Abstract
To assess the clinical significance of lupus anticoagulants (LAs) and antiphospholipid antibodies (aPLs) toward thrombosis and abortions, we measured them in 112 patients whose samples were available at enrollment in the warfarin in the antiphospholipid syndrome (WAPS) study. Enzyme-linked immunosorbent assay (ELISA) and coagulation test values in the highest and lowest tertiles were compared. When considered separately, IgG antibodies to β2-glycoprotein I (aβ2GPI) and prothrombin (aPT) were associated with anamnestic arterial and venous thrombosis, respectively, and those to annexin AV (aAnAV) with abortions. IgM antibodies to protein S and the lupus ratio of the dilute prothrombin time were associated with prospective thrombosis. No other association for IgM antibodies was seen. LA-positive patients who carried aβ2GPI antibodies were at risk of anamnestic arterial and total thrombosis and aPT antibodies to that of anamnestic venous and total thrombosis. LA-positive patients who carried IgG aβ2GPI and aAnAV antibodies were at risk for both anamnestic abortion and prospective thrombosis. Overall, these data support the inclusion of aβ2GPI antibodies in and suggest the removal of anticardiolipin antibodies from the laboratory criteria of the antiphospholipid syndrome. They also suggest that the measurement of aPT and aAnAV antibodies is useful in some selected situations and that there is little role for IgM antibody detection.
Introduction
Arterial and venous thrombosis and recurrent abortions in combination with lupus anticoagulants (LAs) and/or medium to high titers of IgG/IgM anticardiolipin (aCL) and anti-β2-glycoprotein I (aβ2GPI) antibodies define the antiphospholipid syndrome (APS).1 These antibodies are the best known and characterized antiphospholipid (aPL) antibodies, which, despite their name, are not directed against negatively charged phospholipids but recognize several (plasma) proteins with affinity for anionic phospholipids. Among them, β2-glycoprotein I (β2GPI)2–4 and prothrombin (PT)5,6 are the antigenic targets of the majority of aPL antibodies, and subgroups of aβ2GPI and aPT antibodies display LA activity in vitro.3,5,7–9 The association of these antibodies with arterial and venous thrombosis has been widely investigated in the last two decades. In 2003, two systematic reviews of the literature on LA, aCL, aβ2GPI, and aPT antibodies showed that LAs are the strongest risk factors for both arterial and venous thrombosis, whereas such a role did not clearly emerge for the other antibodies, unless in some selected clinical scenarios.10,11 Overall, IgG aCL antibodies at medium to high titer appeared as possible risk factors of arterial thrombosis, aβ2GPI antibodies of venous thrombosis, whereas aPT antibodies did not seem to represent a significant thrombotic risk factor. More recently, retrospective studies have found thrombosis to be associated with the presence of β2GPI-dependent LA activity12 and with the combined positivity for LA, aCL, and aβ2GPI antibodies.13 These data support the concept that the analysis of the aPL antibody profile, rather than of a single test, helps to establish which aPL-positive patients are at risk of thrombosis. Along this line, other so-called aPL antibodies, such as those directed to protein S (PS)14 and annexin AV (AnAV),15 have been studied for their association with thrombosis and miscarriage. Variable results have been reported, partly because of the retrospective design of most studies, which prevented firm conclusions from being drawn.
In the present analysis, we explored the relationship between single/multiple positive tests for LA and IgG/IgM aCL, aβ2GPI, aPT, aPS, and aAnAV antibodies and thrombosis and obstetric complications before and after recruitment in a subgroup of patients enrolled in the warfarin in the antiphospholipid syndrome (WAPS) prospective, multicenter, international study.16
Patients, materials, and methods
Patients
This study was undertaken in the setting of the WAPS study, an international and prospective registry that enrolled 462 patients with persistent LAs and/or moderate to high positive aCL antibodies from 26 centers in Italy, Norway, Poland, Argentina, and Czech Republic.16 Diagnosis of LAs and aCL antibodies was performed locally by each participating center.17,18 No other aPL antibody measurement was required to participate in the WAPS study. Thus, we were unaware of the aPL status of the patients apart from their LA and/or aCL persistent positivity.
All patients gave their written informed consent to participate in the clinical study and to blood testing in accordance with the Declaration of Helsinki. The present study included the 112 patients whose plasma and/or serum samples were centralized at the Ospedali Riuniti of Bergamo (Italy). The study was approved by the ethical committee of each participating center. We measured aCL, aβ2GPI, aPT, aPS, and aAnAV antibodies in all of the enrolled patients. The complete series of 3 coagulation tests (ie, activated partial thromboplastin time[APTT], dilute Russell viper venom time [RVVT], dilute prothrombin time [DPT]) was performed in the plasma of 108 patients; the APTT- and the RVVT- but not the DPT-based tests were carried out in the plasma of 2 patients, and for the remaining 2 cases plasma was not available for coagulation tests.
End points
The analysis was aimed at assessing the association between the antibodies of interest (see “Measurement of aPL antibodies”) and (1) diagnosis of APS (eg, any anamnestic thrombotic and obstetric event qualifying for the syndrome) at recruitment; (2) anamnestic total, arterial, and venous thrombosis; (3) prospective thrombosis; and (4) abortions before recruitment.
All diagnoses of thrombosis (stroke, transient ischemic attack, deep vein thrombosis, pulmonary embolism) had to be objectively documented (for clinical details, see Finazzi et al16 ). Thrombotic events during follow-up have been blinded and validated by an ad hoc committee of experts. The criteria for and classification of these events were prespecified.16
Measurement of aPL antibodies
IgG and IgM aCL, aβ2GPI, and aPT antibodies were measured by commercially available enzyme-linked immunosorbent assay (ELISA; Asserachrom APA, Asserachrom anti-β2GPI, and Asserachrom antiprothrombin, respectively; all kindly provided by Diagnostica Stago, Asnieres, France). ELISA results were expressed in G antiphospholipid (GPL) and M antiphospholipid (MPL) units according to the manufacturer's instructions. Asserachrom APA is a mixture of 3 phospholipids, among which cardiolipin coated the plate as a solid-phase antigen. It is also a β2GPI-dependent ELISA. For both of these reasons, we used this assay to measure aCL antibodies.
IgG and IgM aAnAV and aPS antibodies were measured by prototype ELISAs (both kindly provided by Diagnostica Stago). Results were expressed in milli optical density (mOD).
Measurement of LAs
LAs were detected by lupus ratio (LR) tests, which are quantitative assays for LAs that integrate screening, mixing, and confirmatory procedures into 1 run; they are based on 1 of the phospholipid-dependent clotting principles: APTT, RVVT, or DPT, respectively.19–21 For each of the 3 tests, in-house reagents with dilutions of crude cephalin from porcine brain (a generous gift from Axis-Shield PoC AS, Oslo, Norway) were used. In the APTT-based LR test, crude cephalin diluted 1:50 and 1:800 in Owren buffer was mixed 1:1 with ellagic acid as activator (final concentration 6 mg/L). For the DRVVT-based LR test, crude cephalin was diluted 1:200 and 1:10 000. Russell viper venom (Sigma-Aldrich Norway, A/S, Oslo, Norway) was diluted in imidazole buffer with 1% bovine serum albumin. The reagents for the DPT-based LR test were prepared with recombinant thromboplastin (Innovin; Dade Behring, Marburg, Germany) diluted 1:200 in tris-buffered saline (final dilution). Diluted recombinant thromboplastin was used as reagent with low phospholipid concentration. For a reagent with high phospholipid concentration, crude cephalin (final dilution 1:200) was added to the thromboplastin.21 Regardless of which clotting principle was used, the patient's plasma was mixed 1:1 with pooled normal plasma before testing. For each of the 3 clotting tests, clotting times were then registered with the reagents with low and high phospholipid concentrations. The clotting time with the reagent with low phospholipid concentration was then divided by the clotting time obtained with the reagent at high phospholipid concentration. The ratio was normalized by dividing with the corresponding ratio of the normal pooled plasma. The final result is the LR of that patient's plasma.
Statistical analysis
Continuous data are expressed as median and interquartile range. Discrete variables are presented as percentages. Clinically meaningful cutoff values have been chosen to categorize aCL antibodies (≥ 40 units).
For their association with the clinical end points (see “End points”), values of IgG and IgM antibodies assessed with ELISA have been analyzed as statistical tertiles, whereas upper normal limit values of LR for each coagulation test, corresponding to the 97.5 percentile of a normal population (APPT ≥ 1.10, RVVT ≥ 1.11, DPT ≥ 1.08),20 have been compared with those below the limit.
Clotting assays were also analyzed assessing the effect of 1-unit increases of the LR above the upper normal limit. LA positivity was defined by the value of the LR above the upper normal limit of at least 1 of the following tests: APTT, RVV, and DPT. The relationships between end points and selected antibodies have been considered separately as well as according to their combinations.
Age- and sex-adjusted multivariable logistic models have been fitted with the end points and the selected antibodies alone and in combination as explanatory variables to assess their independent, predictive role. Results are given as odds ratios (ORs) along with their 95% confidence interval (CI). A 2-sided P value less than .05 was considered statistically significant. Because of the explorative nature of the analysis, no correction for multiplicity of tests was performed.
Results
Clinical characteristics of patients recruited in the study
The main demographic, clinical, and laboratory features of the sample population are reported in Table 1. Eighty-seven patients were diagnosed with APS because they had thrombotic and/or obstetric complications, whereas aPL antibodies were present in 25 patients either alone or in combination with clinical manifestations other than those qualifying for APS.
At the time of enrollment, 39 (34.8%) and 23 (20.5%) patients were on anticoagulant and antiplatelet therapy, respectively. Forty-one (36.6%) of 112 patients entered the randomized trial: 19 received high-intensity warfarin and 22 continued the conventional therapy.16
During a median follow-up of 3.67 years, 15 (13.4%) patients had a thrombotic event: deep vein thrombosis in 7 patients (1 of them experienced 3 events), transient ischemic attacks in 4 cases, ischemic cerebral strokes in 3 patients, and nonfatal pulmonary embolism in 1 case. Two of the 15 patients with thrombosis during follow-up also experienced an abortion.
Seven patients had a thrombotic event (4 deep vein thromboses, 1 stroke, and 2 transient ischemic attacks) while they were not receiving an antithrombotic therapy (1 of them had withdrawn warfarin 3 weeks before recurrence of deep vein thrombosis).
Prevalence and tertile distribution of various aPL antibodies
Table 2 reports the values of ranges, medians, and lower/upper limits of the highest tertile of both IgG and IgM antibodies measured in the patients' population for the various tests.
The LR exceeded the upper normal limits in 72, 71, and 92 patients for the APTT-, RVVT-, and DPT-based tests, respectively. Ninety-eight patients (87.5%) were LA positive, since they had at least 1 abnormal coagulation test.
Clinical associations of various aPL antibodies
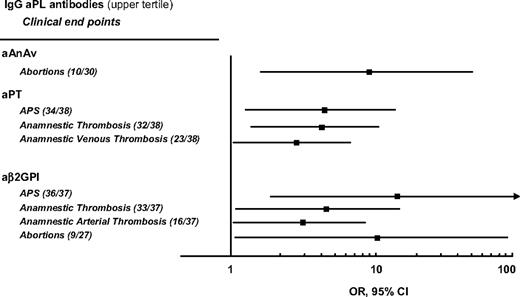
Figure 1 shows the relationships between the outcome events measured before and after recruitment in the study and IgG aβ2GPI, IgG aPT, and IgG aAnAV antibodies, which have been found to be statistically significant. All positive and negative correlations are reported in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Age- and sex-adjusted significant associations of various IgG aPL antibodies in the upper tertile with anamnestic clinical end points. No significant association was found between IgM antibodies in the upper tertile and anamnestic clinical end points. IgG and IgM antibodies were measured at enrollment of 112 patients in the WAPS study. The numbers of patients with events on the number of patients with antibodies in the highest tertile are reported in brackets. APS indicates that arterial thrombosis, venous thrombosis, and obstetric events were combined; total thrombosis indicates that anamnestic arterial and venous thrombosis were combined.
Age- and sex-adjusted significant associations of various IgG aPL antibodies in the upper tertile with anamnestic clinical end points. No significant association was found between IgM antibodies in the upper tertile and anamnestic clinical end points. IgG and IgM antibodies were measured at enrollment of 112 patients in the WAPS study. The numbers of patients with events on the number of patients with antibodies in the highest tertile are reported in brackets. APS indicates that arterial thrombosis, venous thrombosis, and obstetric events were combined; total thrombosis indicates that anamnestic arterial and venous thrombosis were combined.
As to the G isotype, various statistically significant results have been found when we compared the risk of event in the upper tertile with the lower one. In particular, aAnAv antibodies were associated with a 9-fold higher risk of abortion, aPT antibodies were associated with a 3- to 4-fold higher probability of retrospective thrombosis in the study, whereas the probability of full APS diagnosis was 4-fold. As to aβ2GPI antibodies, they were associated with a 3- to 4-fold higher probability of retrospective thrombosis, a 10-fold higher risk of abortion, and a 16-fold higher risk of APS. aPS antibodies in the highest tertile were associated with a significantly reduced risk of anamnestic total thrombosis (OR 0.28, 95% CI 0.09-0.91, P = .035).
The DPT-based assay with a LR above the upper normal limit was also significantly associated with APS (OR 6.62, 95% CI 1.73-25.36, P = .006) and anamnestic thrombosis (OR 4.51, 95% CI 1.22-16.67, P = .024). We also observed a 2.5 times higher risk of prospective thrombosis for each unit increase of the DPT-based LR assay (OR 2.54, 95% CI 1.05-6.19, P = .040). No other coagulation test showed significant associations.
No significant association was seen between the M isotype and anamnestic thrombotic or obstetric events. IgM aPS antibodies in the highest tertile were associated with a significantly reduced risk of anamnestic arterial thrombosis (OR 0.21, 95% CI 0.07-0.67, P = .035). Conversely, the presence of IgM aPS antibodies in the highest tertile was associated with a significantly high risk of prospective thrombosis (OR 6.58, 95% CI 1.19-36.36, P = .031).
Contribution of positivity to IgG aPL antibodies to the risk of thrombosis and abortion in LA-positive patients
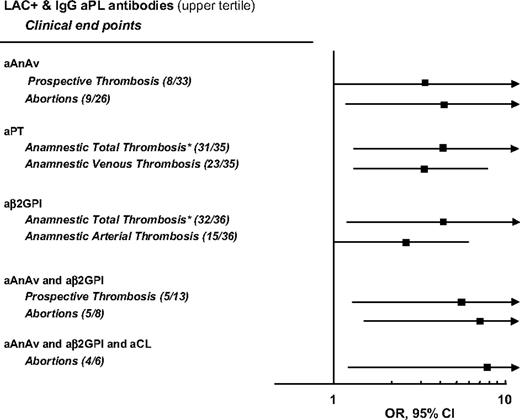
Figure 2 shows the significant relationships between the outcome events measured before and after recruitment in the study in LA-positive patients with high levels (upper tertile) of 1 or more antibodies. Because we obtained a statistically significant result for only 1 test involving IgM antibodies (see “Clinical associations of various aPL antibodies”), we limited the analysis to the G isotype. All positive and negative correlations are reported in Table S2.
Contribution of IgG aPL antibodies in the upper tertile to the risk of thrombosis and abortions of LA-positive patients enrolled in the WAPS study. The numbers of patients with events on the number of patients with LA-positive and IgG antibody in the highest tertile are reported in brackets. Analysis was age- and sex-adjusted. The numbers of patients with events on the number of LA-positive patients with antibodies in the highest tertile are reported in brackets. Total thrombosis indicates that anamnestic arterial and venous thrombosis were combined.
Contribution of IgG aPL antibodies in the upper tertile to the risk of thrombosis and abortions of LA-positive patients enrolled in the WAPS study. The numbers of patients with events on the number of patients with LA-positive and IgG antibody in the highest tertile are reported in brackets. Analysis was age- and sex-adjusted. The numbers of patients with events on the number of LA-positive patients with antibodies in the highest tertile are reported in brackets. Total thrombosis indicates that anamnestic arterial and venous thrombosis were combined.
We had available 31 combinations of laboratory variables ranging from a minimum of 2 (ie, LA-positive patients who carried only 1 IgG antibody in the highest tertile) to a maximum of 6 (ie, LA-positive patients who carried all 5 IgG antibodies in the highest tertile). Overall, 9 (5.8%) of 155 combinations that have been analyzed reached the formal level for statistical significance (P < .05). LA-positive patients belonging to the upper tertile of aAnAv or aPT antibodies had an almost 3- to 4-fold higher risk of thrombosis. LA-positive patients belonging to the upper tertile of aAnAv antibodies had a 4-fold higher risk of abortion. LA-positive patients belonging to the upper tertile of aβ2GPI antibodies had an almost 2- to 4-fold higher risk of thrombosis. LA-positive patients belonging to the upper tertile of aAnAv and aβ2GPI antibodies had an almost 5- to 7-fold higher risk of prospective thrombosis and abortion. Finally, LA-positive patients belonging to the upper tertile of aAnAv, aβ2GPI, and aCL antibodies had an almost 8-fold higher risk of abortion.
Discussion
At the beginning of 2006, an international panel of experts established by majority the inclusion of aβ2GPI antibodies among the criteria of APS in addition to LAs and aCL antibodies.1 The same panel established the inclusion of aPT and antiphosphatidylethanolamine antibodies to be premature, due to the insufficient amount of available evidence. The panel neither took into account the role of other aPL antibodies as laboratory criteria nor considered the significance of aPL antibodies as predictors of occurrence/recurrence of APS events. Our study, performed in the setting of the WAPS study,16 gave us the possibility to investigate the role of 5 aPL antibodies and 3 coagulation tests as diagnostic criteria and predictors of thrombosis and obstetric complications of APS. We considered the clinical significance of each ELISA and coagulation test and evaluated the contribution of ELISA tests to the risk conferred by the presence of LAs. Our study confirms the usefulness of the inclusion of IgG aβ2GPI antibodies among the laboratory diagnostic criteria of APS, since we found that levels of these antibodies in the highest tertile were significantly associated with all of the clinical end points qualifying for the syndrome. Conversely, aCL antibodies did not show any significant association. These observations are well in agreement with the results of a systematic review of the literature on APS,11 which reported a higher frequency of significant associations with thrombosis for aβ2GPI than for aCL antibodies. This difference is apparently difficult to reconcile to the notion that ELISAs for aCL detection are β2GPI dependent and should, therefore, measure aβ2GPI antibodies. In the real case, measurements of aCL and aβ2GPI antibodies are only partly overlapping, and the presence of the antibodies may be detected by one but not the other ELISA system. Several reasons account for this discrepancy: (1) according to the source of β2GPI in the assay, aCL ELISAs may measure antibodies directed against bovine β2GPI, which are clinically irrelevant; (2) since cardiolipin or a mixture of anionic phospholipids are coated on the aCL ELISA plate, antibodies against phospholipid-binding proteins other than β2GPI may be measured in this assay; (3) aβ2GPI ELISAs allow the measurement of antibodies directed against all of the potential binding sites on the molecule, whereas aCL ELISA does not allow the detection of antibodies directed against the fifth domain, which is already engaged in the binding to phospholipids. Based on these clinical and laboratory observations, we may foresee that the next update will retain aβ2GPI antibodies and discard aCL antibodies as laboratory criteria of APS.
Our analysis suggests the possibility that aPT and aAnAV antibodies are potential candidates as laboratory criteria of APS, at least in some selected situations. In fact, the former antibodies were associated with (venous) thrombotic events, which is a somewhat unexpected finding, based on the results of a systematic review of the literature.11 On the other side, this is well in keeping with the results of “in vitro” model systems, which show the ability of aPT antibodies to interfere with the anticoagulant protein C pathway22 and to promote thrombin generation.23 It is also in agreement with the results of a recent prospective study, which reported IgG aPT and aβ2GPI antibodies to be independent risk factors of thrombosis recurrence.24 aAnAV antibodies were found to be associated with the obstetric complications of APS. The observation that aAnAV is the main anticoagulant substance present in the placenta25 provides the plausible pathophysiologic rationale. However, care must be taken before these data are generalized because a large retrospective study reported only a nonsignificant trend for the association between aAnAV antibodies and miscarriage.26
By age- and sex-adjusted logistic regression, we found that IgM aPS antibodies were significantly associated with prospective thrombosis. aPS antibodies are reported in children during or after (viral) infections, such as varicella and chicken pox.27 They are commonly transient and present together with LAs, and only in a few cases they cause purpura fulminans.28 These clinical scenarios are very selective and unusual, which makes the routine detection of IgM aPS antibodies of little use in adult patients suspected of suffering from APS. Even more, the presence of aPS antibodies of both isotypes seems to have a protective effect against anamnestic thrombosis. Thus, the evidence so far available about aPS antibodies is rather conflicting, implying that their significance is still doubtful and that their measurement is presently not recommended outside of clinical studies. No other significant association was observed between IgM aPL antibodies and thrombosis or obstetric complications. These data are, again, in line with the results of two systematic reviews, which reported IgM aCL, aβ2GPI, and aPT antibodies to be less often associated than IgG antibodies with the clinical events of APS.10,11 Very few studies included in these reviews analyzed the relationship between thrombosis and the titer of IgM aCL antibodies. It appeared that the higher the titer, the higher the possibility to find significant correlations. However, the amount of available information was too scanty and limited to allow any meaningful conclusion. A recent large prospective study on IgG and IgM aCL, aβ2GPI, and aPT antibodies did not find any significant association between the M isotype and thrombosis, even when the cutoff of 40 units was used.24 Because of the low risk of missing the occasional patient whose APS is characterized by the isolated presence of IgM antibodies, we suggest limiting the routine measurement of aPL antibodies to the G isotype, at least until well-designed studies establish if and which role IgM aPL antibodies have in the setting of APS. This suggestion goes toward the simplification of the laboratory workout of patients suspected of suffering from APS and represents a step beyond the indications of the international panel of experts to measure aCL and aβ2GPI antibodies of both the G and M isotype.1
In the original WAPS study,16 LA detection was performed locally by each participating center and many different tests, reagents, and instrumentations were used. This variation made it virtually impossible to investigate the clinical significance and contribution of each single coagulation test. In the present study, plasma samples were centralized in order to perform a homogeneous coagulation screening. The use of 3 integrated assays allowed us to analyze plasmas from patients on oral anticoagulation and to detect antibodies with different specificities. Because the great majority (about 90%) of our patients had at least 1 of these assays abnormal, we could not establish the role of LA positivity as an independent risk factor of thrombosis. When the 3 assays were analyzed separately, the DPT-based LR test was significantly associated with both anamnestic and prospective thrombosis. In essence, this is a prothrombin time-based assay performed with diluted thromboplastin: according to the type of reagent, the DPT is highly sensitive and specific for the presence of LAs,29 caused by both aβ2GPI30,31 and aPT antibodies.31 The analysis was repeated to investigate the contribution of IgG aPL antibodies measured by ELISA to the risk of thrombosis conferred by LAs. Again, aCL antibodies gave a very marginal contribution, whereas aβ2GPI antibodies were associated, alone or in various combinations, with the thrombotic and obstetric events of APS. In particular, their presence together with aAnAV antibodies was a risk factor for prospective thrombosis. aPT antibodies also contributed to the risk of thrombosis. aAnAV antibodies alone contributed to the risk of abortions. In general, the risk of event increased with the number of positive ELISA tests.
The results of our study must be viewed critically, in the light of its limitations. Firstly, samples were taken at enrollment in the WAPS trial and serial determinations during follow-up were not available. Secondly, a substantial number of patients were on antithrombotic drugs during the study period, which influenced the incidence of thrombosis16,32–34 and, therefore, affected the strength of associations between laboratory and clinical end points. This is, at least partly, the reason why most significant associations were retrospective. Thirdly, the laboratory tests to detect aPL antibodies are far from a proper standardization. The assays we used are no exception, although care was taken to centralize both the coagulation and the ELISA determinations. As we measured aCL antibodies by an assay that uses a mixture of phospholipids rather than pure cardiolipin as solid-phase antigen, we cannot rule out the possibility that another ELISA may lead to substantially different results and associations. Also, the highly select nature of our patient population—more than three-quarters of them suffered from APS—may have influenced the reported associations. Finally, because of the explorative approach of the analysis, we did not allow for multiplicity of tests. Therefore, we cannot exclude that play of chance could be the explanation of some of the results, but the rarity of the disease along with the bounty of specific tests that were available in the database made this approach a unique opportunity, though scientifically speculative, to produce stimulating results.
In conclusion, this study supports the inclusion of aβ2GPI antibodies among the laboratory criteria of APS.1 Based on our data and on the literature published in the last few years, we go even further and propose to replace aCL measurement by that of aβ2GPI antibodies and to explore the possibility, by means of ad hoc studies, to limit antibody detection to the G isotype. As the measurement of aPT and aAnAV antibodies appeared useful in some selected situations, we prompt further investigation to establish if and to which extent they can also be considered among such criteria.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We wish to thank Dr F. Baudo, Dr T.M. Caimi (Ospedale Niguarda Cà Granda, Milano, Italy), and Drs G. B. Cavallero, M. Bonferroni, and G. Galvagno (Azienda Ospedaliera Santa Croce e Carle, Cuneo, Italy) who provided some of the biologic samples used in the study. Diagnostica Stago provided the ELISA reagents for free.
Authorship
Contribution: M.G. designed research, analyzed data, and wrote the manuscript; G.B., R.M.M., and R.M. analyzed data and wrote the manuscript; E.M.J. and F.W. performed coagulation tests, analyzed data, and wrote the manuscript; G.F., O.M., and T.B. wrote the manuscript; and S.M. performed ELISA tests.
Conflict-of-interest disclosure: O.M. is an employee of Diagnostica Stago-France. The other authors declare no competing financial interests.
Correspondence: Monica Galli, Divisione di Ematologia, Ospedali Riuniti, L.go Barozzi, 1, 24128 Bergamo, Italy; e-mail: monicagalli@virgilio.it.