28−CD95+ (bottom panel) CD8+ T-cell memory populations for each animal individually and pooled data from 4 animals are shown. Bars depict means (± SD). The P values were calculated using Student t test.
Chronic antigen-specific stimulation is indispensable for persistent high expression of PD-1 on CM9+CD8+ T cells. (A) Representative flow cytometry showing the simultaneous detection of CM9- and TL8-specific CD8+ T-cell populations from the same sample as well as their memory status. Histogram depicts the PD-1 expression on total, CM9-specific, and TL8-specific CD8+ T cells from the same sample. (B) Representative plots showing the CM9- and TL8-specific CD8+ T-cell populations from one animal at different time points before and after infection. The animal was challenged with SIVmac251 on day 0. (C) The expression of PD-1 (as % or MFI) before and after infection on total, CM9-specific, and TL8-specific CD8+ T cells is shown. The plasma viral load for all animals is also shown (top left panel). PD-1 expression was analyzed in total (top panel), CD28highCD95+ (middle panel), and CD28−CD95+ (bottom panel) CD8+ T-cell memory populations for each animal individually and pooled data from 4 animals are shown. Bars depict means (± SD). The P values were calculated using Student t test.
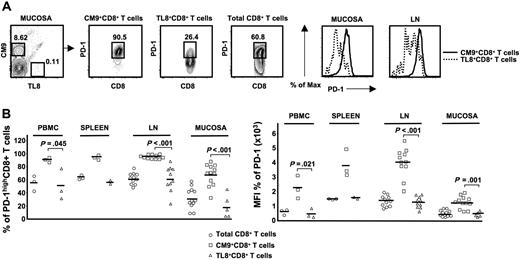
Next, we analyzed the PD-1 expression on CM9- and TL8-specific CD8+ T cells in different tissues from chronically infected monkeys (> 6 months after infection). In all tissues tested, TL8-specific CD8+ T cells had lower levels of PD-1 expression than CM9-specific CD8+ T cells, expressed either as percent positivity or MFI (PBMCs: 51.2% ± 23.9%, n = 3 versus 91.4% ± 3.7%, n = 3, P = .045; spleen: 57.2% ± 2.7%, n = 2 versus 94.9% ± 3.6%, n = 3; LN: 62.7% ± 19.3%, n = 10 versus 95.8% ± 1.3%, n = 11, P = .001; mucosa: 19.9% ± 17.4%, n = 5 versus 68.5% ± 16.3%, n = 12, P = .001) (Figure 3B). The above data collectively show that sustained antigen-specific stimulation mediated through the TCR is primarily responsible for the high expression levels of PD-1 on virus-specific CD8+ T cells.
The requirement for antigen-specific stimulation mediated through the TCR for maintaining the PD-1highCM9+ phenotype is independent of tissue localization. (A) Flow cytometry plots showing the PD-1 expression on total, CM9-specific, and TL8-specific CD8+ T cells in the same sample. Histograms depict the PD-1 expression on CM9- and TL8-specific CD8+ T cells from the same sample, located in mucosa and LN. (B) Pooled data showing the expression of PD-1 as percentage (left panel) or MFI (right panel) on total, CM9-specific, and TL8-specific CD8+ T cells from different tissues. Samples from PBMCs (n = 3), spleen (n = 3), LN (n = 11), and mucosal sites (n = 12) were analyzed. Horizontal lines depict mean values. The P values were calculated using Student t test.
The requirement for antigen-specific stimulation mediated through the TCR for maintaining the PD-1highCM9+ phenotype is independent of tissue localization. (A) Flow cytometry plots showing the PD-1 expression on total, CM9-specific, and TL8-specific CD8+ T cells in the same sample. Histograms depict the PD-1 expression on CM9- and TL8-specific CD8+ T cells from the same sample, located in mucosa and LN. (B) Pooled data showing the expression of PD-1 as percentage (left panel) or MFI (right panel) on total, CM9-specific, and TL8-specific CD8+ T cells from different tissues. Samples from PBMCs (n = 3), spleen (n = 3), LN (n = 11), and mucosal sites (n = 12) were analyzed. Horizontal lines depict mean values. The P values were calculated using Student t test.
PD-1high SIV-specific CD8+ T cells produce multiple cytokines
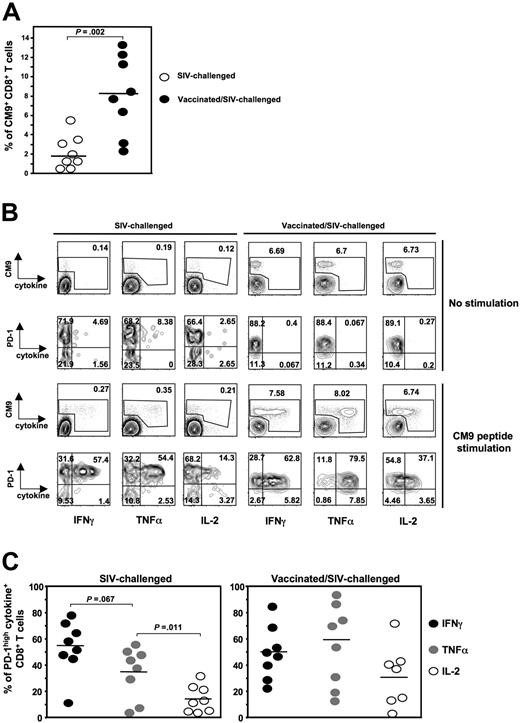
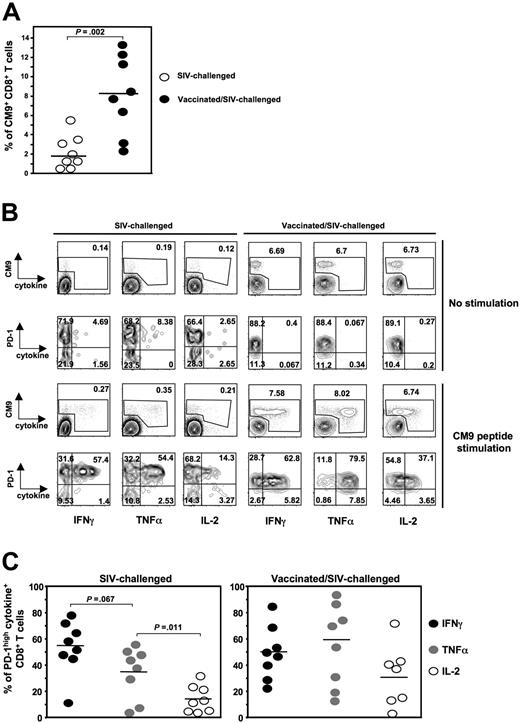
A characteristic of virus-specific CD8+ T-cell exhaustion under conditions of chronic antigen stimulation is their reduced ability to produce cytokines.52 An association between PD-1 expression and lack of cytokine production has been previously proposed.37,38,40 Therefore, we asked whether the expression of PD-1 on SIV-specific CD8+ T cells is linked to their ability to produce cytokines. To this end, we measured the expression of PD-1 and production of IFN-γ, TNF-α, and IL-2 in CM9-specific CD8+ T cells. We performed a polychromatic flow cytometry assay allowing the simultaneous detection of all 3 cytokines and in relation to PD-1 expression on CM9-specific CD8+ T cells after 6-hour stimulation with CM9 peptide. In vitro stimulation with either SEB or CM9 peptide did not induce any significant alteration of the surface expression of PD-1 on CD8+ T cells (data not shown). We used cells from both SIV-challenged (n = 8) and vaccinated/SIV-challenged (n = 8) animals. The percentage of CM9-specific CD8+ T cells was significantly lower in the former animals (2.1% ± 1.82%, n = 8 versus 8.1% ± 4%, n = 8, P = .002), in agreement with previous reports34,35 (Figure 4A). The SIV-challenged group included cells from animals that had been infected for 8 or 12 weeks. In some of these animals, a separate PD-1lowCM9-specific CD8+ T-cell population was observed (Figure 4B left panel). This was not, however, observed in CM9-specific CD8+ T cells from macaques in the vaccinated/SIV-challenged group, with one exception (Figure 4B right panel). The vast majority of cytokine producers fell within the PD-1high gate. The pattern (%) of cytokine+CM9+CD8+ T cells from SIV-challenged animals was similar to that described for HIV-specific CD8+ T cells25,39 with IFN-γ being the most frequent cytokine (54.8% ± 7.4%), followed by TNF-α (34.6% ± 7.2%) and significantly lower levels of IL-2 (14.1% ± 3.7%, P = .011). When cells from the vaccinated/SIV-challenged group were analyzed, TNF-α was found to be the most frequent cytokine (60.1% ± 11.1%), followed by IFN-γ and IL-2. It is worth mentioning that the PD-1lowCM9+CD8+ T-cell population (either from blood, spleen, or LN) observed in one of the vaccinated/SIV-challenged animals exhibited significantly higher production of all 3 cytokines compared with PD-1highCM9+CD8+ T cells (data not shown). This one monkey notwithstanding, our data show that, as in HIV infection,39 PD-1highSIV-specific CD8+ T cells are capable of producing cytokines and there is no direct link between PD-1 expression on these cells and their ability to produce cytokines.
PD-1highCM9+CD8+ T cells are capable of producing multiple cytokines. (A) Dot-plot graph showing the percentage of CM9+CD8+ T cells in PBMCs from SIV-challenged (n = 8) and vaccinated/SIV-challenged (n = 8) animals. (B) Representative flow cytometry showing the production of IFNγ, TNFα, and IL-2 by CM9+CD8+ T cells after 6-hour stimulation with CM9 peptide. Single-live lymphocytes were gated first for CD8- and then for CM9-specific CD8+ T cells by CM9 tetramer. The production of all 3 cytokines was simultaneously measured in CM9+CD8+ T cells and in relation to their PD-1 expression. Cells from one SIV-challenged (left panel) and one vaccinated/SIV-challenged (right panel) animal for both no stimulation and CM9 stimulation conditions are shown. (C) Compiled data showing the percentage of PD-1highCM9+CD8+ T cells that is positive for each one cytokine tested in SIV-challenged (left panel, n = 8) and vaccinated/SIV-challenged (right panel, n = 8) animals. Horizontal lines depict mean values. The P values were calculated using Student t test.
PD-1highCM9+CD8+ T cells are capable of producing multiple cytokines. (A) Dot-plot graph showing the percentage of CM9+CD8+ T cells in PBMCs from SIV-challenged (n = 8) and vaccinated/SIV-challenged (n = 8) animals. (B) Representative flow cytometry showing the production of IFNγ, TNFα, and IL-2 by CM9+CD8+ T cells after 6-hour stimulation with CM9 peptide. Single-live lymphocytes were gated first for CD8- and then for CM9-specific CD8+ T cells by CM9 tetramer. The production of all 3 cytokines was simultaneously measured in CM9+CD8+ T cells and in relation to their PD-1 expression. Cells from one SIV-challenged (left panel) and one vaccinated/SIV-challenged (right panel) animal for both no stimulation and CM9 stimulation conditions are shown. (C) Compiled data showing the percentage of PD-1highCM9+CD8+ T cells that is positive for each one cytokine tested in SIV-challenged (left panel, n = 8) and vaccinated/SIV-challenged (right panel, n = 8) animals. Horizontal lines depict mean values. The P values were calculated using Student t test.
PD-1high SIV-specific CD8+ T cells are characterized by low proliferative ability and increased sensitivity to activation-induced cell death
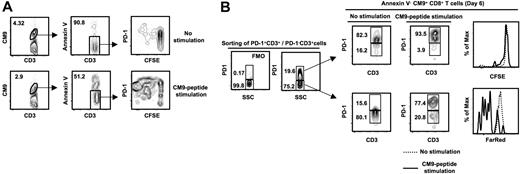
Manipulation of the PD-1:PD-L1 system has been found to alter the ability of human virus-specific CD8+ T cells to proliferate in vitro,38-40 probably by regulating their survival.39 Therefore, we asked whether PD-1 plays any role in the survival and proliferative capacity of SIV-specific CD8+ T cells. CFSE dilution was used to quantify cell division within SIV-specific CD8+ T cells cultured for 6 days in the absence or presence of CM9 peptide. The CFSE profile was examined in the live (annexin V−) compartment of CM9-specific CD8+ T cells and in relation to PD-1 expression (Figure 5A). Contrary to expectations, divided (CFSElow) cells consistently expressed a PD-1high/PD-1intermediate phenotype (Figure 5A). Previous studies have shown that PD-1 is up-regulated upon in vitro activation of both CD4+ and CD8+ T cells.54 As a result, high PD-1 expression on CFSElow CM9+CD8+ T cells could be attributed either to the proliferation of preexisting PD-1highCM9+CD8+ T cells or to its de novo expression on PD-1lowCM9+CD8+ T cells during their in vitro activation with the cognate peptide. To clarify this matter, sorted PD-1highCD3+ and PD-1lowCD3+ were labeled with CFSE (PD-1high) or FarRed (PD-1low) and cultured for 6 days in the absence or presence of the CM9 peptide. We found that CM9-specific CD8+ T cells originating from the PD-1high pool exhibit a low proliferative phenotype (Figure 5B). In sharp contrast, the CM9-specific CD8+ T cells from the PD-1low compartment were able to divide as determined by dilution of FarRed (Figure 5B). These data indicate that the majority of CFSElowCM9+CD8+ T cells shown in Figure 5A were initially from the PD-1low compartment, and that they expressed PD-1 subsequent to their activation. Therefore, despite the expression of PD-1 on proliferating cells, the data are consistent with a negative role for PD-1 in proliferation.
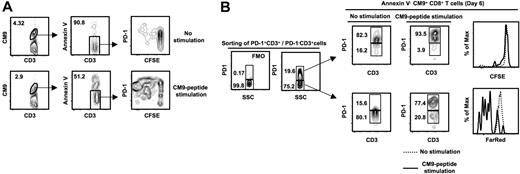
PD-1highCM9+CD8+ T cells are characterized by low proliferative capacity. (A) Flow cytometry plots showing the CFSE profile of annexin V−CM9+CD8+ T cells. Cells were cultured for 6 days in the absence or presence of CM9 peptide. A representative sample from 5 macaques tested is shown. (B) PD-1+CD3+ and PD-1−CD3+ cells were sorted, labeled with CFSE (PD-1+ CD3+) or FarRed (PD-1−CD3+), and cultured in the absence or presence of CM9 peptide. The PD-1 control (FMO) and specific staining in a presort sample is shown on the left. Cells were harvested on day 6 and stained for PD-1 expression. Histograms depict the CFSElow and FarRedlow populations in the annexin V−CM9+CD8+ T cells. A representative example from 3 macaques analyzed is shown.
PD-1highCM9+CD8+ T cells are characterized by low proliferative capacity. (A) Flow cytometry plots showing the CFSE profile of annexin V−CM9+CD8+ T cells. Cells were cultured for 6 days in the absence or presence of CM9 peptide. A representative sample from 5 macaques tested is shown. (B) PD-1+CD3+ and PD-1−CD3+ cells were sorted, labeled with CFSE (PD-1+ CD3+) or FarRed (PD-1−CD3+), and cultured in the absence or presence of CM9 peptide. The PD-1 control (FMO) and specific staining in a presort sample is shown on the left. Cells were harvested on day 6 and stained for PD-1 expression. Histograms depict the CFSElow and FarRedlow populations in the annexin V−CM9+CD8+ T cells. A representative example from 3 macaques analyzed is shown.
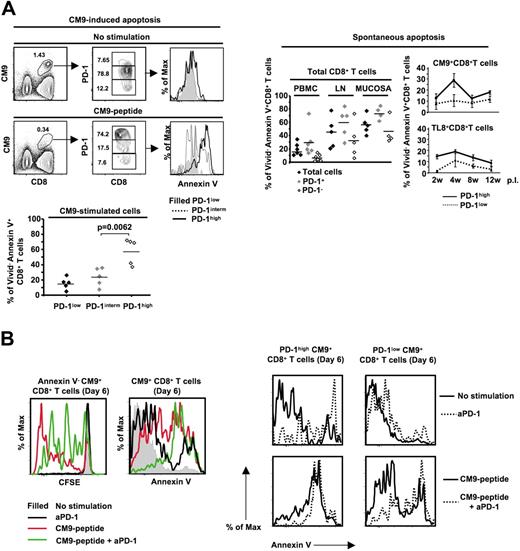
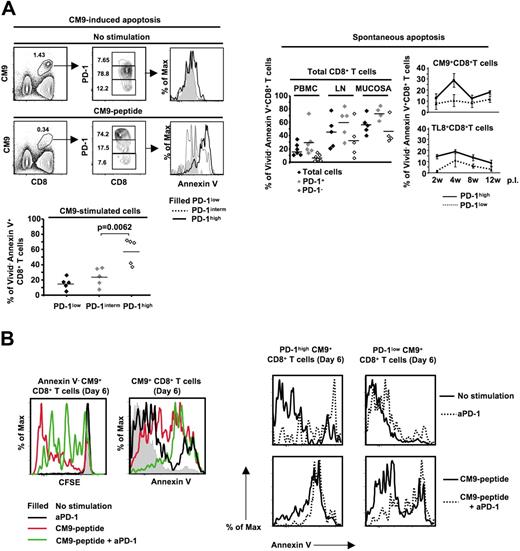
Low proliferation of PD-1high cells could be due to increased in vitro death upon activation.55 Therefore, we analyzed the susceptibility of CM9-specific CD8+ T cells to cell death after stimulation with CM9 peptide for 14 to 16 hours. CM9-specific CD8+ T cells that were negative for Vivid, an amine reactive viability dye,56 further up-regulated PD-1 upon stimulation with CM9 (Figure 6A left panel). This up-regulation of PD-1 was associated with extensive sensitivity to cell death, estimated by annexin V positivity (57.4% ± 7.5% annexin V+, n = 5, versus 23.4% ± 5.3% annexin V+, n = 5, for PD-1high and PD-1intermCM9+CD8+ T cells, respectively, P = .006) (Figure 6A left panel). PD-1high cells were also more sensitive than PD-1low cells to spontaneous cell death within total, CM9-specific, and TL8-specific CD8+ T-cell populations (Figure 6A right panel). We further investigated the role of PD-1 in the survival of CM9-specific CD8+ T cells by stimulating them with CM9 peptide in the absence or presence of a cross-reactive monoclonal antihuman PD-1 antibody. The presence of the anti-PD-1 resulted in reduced proliferation of CM9-specific CD8+ T cells in a 6-day culture (Figure 6B left panel). This reduction was accompanied by increased sensitivity to cell death (Figure 6B left panel). Furthermore, the effect of treatment with anti-PD-1 was observed mainly in the PD-1highCM9+CD8+ T-cell compartment, either in the absence or presence of stimulation with CM9 peptide (Figure 6B right panel). Overall, our data indicate that PD-1 is a negative regulator of SIV-specific CD8+ T-cell proliferation, likely by regulating their in vitro survival.
PD-1high CM9-specific CD8+ T cells are highly susceptible to activation-induced cell death. (A) Flow cytometry showing CM9+CD8+ T cells as well as their PD-1 expression cultured in the absence or presence of CM9 peptide for 18 to 22 hours (left panel, top row). Histograms depict the levels of annexin V positivity in Vivid− CM9+CD8+ T cells. Pooled data showing the percentage of Vivid−annexin V+CM9+CD8+ T cells from 4 macaques, in the PD-1low, PD-1interm, and PD-1high compartments are shown below. The spontaneous apoptosis of total, CM9-specific, and TL8-specific CD8+ T cells cultured for 14 hours is shown in the right panel. SIV-specific CD8+ T cells from 4 animals at different time points after infection were analyzed. Horizontal lines and bars depict mean values. The P values were calculated using Student t test. (B) Histograms representing the CFSE dilution in annexin V−CM9+CD8+ T cells cultured alone or stimulated with CM9 peptide in the absence or presence of an anti-PD-1 antibody (20 μg/mL) (left panel). The levels of annexin V positivity on CM9+CD8+ T cells, under the same treatment conditions, are also shown (left panel). The effect of treatment with the anti-PD-1 on the PD-1high and PD-1low CM9+CD8+ T cells in a 6-day culture is shown in the right panel. Data from 1 of 2 macaques tested are shown.
PD-1high CM9-specific CD8+ T cells are highly susceptible to activation-induced cell death. (A) Flow cytometry showing CM9+CD8+ T cells as well as their PD-1 expression cultured in the absence or presence of CM9 peptide for 18 to 22 hours (left panel, top row). Histograms depict the levels of annexin V positivity in Vivid− CM9+CD8+ T cells. Pooled data showing the percentage of Vivid−annexin V+CM9+CD8+ T cells from 4 macaques, in the PD-1low, PD-1interm, and PD-1high compartments are shown below. The spontaneous apoptosis of total, CM9-specific, and TL8-specific CD8+ T cells cultured for 14 hours is shown in the right panel. SIV-specific CD8+ T cells from 4 animals at different time points after infection were analyzed. Horizontal lines and bars depict mean values. The P values were calculated using Student t test. (B) Histograms representing the CFSE dilution in annexin V−CM9+CD8+ T cells cultured alone or stimulated with CM9 peptide in the absence or presence of an anti-PD-1 antibody (20 μg/mL) (left panel). The levels of annexin V positivity on CM9+CD8+ T cells, under the same treatment conditions, are also shown (left panel). The effect of treatment with the anti-PD-1 on the PD-1high and PD-1low CM9+CD8+ T cells in a 6-day culture is shown in the right panel. Data from 1 of 2 macaques tested are shown.
Discussion
The aim of the current study was to investigate the expression of the surface receptor PD-1, a negative regulator of T-cell activation and proliferation,41 on SIV-specific CD8+ T cells, as well as its role in regulating their function and proliferation. Our data revealed high and persistent expression of PD-1 on cells recognizing the CM9 epitope of the SIV Gag protein in a manner that is independent of their maturation and localization, consistent with our prior findings with HIV-specific CD8+ T cells.39 Specifically, we found that the level of PD-1 expression (as either % or MFI) is identical on CD28highCD95+ and CD28−CD95+ memory subpopulations of CM9-specific CD8+ T cells. Furthermore, CM9-specific CD8+ T cells express significantly higher levels of PD-1 than does the total CD28highCD95+CD8+ T-cell population, indicating that not all memory CD8+ T cells express high levels of PD-1. What mediates the high expression of PD-1 on CM9-specific CD8+ T cells? By comparing PD-1 expression on CM9- and TL8-specific CD8+ T cells during acute infection, we were able to show that persistent antigen-specific stimulation mediated through the TCR is a prerequisite for high PD-1 expression. Approximately 3 to 5 weeks into SIV infection, the TL8 epitope undergoes a common escape mutation50 resulting in loss of TCR recognition. In our studies, we found that PD-1 expression initially increased on TL8-specific cells, but then decreased coincident with loss of TCR recognition of the escape variant (Figure 2C). There was also a decrease in the total number of TL8-specific cells after mutational escape at the TL8 epitope, and during this contraction phase, PD-1highTL8+CD8+ T cells were found to be more sensitive to spontaneous apoptosis compared with PD-1lowTL8+CD8+ T cells, thereby implying that PD-1 was actively involved in depletion of TL8+CD8+ T cells (Figure 6A). Mutational escape at the CM9 epitope did not occur, and the resultant chronic TCR-dependent stimulation led to persistently high numbers of CM9-specific CD8+ T cells that maintained high expression of PD-1 (Figure 2C).
The need for persistent antigenic-specific TCR stimulation for PD-1 expression could explain why HIV-specific, compared with CMV-specific, CD8+ T cells exhibit higher levels of PD-1.39 We did not observe any difference between PD-1 levels on total CD8+ T cells from noninfected and SIV-infected animals in all memory populations tested. Furthermore, the expression of PD-1 on total central and effector memory subsets of CD8+ T cells from SIV-infected animals was rather uniform. Therefore, our data indicate that neither the chronic general activation of the immune system that is observed in HIV and SIV infection57,58 nor the mechanism(s) underlying this activation is capable of inducing expression of PD-1. Interestingly, CM9-specific CD8+ T cells located in mucosa were found to express lower levels of PD-1 compared with these cells in other tissues (Figures 1B and 3B). Since the gastrointestinal tract is a major site for SIV replication,59 one would have predicted higher PD-1 expression in this compartment. Because PD-1 expression is linked to antigen-specific TCR stimulation, these findings suggest that antigen presentation of SIV may be impaired at mucosal sites, possibly due to infection and depletion of local dendritic cells.60 Alternatively, it is possible that accelerated cell death of PD-1highCM9+CD8+ T cells occurs in situ at mucosal sites, resulting in lower detection of cells expressing PD-1 in the live cell gate. A final possibility is that the techniques needed to isolate T lymphocytes from mucosal samples affect the expression of PD-1. Ultimately, immunocytochemistry studies will be needed to determine whether PD-1 expression at mucosal sites is lower than in the blood and lymph nodes, and whether it is associated with high expression of PD-1 ligands and increased cell death of CD8+ T cells.
Whether PD-1 expression on virus-specific CD8+ T cells is directly associated with their capacity for cytokine production is uncertain. Although the frequency of PD-1 expression on the different virus-specific CD8+ T cells was found to correlate with their ability to produce cytokines (IFN-γ, TNF-α, and IL-2),38-40 we have been unable to establish a direct link between these 2 parameters.39 In agreement with previous studies,34,36 we found significantly higher frequencies of circulating CM9+CD8+ T cells in vaccinated/SIV-challenged monkeys compared with unvaccinated/SIV-challenged ones. Within this context, our data show that PD-1highCM9+CD8+ T cells are capable of producing IFN-γ, TNF-α, and IL-2, indicating that PD-1 expression is not directly linked to this component of SIV-specific CD8+ T-cell exhaustion. In contrast to the production of cytokines by PD-1low HIV-specific CD8+ T cells,39 PD-1lowCM9+CD8+ T cells from SIV-challenged animals did not produce significant amounts of cytokines. However, we did find one vaccinated/SIV-challenged monkey that controlled viremia and possessed a clear population of PD-1lowCM9+CD8+ T cells that were potent producers of all 3 cytokines. This was true for cells isolated from blood, spleen, or LN of this animal, indicating that this was not a tissue-specific phenomenon. This one monkey notwithstanding, the weight of our data indicates the lack of a direct link between cytokine function and PD-1 expression in SIV, as well as HIV, infection. We did find that the pattern of cytokines produced differed between SIV-challenged and vaccinated/SIV-challenged animals (Figure 4C), but no significant difference was found for each individual cytokine between the 2 groups. This suggests that the quality of the CM9+CD8+ T-cell cytokine response induced under these 2 conditions is similar.
We found that PD-1highCM9+CD8+ T cells were characterized by low proliferative capacity. This phenotype was associated with extensive susceptibility of these cells to death upon antigen-specific TCR stimulation. Altered proliferation of virus-specific CD8+ T cells was recently described under in vitro manipulation of the PD-1:PD-L1 axis,38-40 probably through regulation of their survival ability.39 As in humans, use of a monoclonal anti-PD-1 antibody resulted in reduced proliferation of SIV-specific CD8+ T cells. Again, this reduction was associated with higher annexin V positivity specifically within the PD-1high compartment. The proportion of antigen-specific CD8+ T cells that enter cell division was determined by their susceptibility to cell death shortly after in vitro stimulation according to a recent study.55 Our data are in agreement with that study and show that survival is a critical regulator of SIV-specific CD8+ T-cell proliferation and point to PD-1 as a negative regulator of this process. In conclusion, our study revealed high expression of PD-1 on SIV-specific CD8+ T cells, which may contribute to the failure of these cells to control the virus fully. The overall pattern of PD-1 expression and the functional attributes of PD-1+ T cells in SIV infection closely mimic those in HIV infection. Therefore, our findings justify the use of the SIV/macaque model to test whether manipulation of the PD-1:PD-L1 axis will lead to restoration of the function of SIV-specific CD8+ T cells and improved clinical outcome, and whether such interventions may result in unfavorable events when PD-1+ T cells of other specificities are released from their negative regulatory control.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
D.A.P. is a Medical Research Council (United Kingdom) Senior Clinical Fellow.
National Institutes of Health
Authorship
Contribution: C.P. designed, performed, and analyzed experiments and wrote the paper; D.A.P., D.R.A., C.G., E.G., and V.C. participated in performing the experiments; J.M., V.C., M.V., E.T., and G.F. contributed samples; M.R. and D.C.D. contributed to experimental design and reagents; S.H.M. and S.J.D. contributed reagents; R.A.K. designed experiments and wrote the paper. All authors discussed the results and contributed to the paper writing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Constantinos Petrovas, Vaccine Research Center, NIAID, NIH, 40 Convent Dr, Bethesda, MD 20892; e-mail: petrovasc@mail.nih.gov.