Abstract
Phosphoinositide 3-kinases (PI3Ks) have been considered important in leukocyte motility. PI3Kγ, the class IB PI3K, expressed prominently in leukocytes and also in endothelial cells, mediates leukocyte functional responses induced by chemoattractants. To reveal its role in leukocyte recruitment, we used intravital microscopy to directly visualize leukocyte rolling, adhesion, and emigration in postcapillary venules in PI3Kγ-deficient (PI3Kγ-/-) mice. We report here that PI3Kγ deficiency had no significant effects on leukocyte rolling flux or rolling velocity and minor effects on adhesion (30% to 35%) in response to CXC chemokine MIP-2 (CXCL2) or KC (CXCL1). However, leukocyte emigration was severely impaired in PI3Kγ-/- mice in an early (first 90 minutes) response to MIP-2 or KC. Chimeric mice receiving bone marrow transplants revealed that this early response was entirely dependent upon PI3Kγ in neutrophils but not parenchymal cells (endothelium and others). Identical responses were observed when endogenous chemokine production was induced by TNFα; leukocyte emigration was reduced in PI3Kγ-/- mice. More prolonged responses to MIP-2 (for 4 to 5 hours) or TNFα (6 to 8 hours) were almost entirely PI3Kγ independent and largely dependent on PI3Kδ. Our results reveal that leukocyte emigration response to CXC chemokines is entirely dependent upon PI3Kγ or PI3Kδ, but these are nonoverlapping, temporally distinct events in inflamed tissues in vivo.
Introduction
Phosphoinositide 3-kinases (PI3Ks) are important cellular lipid kinases that convert phosphatidylinositol-4,5-bisphosphate to phosphatidylinositol-3,4,5-trisphosphate (PIP3), an important intracellular second messenger crucially involved in a wide variety of cellular functions and responses.1,2 There are 4 members in the PI3K class I family, in which the catalytic subunits are p110α, p110β, p110γ, and p110δ isoforms.1,2 Upstream of the signaling pathways, the p110α and p110β isoforms are activated by tyrosine kinases, and these 2 types of PI3Ks are expressed in many cell types. PI3Kγ (the p110γ isoform) is predominantly expressed in leukocytes, and chemoattractants, including chemokines, proinflammatory lipids, and bacterial products, activate G protein–coupled receptors leading to PI3Kγ activation via the βγ subunits.2,3 Extensive research efforts over the last few years have demonstrated that PI3Kγ is critical to the inflammatory response and more specifically in leukocyte functions including leukocyte chemotaxis.3–8 These phenotypes were best revealed in PI3Kγ-deficient (PI3Kγ-/-) mice whose neutrophils had impaired chemotactic response toward a number of chemoattractants (IL-8, fMLP, C5a, and macrophage inflammatory protein-1α [MIP-1α]) in vitro and attenuated in general leukocyte recruitment to the peritoneal cavity in response to peritonitis.4–6 However, the responses to chemoattractants were not always completely inhibited (at times less than 30% reduced) or in the case of the pleiotropic inflammatory stimulus, carrageenan, not inhibited at all.4–6
PI3Kδ (the p110δ isoform) is also predominantly expressed in hematopoietic cells,9,10 and recent work suggests that PI3Kδ also contributes to chemoattractant-induced neutrophil migration.11 Boulven et al8 demonstrated that the production of PIP3 in response to chemoattractants is biphasic, with the first 30-second response being entirely dependent upon PI3Kγ activity, whereas the delayed, 5-minute phase is entirely dependent upon p110δ. The most surprising finding was that not only was p110δ responsible for much of the chemoattractant-induced chemotaxis, but PI3Kγ was not involved. It is difficult to consolidate these observations with a clear impairment in chemotaxis in PI3Kγ-/- mice. Puri et al12,13 reported an essential role for both PI3Kγ and PI3Kδ in endothelium, suggesting a partnership between these isoforms in vivo in endothelial cells. Another possibility is that PI3Kγ and PI3Kδ may serve functionally or temporally distinct roles in the recruitment cascade.
Leukocyte recruitment is a multistep recruitment process that is optimally visualized and studied in vivo using intravital microscopy. Making use of this approach, we directly visualized and measured each of the steps of the leukocyte recruitment paradigm, including leukocyte rolling flux, rolling velocity, adhesion, and emigration in response to CXC chemokines in vivo in PI3Kγ-/- mice and in mice treated with a PI3Kδ inhibitor and compared the results with wild-type (WT) mice. Because PI3Kγ is also expressed at low level in endothelial cells and is thought to play a role in TNFα as well as shear stress-induced activation of c-Jun NH2-terminal kinase (JNK) mitogen-activated protein kinase (MAPK)-mediated neutrophil attachment to endothelial cells,13,14 we also asked whether endothelial PI3Kγ plays a role in chemokine-induced leukocyte emigration in vivo. Our data demonstrate that PI3Kγ is important in early CXC chemokine responses, whereas PI3Kδ replaces and maintains the delayed chemokine-induced neutrophil recruitment into inflamed tissues in vivo. There was no temporal overlap for PI3Kγ and PI3Kδ in vivo, but rather their chemotactic responses were distinct sequential events.
Materials and methods
Animals
C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA). PI3Kγ-/- mice on the C57BL/6 background were made by J.M.P.,6 transferred to the University of Calgary Health Sciences Center, and housed in a specific pathogen-free environment. The male mice between 8 and 16 weeks old were used in this study. All animal protocols were approved by the Animal Care Committee of the University of Calgary and met the standards of the Canadian Association of Animal Care.
Two types of chimeric mice were generated following the standard protocols in our laboratory.15 In brief, bone marrow cells were isolated from 6- to 8-week-old donor mice euthanized by spinal cord displacement. The bone marrow cell suspensions (8 × 106 cells) from donor PI3Kγ-/- and C57BL/6 WT mice were injected into the tail vein of C57BL/6 WT and PI3Kγ-/- mice, respectively. Prior to bone marrow cell injection, recipients were irradiated with 2 doses of 5 Gy γ-ray (Gammacell, 137Cs γ-irradiation source) with a 3-hour interval between the 2 irradiations. These chimeric mice were housed in a specific pathogen-free environment for 6 to 8 weeks to allow full humoral reconstitution before they were used in experiments. Initial experiments confirmed that about 99% of leukocytes were from donor mice.15 Also using this exact protocol, we have established that in 6 weeks after irradiation, the irradiated WT mice receiving bone marrow from donor WT mice behaved exactly like nonirradiated WT mice, and the irradiated mutant mice receiving bone marrow from mutant mice behaved identically to nonirradiated mutant mice.15,16
Intravital microscopy
Male mice were anesthetized with an intraperitoneal injection of a mixture of 10 mg/kg xylazine (Bayer, Animal Health, Toronto, ON, Canada) and 200 mg/kg ketamine hydrochloride (Rogar/STB, Montreal, QC, Canada). For all protocols, the left jugular vein was cannulated to administer additional anesthetic or drugs when necessary. The mouse cremaster muscle preparation was used to study the behavior of leukocytes in the microcirculation and adjacent muscle tissue as previously described.16,17 Briefly, an incision was made in the scrotal skin to expose the left cremaster muscle, which was then carefully dissected free of the associated fascia. The cremaster muscle was cut longitudinally with a cautery. The testicle and the epididymis were separated from the underlying muscle and were moved into the abdominal cavity. The muscle was then held flat on an optically clear viewing pedestal and was secured along the edges with 4-0 suture. The exposed tissue was superfused with 37°C-warmed bicarbonate-buffered saline (pH 7.4). An intravital microscope (Axioskop; Carl Zeiss Canada, Don Mills, ON, Canada) with a 25× objective lens (Weltzlar L25/0.35; E. Leitz, Munich, Germany) and a 10× eyepiece was used to examine the cremasteric microcirculation. A video camera (5100 HS; Panasonic, Osaka, Japan) was used to project the images onto a monitor, and the images were recorded for playback analysis using a videocassette recorder.
Single unbranched cremasteric venules (25 to 40 μm in diameter) were selected, and to minimize variability the same section of cremasteric venule was observed throughout the experiment. The number of rolling, adherent, and emigrated leukocytes was determined offline during video playback analysis. Rolling leukocytes were defined as those cells moving at a velocity less than that of erythrocytes within a given vessel. The flux of rolling cells was measured as the number of rolling leukocytes passing by a given point in the venule per minute. Leukocyte rolling velocity was measured for the first 20 leukocytes entering the field of view at the time of recording and calculated from the average time required for a leukocyte to roll along a 100-μm length of venule. A leukocyte was considered to be adherent if it remained stationary for at least 30 seconds, and total leukocyte adhesion was quantified as the number of adherent cells within a 100-μm length of venule in 5 minutes. Leukocyte emigration was defined as the number of cells in the extravascular space within a 200 × 300 μm2 area, adjacent to the observed venule. More than 90% of these emigrated cells were neutrophils.18 Only cells adjacent to and clearly outside the vessel under study were counted as emigrated.
Induction of leukocyte recruitment in cremaster muscle
To induce neutrophil recruitment with a single CXC chemokine in the cremasteric muscle preparation, 3 approaches were taken. The first approach was the superfusion of the cremasteric muscle preparation with a neutrophil chemokine solution. After an initial baseline recording, the cremaster muscle was superfused with either MIP-2 (CXCL2; R&D Systems, Minneapolis, MN) or keratinocyte-derived chemokine (KC, CXCL1; R&D Systems) at 5 nM in superfusion buffer at 37°C. The neutrophil recruitment was monitored at different time points up to 90 minutes. This approach has been shown to induce neutrophil emigration into the tissue.19 Some mice were orally administered with PI3Kδ inhibitor IC87114 at 25 mg/kg or the same amount of vehicle (PEG-400) for 2 hours before the perfusion of MIP-2 solution on the cremaster muscle preparations. The second approach was to determine leukocyte recruitment induced by a chemokine at later time points by intrascrotal injection of neutrophil chemokines of either MIP-2 or KC (1 μg in 200 μL sterile saline). In some experiments, pan-PI3K inhibitor LY294002 (10 mg/kg) was injected peritoneally into mice 20 minutes prior to MIP-2 local injection, and the control mice were treated with the same amount of vehicle (DMSO). In some experiments, 1 hour prior to chemokine local injection, the mice were orally administered with selective PI3Kδ inhibitor IC87114 at 25 mg/kg or the same amount of vehicle (PEG-400) as described.12 The neutrophil recruitment was determined at 2 to 2.5 hours and 4 to 5 hours after chemokine local injection. Thirdly, to determine whether leukocyte recruitment was impaired in response to endogenous chemokines, recombinant mouse TNFα (0.5 μg; R&D Systems) in 200 μL sterile saline was injected intrascrotally into PI3Kγ-/-, C57BL/6 WT, or chimeric mice. This cytokine induces both KC and MIP-2 production.20–22 The leukocyte rolling flux, rolling velocity, adherence, and emigration were measured in the cremasteric venule at 4 hours, 4.5 hours, 5 hours, and 7.5 hours after the injection. The control mice were injected with the same volume of saline alone.
Statistical analysis
The results were expressed as means ± SEM. Student t test was applied to compare the statistical difference within 2 groups. A probability (P) value of less than .05 was considered statistically significant.
Results
PI3Kγ is crucial for neutrophil emigration in response to chemokines
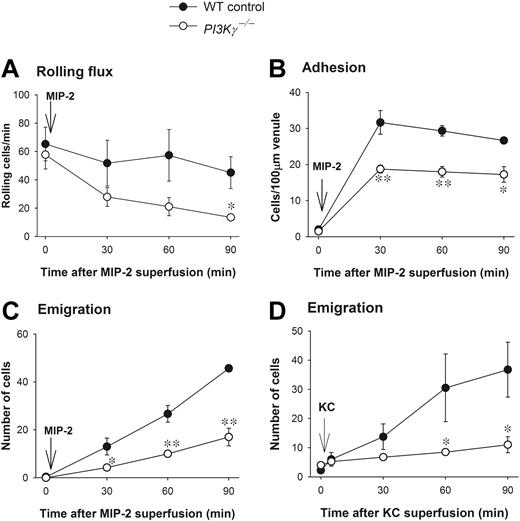
There were no differences in any leukocyte parameters, including rolling, adhesion, emigration, or circulating counts under basal conditions in WT and PI3Kγ-/- mice (Figure 1 and data not shown). When the cremaster muscle was superfused with the CXC chemokine MIP-2, leukocyte rolling flux (Figure 1A) and rolling velocity (data not shown) were comparable in postcapillary venules in WT and PI3Kγ-/- mice. The drop in leukocyte rolling flux over time was more notable in PI3Kγ-/- mice but only reached significance at 90 minutes. Leukocyte adhesion increased substantially in both PI3Kγ-/- and WT mice but somewhat more (30% to 35%) in the latter (Figure 1B). The leukocyte emigration showed a dramatic difference between the 2 genotypes. WT neutrophils began to emigrate into the cremasteric tissue at 30 minutes of MIP-2 superfusion (Figure 1C). At 60 minutes of MIP-2 superfusion, about 25 to 30 WT cells and, at 90 minutes, about 45 WT cells were seen per field of view. By contrast, only 15 to 20 PI3Kγ-/- neutrophils emigrated into the tissue per field of view over the 90 minutes of MIP-2 superfusion (60% to 70% decrease). Nevertheless, it is interesting that in PI3Kγ-/- mice there was a slow but gradual increase in the emigrated neutrophils in the tissue (Figure 1C). Using another CXC chemokine, KC, we found that KC superfusion (5 nM) induced neutrophil emigration similar to MIP-2 and was similarly impaired in PI3Kγ-/- mice (Figure 1D). Interestingly, with KC superfusion, the adhesion was not significantly different between the WT and PI3Kγ-/- mice; however, the adhesion values in PI3Kγ-/- mice had a tendency to be lower. Nevertheless, the major neutrophil defect in PI3Kγ-/- mice was the ability to emigrate out of the vasculature. Finally, histology revealed that more than 90% of the leukocytes in the tissue were neutrophils (data not shown).
Leukocyte recruitment in CXC chemokine-superfused cremasteric postcapillary venules in WT and PI3Kγ-/- mice. (A) The flux of rolling leukocytes, (B) leukocyte adhesion, (C) leukocyte emigration in response to MIP-2, and (D) leukocyte emigration in response to KC in cremasteric postcapillary venules are shown. After measurement of basal leukocyte recruitment (at time 0), leukocyte recruitment was induced by superfusion of the cremaster muscle preparation with 5 nM MIP-2 or KC as indicated (arrow) and the recruitment parameters determined in cremasteric venules (n = 3 to 4 in each group). *P < .05 and **P < .01 as compared with each value in WT mice. Error bars represent means plus or minus SEM.
Leukocyte recruitment in CXC chemokine-superfused cremasteric postcapillary venules in WT and PI3Kγ-/- mice. (A) The flux of rolling leukocytes, (B) leukocyte adhesion, (C) leukocyte emigration in response to MIP-2, and (D) leukocyte emigration in response to KC in cremasteric postcapillary venules are shown. After measurement of basal leukocyte recruitment (at time 0), leukocyte recruitment was induced by superfusion of the cremaster muscle preparation with 5 nM MIP-2 or KC as indicated (arrow) and the recruitment parameters determined in cremasteric venules (n = 3 to 4 in each group). *P < .05 and **P < .01 as compared with each value in WT mice. Error bars represent means plus or minus SEM.
Endothelial PI3Kγ does not regulate leukocyte recruitment in response to MIP-2
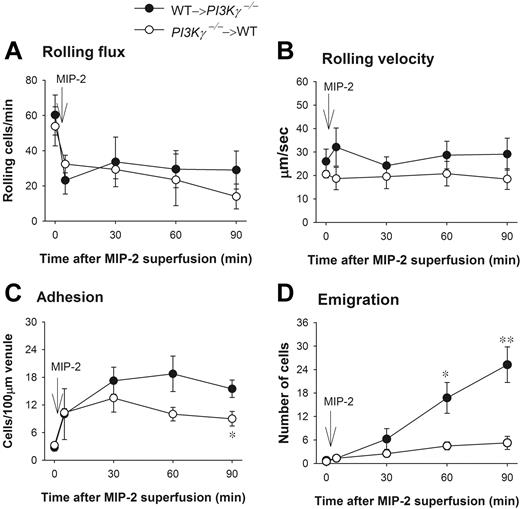
PI3Kγ has been reported to be expressed in endothelial cells and to play a role in TNFα-induced leukocyte recruitment13 and in shear stress–dependent activation of JNK in endothelial cells.14 To test whether endothelial PI3Kγ had any function in the dramatic difference in leukocyte emigration in response to CXC chemokines observed in WT versus PI3Kγ-/- mice, we made chimeric mice that lacked PI3Kγ in the leukocytes and had WT endothelial cells (referred to as PI3Kγ-/-→WT). We also made the opposite chimeric mice in which the PI3Kγ-/- mice received bone marrow transplants from WT mice (referred to as WT→PI3Kγ-/-). The latter mice lack PI3Kγ in endothelium. Leukocyte rolling flux, rolling velocity, and adhesion were similar in both types of chimeric mice (Figure 2A,B) and not different from WT mice in responses to MIP-2. Adhesion was somewhat reduced in mice in which leukocytes lacked PI3Kγ, reaching significance at 90 minutes (Figure 2C). In those chimeric mice that lacked PI3Kγ only in their leukocytes, emigration out of the vasculature was severely impaired in response to MIP-2 as compared with chimeric mice that lacked PI3Kγ in endothelial cells (Figure 2D). These 2 phenotypes (PI3Kγ-/-→WT and WT→PI3Kγ-/-) in response to MIP-2 were very similar to the PI3Kγ-/- and WT responses to CXC chemokine, respectively, seen in Figure 1C. Clearly, the defect of leukocyte emigration in response to MIP-2 was primarily in leukocytes but not in endothelium. We previously demonstrated that in 6 weeks after irradiation, the irradiated WT mice receiving bone marrow from donor WT mice behaved exactly like nonirradiated WT mice, and the irradiated mutant mice receiving bone marrow from mutant mice behaved identically to nonirradiated mutant mice.15,16
Leukocyte recruitment in chemokine MIP-2-superfused cremasteric postcapillary venules in chimeric mice. (A) The flux of rolling leukocytes, (B) leukocyte rolling velocity, (C) adherent, and (D) emigrated leukocytes in MIP-2-superfused cremasteric postcapillary venules of chimeric mice are shown. WT and PI3Kγ-/- mice were reconstituted with PI3Kγ-/- and WT bone marrow and indicated as PI3Kγ-/-→WT and WT→ PI3Kγ-/-, respectively. After measurement of basal leukocyte recruitment (at time 0), leukocyte recruitment was induced by superfusion of the cremaster muscle preparation with 5 nM MIP-2 (arrow) and the recruitment parameters determined in cremasteric venules from these chimeric mice (n = 4 in each group). *P < .05 and **P < .01 as compared with each group of opposite chimeric mice. Error bars represent means plus or minus SEM.
Leukocyte recruitment in chemokine MIP-2-superfused cremasteric postcapillary venules in chimeric mice. (A) The flux of rolling leukocytes, (B) leukocyte rolling velocity, (C) adherent, and (D) emigrated leukocytes in MIP-2-superfused cremasteric postcapillary venules of chimeric mice are shown. WT and PI3Kγ-/- mice were reconstituted with PI3Kγ-/- and WT bone marrow and indicated as PI3Kγ-/-→WT and WT→ PI3Kγ-/-, respectively. After measurement of basal leukocyte recruitment (at time 0), leukocyte recruitment was induced by superfusion of the cremaster muscle preparation with 5 nM MIP-2 (arrow) and the recruitment parameters determined in cremasteric venules from these chimeric mice (n = 4 in each group). *P < .05 and **P < .01 as compared with each group of opposite chimeric mice. Error bars represent means plus or minus SEM.
PI3Kγ is not important in leukocyte recruitment in a 4- to 5-hour chemokine response
We also observed that there was some PI3Kγ-independent neutrophil emigration in response to CXC chemokines and that the PI3Kγ-independent neutrophil emigration had a tendency to increase with time (Figure 1C,D). Although it is difficult to maintain cremasteric preperations for longer than 90 minutes without some deterioration related to the preparation, when some preparations were maintained for up to 150 to 180 minutes and visualized at these later time points the response to MIP-2 became more notable in the PI3Kγ mice (data not shown). Therefore, in the next series of experiments, MIP-2 was injected intrascrotally into the tissues, and at 4 hours the cremaster muscle was prepared for intravital microscopy. Figure 3A revealed that neutrophil emigration in PI3Kγ-/- mice had reached similar levels to WT mice over 4 to 5 hours after MIP-2 administration, suggesting a complete PI3Kγ-independent neutrophil emigration at these time points. MIP-2 treatment caused identical changes in leukocyte rolling flux, rolling velocity, and adhesion in WT and PI3Kγ-/- mice (not shown). Similar PI3Kγ-independent neutrophil emigration was also observed after using another neutrophil chemokine KC (Figure 3B).
Leukocyte emigration in CXC chemokine 4- to 5-hours-treated cremasteric postcapillary venules in WT and PI3Kγ-/- mice. The number of emigrated neutrophils in cremasteric postcapillary venules after intrascrotal injection of (A) MIP-2 and (B) KC in WT and PI3Kγ-/- mice was determined. These chemokines were injected as a single dose of 1 μg in 200 μL saline (n = 3 to 7 in each group). The control WT mice were injected with 200 μL saline (n = 4). Leukocyte emigration was determined by intravital microscopy at 4, 4.5, and 5 hours after the treatment. **P < .01 as compared with WT or PI3Kγ-/- mice treated with MIP-2 or KC. Error bars represent means plus or minus SEM.
Leukocyte emigration in CXC chemokine 4- to 5-hours-treated cremasteric postcapillary venules in WT and PI3Kγ-/- mice. The number of emigrated neutrophils in cremasteric postcapillary venules after intrascrotal injection of (A) MIP-2 and (B) KC in WT and PI3Kγ-/- mice was determined. These chemokines were injected as a single dose of 1 μg in 200 μL saline (n = 3 to 7 in each group). The control WT mice were injected with 200 μL saline (n = 4). Leukocyte emigration was determined by intravital microscopy at 4, 4.5, and 5 hours after the treatment. **P < .01 as compared with WT or PI3Kγ-/- mice treated with MIP-2 or KC. Error bars represent means plus or minus SEM.
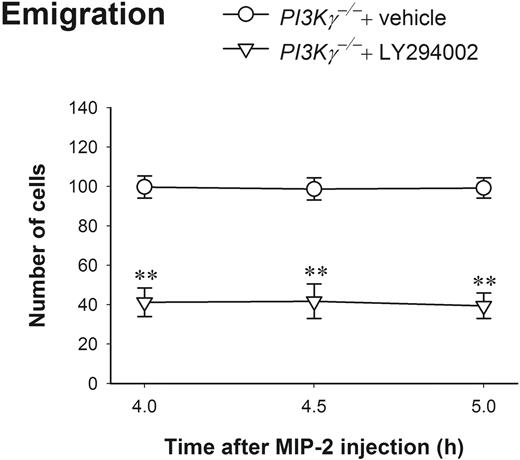
Using the pan-PI3K inhibitor LY294002 at a dosage that dramatically blocks neutrophil and eosinophil infiltration in an inflammation mouse model,23 we found that this compound can effectively block about 60% of PI3Kγ-independent neutrophil emigration (Figure 4). These results reveal that at 4 to 5 hours, neutrophil chemokines induce complete PI3Kγ-independent neutrophil emigration, but it is still PI3K-dependent, suggesting PI3Ks other than PI3Kγ might be involved.
The effect of LY294002 on leukocyte emigration induced by 4- to 5-hour MIP-2 treatment in PI3Kγ-/- mice. The number of emigrated neutrophils in cremasteric postcapillary venules after intrascrotal injection of MIP-2 (1 μg in 200 μL saline) in PI3Kγ-/- mice with or without LY294002 was determined (n = 4 in each group). The mice were pretreated with intraperitoneal injection of either LY294002 (10 mg/kg body weight in 0.5 mL saline) or vehicle DMSO 20 minutes before MIP-2 intrascrotal injection. Leukocyte emigration was determined by intravital microscopy at 4 hours, 4.5 hours, and 5 hours after MIP-2 treatment. **P < .01 as compared with PI3Kγ-/- mice treated with only vehicle. Error bars represent means plus or minus SEM.
The effect of LY294002 on leukocyte emigration induced by 4- to 5-hour MIP-2 treatment in PI3Kγ-/- mice. The number of emigrated neutrophils in cremasteric postcapillary venules after intrascrotal injection of MIP-2 (1 μg in 200 μL saline) in PI3Kγ-/- mice with or without LY294002 was determined (n = 4 in each group). The mice were pretreated with intraperitoneal injection of either LY294002 (10 mg/kg body weight in 0.5 mL saline) or vehicle DMSO 20 minutes before MIP-2 intrascrotal injection. Leukocyte emigration was determined by intravital microscopy at 4 hours, 4.5 hours, and 5 hours after MIP-2 treatment. **P < .01 as compared with PI3Kγ-/- mice treated with only vehicle. Error bars represent means plus or minus SEM.
PI3Kδ is responsible for the PI3Kγ-independent neutrophil emigration induced by MIP-2
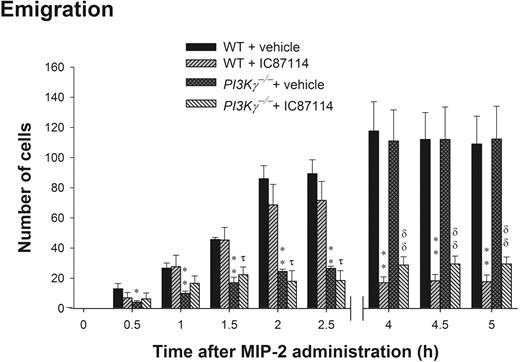
PI3Kδ has been shown to also contribute to neutrophil recruitment in vivo12 and neutrophil chemotaxis in vitro.11 We administered selective PI3Kδ inhibitor IC87114 at 25 mg/kg into PI3Kγ-/- mice prior to MIP-2 local addition. This compound at this concentration has been demonstrated to be a potent and selective inhibitor of PI3Kδ but not p110α, p110β, or p110γ isoforms or MAPK in neutrophils.11,12 Figure 5 is a summation of 3 separate experiments in which mice either received MIP-2 and were examined at 0 to 90 minutes, 2 to 2.5 hours, or 4 to 5 hours. In WT mice, there was a steady increase in the number of emigrated cells found in the cremaster muscle preparation. In PI3Kγ-/- mice there was a profound reduction in neutrophils over the first 2.5 hours, but then there was a sharp increase in the number of neutrophils into the tissue over the next 1.5 hours. IC87114 had absolutely no inhibitory effect on emigration over the first 90 minutes and was only marginally inhibited at 2.5 hours. However, over the remaining time (4 to 5 hours), absolutely no emigration was noted in WT mice treated with IC87114, and the cells that had reached the extravascular space left the field of view as they moved further away from the vessel. By 5 hours, IC87114 substantially inhibited MIP-2-induced neutrophil emigration (by 70% to 80%) in WT mice (Figure 5). To determine whether there was redundancy or overlap between the PI3Kγ and PI3Kδ isoforms, we administered IC87114 into PI3Kγ-/- mice. IC87114 decreased the emigration in PI3Kγ-/- mice to the same extent as in WT mice (Figure 5). The inhibitory effect of IC87114 could entirely account for the inhibitory effect of LY294002, suggesting that the PI3Kγ-independent neutrophil emigration induced by MIP-2 was largely due to the function of PI3Kδ.
The role of PI3Kδ in leukocyte emigration in cremasteric postcapillary venules in response to 3 separate time points (0 to 90 minutes, 2 to 2.5 hours, and 4 to 5 hours) of MIP-2 local administration in WT and PI3Kγ-/- mice. Mice were orally administered with 25 mg/kg PI3Kδ inhibitor IC87114 or same amount of vehicle PEG-400 prior to MIP-2 superfusion on the cremaster muscle preparation for 0 to 1.5 hours or prior to MIP-2 intrascrotal injection (1 μg in 200 μL saline) and cremaster muscle preparation at 2 to 2.5 hours or 4 to 5 hours. Leukocyte emigration was determined at the indicated time points after MIP-2 treatment in WT, PI3Kγ-/-, WT + IC87114, or PI3Kγ-/- + IC87114 mice. Each group contains at least 3 mice. *P < .05 and **P < .01, respectively, as compared with each vehicle-treated group of WT mice. τ, P < .05 as compared with IC87114-treated WT mice. δδ, P < .01 as compared with vehicle-treated PI3Kγ-/- mice. Error bars represent means plus or minus SEM.
The role of PI3Kδ in leukocyte emigration in cremasteric postcapillary venules in response to 3 separate time points (0 to 90 minutes, 2 to 2.5 hours, and 4 to 5 hours) of MIP-2 local administration in WT and PI3Kγ-/- mice. Mice were orally administered with 25 mg/kg PI3Kδ inhibitor IC87114 or same amount of vehicle PEG-400 prior to MIP-2 superfusion on the cremaster muscle preparation for 0 to 1.5 hours or prior to MIP-2 intrascrotal injection (1 μg in 200 μL saline) and cremaster muscle preparation at 2 to 2.5 hours or 4 to 5 hours. Leukocyte emigration was determined at the indicated time points after MIP-2 treatment in WT, PI3Kγ-/-, WT + IC87114, or PI3Kγ-/- + IC87114 mice. Each group contains at least 3 mice. *P < .05 and **P < .01, respectively, as compared with each vehicle-treated group of WT mice. τ, P < .05 as compared with IC87114-treated WT mice. δδ, P < .01 as compared with vehicle-treated PI3Kγ-/- mice. Error bars represent means plus or minus SEM.
PI3Kγ and PI3Kδ have temporally distinct roles in neutrophil emigration in response to endogenous chemokines produced by TNFα
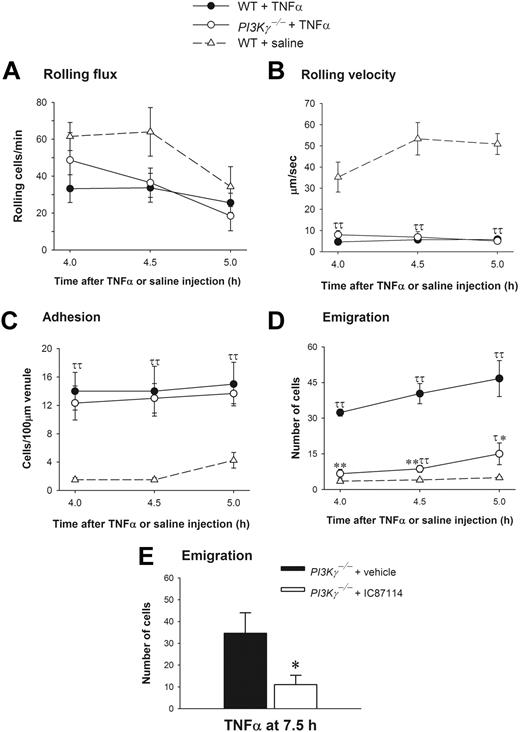
PI3Kγ and PI3Kδ have both been reported to play a role in E-selectin–dependent leukocyte rolling and accumulation in TNFα-stimulated muscle venules.12,13 However, in the latter model where TNFα is administered at low doses, there was limited emigration unless an exogenous chemoattractant (such as LTB4) was also provided. Under these conditions, PI3Kγ-/- mice did not respond to the TNFα/LTB4 stimulus. Whether this was related to the impaired rolling or a direct effect on emigration remained unclear. Higher concentrations of TNFα (0.5 μg per animal injection) have been used by us and others15,16,24,25 to directly study neutrophil emigration (independent of administration of exogenous chemoattractants). Because data suggest that PI3K may vary in importance depending on whether the exogenously (artificially) added chemotactic gradients are shallow or steep,26,27 the endogenously (naturally) produced chemokine gradients via TNFα stimulation takes on added importance. This amount of TNFα causes the synthesis of endogenous chemokines, including MIP-2 and KC, sufficient to induce neutrophil emigration at approximately 4 hours.20–22 When this approach was taken and WT and PI3Kγ-/- mice were treated intrascrotally with 0.5 μg TNFα, the leukocyte rolling flux (Figure 6A) and rolling velocity (Figure 6B) responses were identical in cremasteric venules at 4.0 hours, 4.5 hours, and 5.0 hours in both WT and PI3Kγ-/- mice. The rolling velocity of leukocytes was about 35 to 53 μm per second under control conditions in WT control mice, and a very profound and similar 80% to 90% decrease in rolling velocity was observed in both WT and PI3Kγ-/- mice following TNFα administration (Figure 6B), suggesting that the rolling defect attributed to endothelium in PI3Kγ-/- mice reported previously13 is overcome by higher TNFα concentrations. A dramatic increase in leukocyte adhesion in postcapillary venules following TNFα treatment was noted in both WT and PI3Kγ-/- animals (Figure 6C). Despite the lack of any impairment in any of the preceding steps of the leukocyte recruitment cascade in PI3Kγ-/- mice, a very significant difference in leukocyte emigration was noted in WT and PI3Kγ-/- mice in response to TNFα (Figure 6D). We observed about 32 to 47 emigrated neutrophils per field of view in WT mice whereas only 7 to 15 cells emigrated in PI3Kγ-/- mice. To determine whether the importance of PI3Kγ is lost over time, much like it was with exogenous chemokines, TNFα stimulation was extended to 7.5 hours. Neutrophil emigration was substantially increased in vehicle-treated PI3Kγ-/- mice (about 35 cells), and this increased emigration was again inhibited with IC87114 in these mice (Figure 6E). This suggests that upon prolonged stimulation of TNFα, neutrophil emigration response switched from PI3Kγ dependent at early time points (at 4 to 5 hours) to PI3Kγ independent but PI3Kδ dependent at later time points (7.5 hours).
Leukocyte recruitment in cremasteric postcapillary venules of saline-treated WT and TNFα-treated WT and PI3Kγ-/- mice. (A) The leukocyte rolling flux, (B) rolling velocity, (C) adhesion, and (D,E) emigration are shown. Leukocyte recruitment was induced by intrascrotal injection of TNFα (0.5 μg in 200 μL saline) and the recruitment parameters determined in cremasteric venules from WT (n = 3) and PI3Kγ-/- mice (n = 3) at 4 hours, 4.5 hours, 5 hours, and 7.5 hours. In panel E, PI3Kγ-/- mice were pretreated with either vehicle PEG-400 or IC87114 at 1 hour prior to TNFα injection. The WT control mice (n = 4) were injected only with saline. τ, P < .05 and ττ, P < .01 as compared with saline-injected WT control group. *P < .05 and **P < .01 as compared with TNFα-treated WT mice (D) or TNFα-treated PI3Kγ-/- mice with PEG-400 (E). Error bars represent means plus or minus SEM.
Leukocyte recruitment in cremasteric postcapillary venules of saline-treated WT and TNFα-treated WT and PI3Kγ-/- mice. (A) The leukocyte rolling flux, (B) rolling velocity, (C) adhesion, and (D,E) emigration are shown. Leukocyte recruitment was induced by intrascrotal injection of TNFα (0.5 μg in 200 μL saline) and the recruitment parameters determined in cremasteric venules from WT (n = 3) and PI3Kγ-/- mice (n = 3) at 4 hours, 4.5 hours, 5 hours, and 7.5 hours. In panel E, PI3Kγ-/- mice were pretreated with either vehicle PEG-400 or IC87114 at 1 hour prior to TNFα injection. The WT control mice (n = 4) were injected only with saline. τ, P < .05 and ττ, P < .01 as compared with saline-injected WT control group. *P < .05 and **P < .01 as compared with TNFα-treated WT mice (D) or TNFα-treated PI3Kγ-/- mice with PEG-400 (E). Error bars represent means plus or minus SEM.
Endothelial PI3Kγ is not important for leukocyte emigration in response to higher concentrations of TNFα
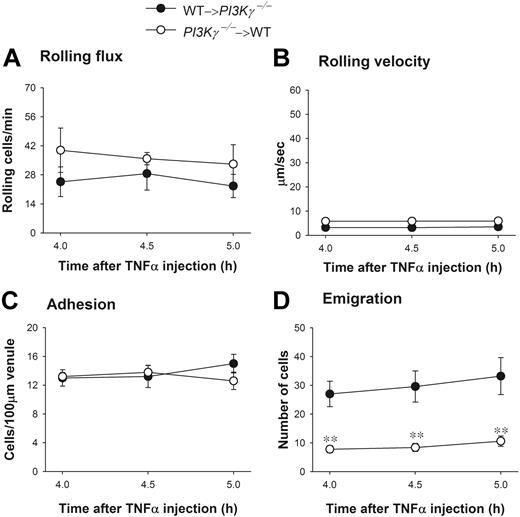
To test whether endothelial PI3Kγ had any function in the dramatic difference in leukocyte emigration observed in WT versus PI3Kγ-/- mice, we again tested chimeric mice that (1) lacked PI3Kγ in the leukocytes (referred to as PI3Kγ-/-→WT) or (2) lacked PI3Kγ in endothelium (referred to as WT→PI3Kγ-/-). Upon TNFα administration, leukocyte rolling flux, rolling velocity, and adhesion were similar in both types of chimeric mice (Figure 7A-C) and not different from WT responses (Figure 6). In those chimeric mice that lacked PI3Kγ only in their leukocytes, emigration out of the vasculature was severely impaired in response to TNFα injection as compared with the opposite chimeric mice that lacked PI3Kγ in endothelial cells (Figure 7D). These 2 phenotypes (PI3Kγ-/-→WT and WT→PI3Kγ-/-) in response to TNFα were very similar to the PI3Kγ-/- and WT mice, respectively (Figure 6D). Clearly, the defect of leukocyte emigration in response to TNFα-associated endogenous chemokines was primarily in leukocytes not in endothelium.
Leukocyte recruitment in cremasteric postcapillary venules of TNFα-treated chimeric mice. (A) The leukocyte rolling flux, (B) rolling velocity, (C) adhesion, and (D) emigration are shown. WT and PI3Kγ-/- mice were reconstituted with PI3Kγ-/- and WT bone marrow, indicated as PI3Kγ-/-→WT and WT→ PI3Kγ-/-, respectively. Leukocyte recruitment was induced by intrascrotal injection of TNFα (0.5 μg in 200 μL saline) and the recruitment parameters determined in cremasteric venules from these chimeric mice (n = 5 in each group) at 4 hours, 4.5 hours, and 5 hours. **P < .01 as compared with groups of opposite chimeric mice. Error bars represent means plus or minus SEM.
Leukocyte recruitment in cremasteric postcapillary venules of TNFα-treated chimeric mice. (A) The leukocyte rolling flux, (B) rolling velocity, (C) adhesion, and (D) emigration are shown. WT and PI3Kγ-/- mice were reconstituted with PI3Kγ-/- and WT bone marrow, indicated as PI3Kγ-/-→WT and WT→ PI3Kγ-/-, respectively. Leukocyte recruitment was induced by intrascrotal injection of TNFα (0.5 μg in 200 μL saline) and the recruitment parameters determined in cremasteric venules from these chimeric mice (n = 5 in each group) at 4 hours, 4.5 hours, and 5 hours. **P < .01 as compared with groups of opposite chimeric mice. Error bars represent means plus or minus SEM.
Discussion
The molecular mechanisms underlying each step of the leukocyte recruitment cascade in vivo have been elucidated primarily using intravital microscopy in translucent tissues like the cremaster muscle.28–30 This recruitment cascade consists of initial leukocyte tethering and rolling on the endothelium followed by the leukocyte adhesion and emigration across the endothelium. The adhesion step is dependent on the presence of chemokines or other chemoattractants on the surface of inflamed endothelial cells.28–31 Although emigration is also deemed a step induced by chemotactic molecules, the main source of these molecules has generally been deemed to be outside the vasculature. Regardless of the source, chemoattractants are important in multiple steps of the recruitment cascade.
Cellular lipid kinases PI3Kγ and PI3Kδ have both been implicated in the neutrophil recruitment cascade as a result of inhibition of both leukocyte and endothelial PI3K activity.11–13 In this study using intravital microscopy and mice either lacking PI3Kγ or inhibiting PI3Kδ activity, we demonstrate very specific and time-dependent roles for PI3Kγ and PI3Kδ in neutrophils. Our data suggest that neutrophil PI3Kγ is extremely important in the immediate emigration in vivo in response to various CXC chemokines. In addition, the PI3Kγ-/- neutrophils failed to respond to the proinflammatory cytokine TNFα, which stimulates the production of endogenous neutrophil chemokines in the tissue.20–22,32 However, this lipid kinase is unimportant in regulating leukocyte rolling along the endothelium and has minor effects on adhesion in the postcapillary venules in vivo. Moreover, results from the chimeric mice revealed that it is the leukocyte PI3Kγ but not the endothelial PI3Kγ that is the key component in the regulation and support of leukocyte emigration into the inflamed tissue in response to exogenous or endogenous CXC chemokines. We also demonstrate that deficiency in PI3Kγ failed to prevent leukocyte emigration in delayed response to MIP-2. This causes PI3Kγ-independent, PI3Kδ-dependent neutrophil emigration at later time points.
Members of the PI3K family are important lipid kinases that affect numerous cell functions. Among them, PI3Kγ is considered to be critical in the inflammatory response. Central to the inflammatory response is the recruitment of neutrophils from the bloodstream to the site of infection or injury. However, neutrophil recruitment can be affected at multiple sites, including neutrophil rolling along the endothelium, then adhesion to the endothelial cells and, finally, neutrophil transendothelial migration and migration in the tissue toward the site of infection or injury.33–35 Neutrophil rolling along the endothelial wall is mediated by selectins (P-, E-, and L-selectin). Our data clearly demonstrated that the rolling is entirely independent of PI3Kγ. Leukocyte adhesion is primarily mediated by integrins and, in the case of neutrophils, β2 integrins; however, PI3Kγ had only a minor role (MIP-2, KC) or no role (TNFα) in this process. This observation is intriguing when one considers that the β2 integrins are activated by G protein-coupled chemokine receptors and both are important in chemotaxis. Clearly, integrin-dependent adhesion differs from integrin-dependent chemotaxis, and only the latter requires PI3K activity. By using CXCL1 (KC) infusion, Smith et al demonstrated a 50% to 80% decrease in neutrophil adhesion in PI3Kγ-/- mice.36 In their study, CXCL1 was infused intravenously and leukocyte adhesion was determined 1 minute before and after CXCL1 administration.36 In our study, we either applied exogenous chemokines or used TNFα to stimulate endogenous chemokine production, and we focused on the adhesion and emigration over many hours. Recently, we reported that integrin αLβ2 or LFA-1 was responsible for adhesion whereas αMβ2 or Mac-1 was more important for chemotaxis in response to MIP-2, suggesting that PI3K may be involved in Mac-1 rather than LFA-1 functions.37 However, emigration into tissues was delayed by only 30 minutes but not prevented in Mac-1-/- mice whereas the PI3Kγ-/- mice also had delayed emigration but this was more prolonged, suggesting that PI3Kγ may have numerous functions including the regulation of Mac-1.
Leukocyte transendothelial migration and chemotaxis in tissue is mediated by chemoattractants, which induce cell activation via G protein-coupled receptors. PI3Kγ is downstream of the βγ subunit of G protein-coupled cell surface receptors. Through the production of PIP3, which recruits a number of intracellular signaling molecules or effectors to the inner leaflet of cell membrane, PI3Kγ mediates cellular chemotaxis. In this regard, PI3Kγ is important in leukocyte actin reorganization and shape changes leading to chemotaxis.4–7,38 These studies demonstrated that PI3Kγ-/- neutrophils have defects in chemotaxis toward chemoattractants such as IL-8, fMLP, C5a, and MIP-1α in vitro. PI3Kγ-/- leukocytes were also defective in recruitment into the tissues, although it was unclear which step in the recruitment was impaired. Using intravital microscopy, we observed a major role for PI3Kγ in leukocyte emigration in response to exogenous chemokines or in response to TNFα, which stimulates endogenous chemokine production.20–22,32
We noted that PI3Kγ deficiency did not completely abolish leukocyte emigration in vivo. For example, there was a small but significant increase in emigration in PI3Kγ-/- leukocytes in response to TNFα as compared with saline control group (Figure 6D). Even more obvious was the fact that, when neutrophil chemokines were administered for extended time periods (4 to 5 hours), the chemokine-induced neutrophil emigration switched from PI3Kγ-dependent to PI3Kγ-independent but PI3Kδ-dependent emigration. Boulven et al also observed distinct, time-dependent activity for PI3Kγ and PI3Kδ, the former mediating the first 30 seconds of PIP3 formation whereas the latter mediated PIP3 formation at 5 minutes.8 However, some important differences are evident. While Boulven and colleagues determined that only PI3Kδ was essential for chemotaxis, our own data suggested a role for PI3Kγ over the first 2.5 hours of leukocyte recruitment whereas PI3Kδ played a role at approximately 4 to 5 hours for chemotaxis in response to chemoattractants. These discrepant results almost certainly relate to the more complex in vivo versus in vitro conditions. We now know that the substratum on which neutrophils crawl,39,40 the presence of shear,41,42 and the presence of endothelium16,37 all affect chemotaxis and migration across the vasculature whereas in vitro the conditions are very specific with specific substrata, no shear, and no endothelium. Indeed, we recently found that certain proteins (eg, LSP1) in leukocytes are not at all important in leukocyte transendothelial migration (dependent upon endothelial LSP1)16 but are very important in leukocyte chemotaxis in extravascular space (our own preliminary data). Clearly, transendothelial migration has a chemotactic component but also a complex series of adhesion interactions with both integrin ligands and also junctional proteins. Another important difference between our work and that of Boulven is the different classes of chemotactic molecules (fMLP versus KC and MIP-2) used.
Using PI3Kγ-/- and PI3Kδ-/- mice and intravital microscopy, Puri et al recently showed that both PI3Kγ and PI3Kδ played an important role in E-selectin–mediated neutrophil rolling attachment in cremasteric venules in vivo in response to TNFα local treatment.12,13 Interestingly, PI3Kγ and PI3Kδ activity was not involved in NF-κB–mediated E-selectin synthesis and expression but was involved in the function of E-selectin, that is, E-selectin–mediated neutrophil slow rolling in the venules. It was postulated that the impaired slow rolling was potentially dependent on altered distribution patterns of E-selectin on the endothelial surface (eg, lack of clustering), and this may have led to the indirect downstream impairment of leukocyte emigration when an additional chemoattractant was added to TNFα. In the present study, we report that higher concentration of TNFα overcomes the PI3Kγ defect in leukocyte rolling flux and rolling velocity but could not overcome the defect in emigration. Clearly, PI3Kγ likely underlies rolling at lower concentrations of TNFα and also plays an essential role in emigration at higher doses of TNFα.
In summary, in this study by using PI3Kγ-/- animals, bone marrow transplantation, exogenous neutrophil chemokines, and TNFα stimulation, we demonstrate that PI3Kγ plays an important role in neutrophil emigration but not rolling and limited adhesion in postcapillary venules in vivo. It is leukocyte but not endothelial PI3Kγ that is critically involved in the early neutrophil emigration into the inflamed tissues. By contrast, the delayed neutrophil emigration in response to neutrophil chemokines is independent of the function of PI3Kγ but is PI3Kδ dependent.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by a Canadian Institutes of Health Research (CIHR) group grant, Heart and Stroke Foundation of Canada, and a research fellowship from Alberta Heritage Foundation for Medical Research (AHFMR) (L.L.). P.K. is an AHFMR scientist, Canada Research Chair recipient, and the Calvin, Phoebe and Joan Snyder Chair in Critical Care Research.
We thank Bryan Heit for testing the effectiveness of LY294002 in in vitro chemotaxis assay and Lori Zbytnuik, Dean Brown, and Krista McRae for their expert assistance in animal care.
Authorship
Contribution: L.L. designed experiments, performed research, collected and analyzed data, and wrote the paper; K.D.P. contributed a vital reagent and participated in the writing of the paper; J.M.P. contributed PI3Kγ-/- mice; and P.K. supervised the project, designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paul Kubes, Immunology Research Group, Department of Physiology and Biophysics, University of Calgary, 3330 Hospital Drive NW, Calgary, Alberta, T2N 4N1, Canada; e-mail: pkubes@ucalgary.ca.