Abstract
Follicular dendritic cells (FDCs) form a reticular FDC network in the lymphoid follicle that is essential for the retention and presentation of native antigens in the form of antigen-antibody immune complexes (ICs) to B cells during secondary immune response. Although the presence of migrating precursors of FDCs has been hypothesized, their entity has not been elucidated. Here we report the identification of murine splenic CD19−CD11c−CD35+B220+ cells as an inducer of FDC network formation. We demonstrated that CD19−CD11c−CD35+B220+ cells, together with stromal cells, had the remarkable ability to form lymphoid-follicle–like structures that contained B220+FDC-M1+ reticular cells originally derived from CD19−CD11c−CD35+B220+ cells in the CD35+ reticulum. Our results indicate that CD19−CD11c−CD35+B220+ cells function as an inducer of FDC network formation and that the interaction between CD19−CD11c−CD35+B220+ cells and stromal cells is required to initiate lymphoid follicle formation.
Introduction
Follicular dendritic cells (FDCs) represent a unique subset of antigen-trapping cells that constitute a major part of the nonlymphoid component of the germinal center (GC) of peripheral lymphoid tissues and of ectopically formed lymphoid follicles.1 The functions of FDCs are largely dependent on the trapping of immune complexes (ICs) via complement receptors and Fc receptors, and on the expression of adhesion molecules such as vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1).2 Physical contact between FDCs and B cells via these adhesion molecules plays a critical role in the sequential events of B-cell differentiation; B cells with low affinity for antigens trapped on FDCs die from apoptosis, whereas those with high affinity survive and mature into plasmablast and memory B cells.3 These characteristics underscore the pivotal role of FDC GC reactions and ensure prompt and effective response to pathogenic antigens.4–7
Accumulating evidence has indicated that the interactive processes involving chemokines and cytokines of the tumor necrosis factor superfamily (lymphotoxins/tumor necrosis factor [TNF]) play a critical role in the orientation of the splenic microarchitecture, such as compartmentalization of T and B cells, formation of marginal zone (MZ), and development of reticular morphology known as the FDC network in secondary lymphoid organs.8–21 Ablation of such signals in adult animals leads to the reduction, although not the complete eradication, of immune response characterized by reduced isotype switching, delayed affinity maturation, and compromised B-cell memory.22 Therefore, the proper formation of microarchitecture within secondary lymphoid organs, including the FDC network, is essential for efficient and/or rapid immune response.
Although FDCs are considered to be the major stromal cells of the lymphoid follicle, a body of evidence also supports the apparently contradictory hypothesis that FDCs in secondary lymphoid tissues originate in bone marrow (BM)–derived precursor cells,23,24 and stimulatory signals from lymphocytes are essential for the development of the splenic FDC network.1,25–27 Even though IC-competent cells have been suggested to play a critical role at the very early stage of FDC network formation,22 the characteristics of such IC-competent cells remain an enigma, and the developmental process of FDCs to form the reticular network remains unknown.
Here, we demonstrate that the intradermal injection of CD19−CD11c−CD35+B220+ cells, together with CD45−CD35− stromal cells, induces lymphoid-follicle–like structure formation. Taking advantage of this technique, we provide evidence that the strong association between these cells initiates FDC network formation within the CD35+ reticulum. In addition, the CD19−CD11c−CD35+B220+ cells migrate into the splenic follicle, where they differentiate into FDC-M1+ reticulum upon adoptive transfer. Our findings suggest that CD19−CD11c−CD35+B220+ cells, identified in the spleen of adult mice, function as an inducer of FDC network formation.
Materials and methods
Mice
C57BL/6J-Jcl and BALB/c mice purchased from Clea (Tokyo, Japan) at 4 weeks old were housed under specific pathogen-free conditions until they were 5-8 weeks old. Fetuses (14.5 days postconception) of C57BL/6J-Jcl mice were purchased from Clea. Green fluorescence protein (GFP)-transgenic (Tg) mice were a gift from Dr Masaru Okabe (Osaka University, Osaka, Japan).28 TNF-knockout (TNF-KO) mice were a gift from Dr Yoichiro Iwakura (The University of Tokyo, Tokyo, Japan). All animal experiments were performed in accordance with the guidelines and regulations of RIKEN Yokohama and were approved by the institute's animal use committee.
Antibodies
The following antimouse monoclonal antibodies (mAbs) were purchased from BD Pharmingen (San Diego, CA): fluorescein isothiocyanate (FITC)-conjugated anti-I-Ab, phycoerythrin (PE)- or biotin-conjugated anti-CD11c, FITC-conjugated anti-CD80, FITC-conjugated anti-CD86, FITC-conjugated anti-CD11b, PE- or biotin-conjugated anti-B220, FITC- or biotin-conjugated anti-Gr-1, PE-conjugated anti-NK1.1, PE-conjugated anti-CD21/CD35, biotin-conjugated anti-CD19, biotin-conjugated anti-CD8, biotin-conjugated anti-CD35 (Cr-1, 8C12), FcγIII/II receptor, and antimouse follicular dendritic cell marker 1 (FDC-M1). FITC- or biotin-conjugated anti-F4/80 mAb and purified anti-CD16/32 mAbs were from e-Bioscience (San Diego, CA). Biotin-conjugated antirat IgG and streptavidin (SA)–conjugated horseradish peroxidase (HRP) were from Dako North America (Carpinteria, CA). SA-conjugated Cy5, SA-conjugated Cy3, HRP-conjugated antirabbit IgG, Cy3-conjugated antirabbit IgG, Cy3-conjugated antigoat IgG, and goat anti-HRP polyclonal antibody were from Jackson ImmunoResearch Laboratories (West Grove, PA). SA-conjugated Alexa Fluor 488, Alexa-Fluor-488- or Cy3-conjugated phalloidin, Alexa-Fluor-488-conjugated anti-GFP IgG, and SA-conjugated Qdot605 were from Invitrogen (Carlsbad, CA). HRP-conjugated anti-OVA antibody was from Rockland Immunochemicals (Gilbertsville, PA). Rabbit anti-OVA antibody was from Chemicon International (Temecula, CA). Alexa-488-conjugated rabbit anti-GFP antibody was from Invitrogen. Biotin-conjugated anti-CD68 mAb was from Serotec (Oxford, United Kingdom).
Cell preparation
Single-cell suspensions of splenocytes were incubated with ammonium chloride lysis (ACK) buffer (Biosource International, Camarillo, CA) to eliminate erythrocytes. For the isolation of CD35+B220+ cells, CD11c−, CD19−, and Gr-1− splenocytes were enriched by negative magnetic selection using a MACS system (Miltenyi Biotec, Auburn, CA). Purity of the cells after 2 selection cycles was more than 96% as determined by flow cytometry. The resulting CD11c−, CD19−, and Gr-1− cells were resuspended in Dulbecco's modified Eagle's medium (Invitrogen) containing 50 U/mL penicillin, 50 mg/mL streptomycin, 0.15 mM arginine, 0.27 mM asparagine, and 10% fetal bovine serum (FBS) (Hyclone, Logan, UT) (complete medium), and plated at a density of 1.0 × 106 cells/cm2 in culture flasks. After 72 hours, nonadherent cells were removed, and the remaining loosely adherent cells were collected by gentle pipetting. The cells were labeled with biotin-conjugated anti-CD19, anti-CD3, anti-CD11c, anti-Gr-1, anti-NK1.1, anti-CD11b, and anti-CD8 mAbs and negatively selected with SA-conjugated MACS beads and large cell separation columns (LC column; Miltenyi Biotec). The resulting population was treated with biotin-conjugated anti-B220 mAb followed by SA-conjugated MACS beads to positively select B220-expressing adherent cells. More than 1 × 104 cells/body of the CD35+B220+ phenotype could be obtained in each experiment. The viability of the obtained cells was estimated to be higher than 90% on the basis of propidium iodide (PI) staining and the Trypan blue exclusion test. The purity of CD35+ cells was 87% to 92% as determined by flow cytometry. In another experiment, loosely adherent cells were sequentially incubated with rat anti-FDC-M1 and biotin-conjugated antibodies (antirat IgG and anti-CD19, anti-CD3, anti-CD11c, anti-Gr-1, anti-NK1.1, anti-CD11b, and anti-CD8 mAbs) on ice, and negatively selected with anti-biotin MACS beads and an LC column. The resulting population was treated with biotin-conjugated anti-B220 mAb followed by MACS beads to positively select B220-expressing adherent cells. This cell population was referred to as FDC-M1−CD35+B220+ cells hereafter.
DCs were generated from BM cells as described with modifications.29 In brief, BM cells from femurs and tibias after lysis of erythrocytes were cultured on 24-well plates (Corning, Corning, NY) in complete medium containing 20 ng/mL murine rGM-CSF (Peprotech, Rocky Hill, NJ) and murine rIL-4 (Peprotech). On days 3 and 5, nonadherent cells were removed by pipetting. On day 9, nonadherent and loosely adherent DCs were harvested by gentle pipetting.
B cells were obtained from single-cell suspension of splenocytes using positive magnetic selection with biotin-conjugated anti-CD19 mAb and SA-conjugated MACS beads. CD4+ T cells were prepared from mesenteric lymph nodes of naive BALB/c mice using a CD4+ T cell isolation kit (R&D Systems, Minneapolis, MN).
To obtain brain-derived stromal cells, brains from C57BL/6J-Jcl mice (14.5 dpc) were cut into small pieces and cultured on tissue culture plates in a 1:1 mixture of RPMI 1640 medium (Invitrogen) containing 50 U/mL penicillin, 50 mg/mL streptomycin, nonessential amino acids, and 10% FBS and α-minimal essential medium (α-MEM) containing 20% FBS (stromal medium). On days 3 and 6, cells were replated, and on day 11, CD45+CD35+ cells were negatively selected with the MACS system. The resulting cells resuspended in stromal medium were cultured overnight with occasional washing to remove nonadherent cells and used as brain stromal cells within 4 days.
Splenic stromal cells from naive C57BL/6J-Jcl mice were obtained by perfusing spleens with PBS containing 5% FBS and 0.5 mg/mL collagenase (Worthington Biochemical, Lakewood, NJ). The spleens were cut into small pieces with a scalpel and incubated in the same medium for 45 minutes at 30°C with gentle shaking. The collagenase-treated splenic cells were washed twice with PBS and erythrocytes were eliminated with ACK buffer. Splenocytes were incubated with antibodies (anti-CD3, anti-CD19, anti-B220, anti-CD11c, anti-CD11b, anti-CD45, and anti-CD35), followed by negative selection with the MACS system. The resulting splenic cells resuspended in stromal medium were cultured overnight with occasional washing to remove nonadherent cells and used as splenic stromal cells within 3 passages. The TEL-2 stromal cell line was a gift from Dr Takeshi Watanabe (RIKEN, Yokohama, Japan), and cultured with stromal medium.
Observation and photography
An Olympus fluorescence microscope (BX51 with UPlanAPO [4 × 0.16] lens, Olympus, Tokyo, Japan) equipped with a DP70 digital camera, a confocal microscope (Leica, Mannheim, Germany; TCS SP2 AOBS system with 63 × 1.32 oil-immersion lens, Leica confocal software), or a DeltaVision RT system (Applied Precision, Issaquah, WA; Olympus IX70 fluorescence microscope equipped with UPlanApo 60 × 1.4 or 20 × 0.75 lens) was used for observation and photography. Images obtained with the Olympus fluorescence microscope were analyzed with DP Manager software (Olympus). Using the DeltaVision RT system, original fluorescence micrographs of serial optical sections were taken every 0.2 mm in the Z-axis direction and digitized. To study cell surface signals in detail, original images were deconvoluted and reconstructed to obtain 3D images of the samples. Time-lapse observation was performed with the DeltaVision RT system equipped with a heater and a CO2 chamber. Differential interference contrast (DIC) images only or DIC images and fluorescence images were acquired every 2 minutes (Figure 2) or 20 minutes (Figure 3) using a ×60 objective (UPlanApo 60 × 1.4; Olympus). The images were analyzed with SoftWoRx (Applied Precision).
Incubation of CD35+B220+ cells with stromal cells
Stromal cells (1 × 104 cells/dish) were plated on a 35-mm glass-bottomed dish (Matsunami Glass, Osaka, Japan) and cultured for 30 minutes to induce adherence. The stromal cells were overlaid with CD35+B220+ cells (1 × 103 cells), and the culture was incubated for 5 minutes before observation. In one experiment, stromal cells derived from GFP-Tg mice or C57BL/6J-Jcl mice were cocultured with unstained CD35+B220+ cells or CD35+B220+ cells stained with PKH26-fluorescence dye (1 × 103 cells in 25 μL of 2 × 10−4 M dye; Sigma) for 24 hour or 5 days on a glass-bottomed dish. In another experiment, TEL-2 stromal cells and CD35+B220+ cells derived from GFP-Tg mice were cocultured for time-lapse imaging. Immunofluorescence or live cell images were obtained with the DeltaVision RT system.
Adoptive transfer studies
Unlabeled CD35+B220+ cells or CD35+B220+ cells (1 × 105 cells) labeled with PKH26 were resuspended in Ringer's solution (Otsuka, Tokyo, Japan) for intravenous injection into naive C57BL/6J-Jcl mice. We chose PKH26 because it binds specifically to the cell membrane and has a long fluorescence lifetime. Seventy percent ethanol-fixed PKH26-labeled CD35+B220+ cells were used as negative control. Spleens collected from the mice 8 or 15 days after the intravenous injection were frozen in OCT compound (Sakura, Tokyo, Japan). For control experiments, WEHI3 cells were stained with PKH26 and fixed with 70% ethanol. Intensity was assessed with flow cytometry (Figure S1; available on the Blood website; see the Supplemental Materials link at the top of the online article). In another experiment, CD35+B220+ cells derived from GFP-Tg mice were injected intravenously into naive C57BL/6J-Jcl mice. Spleen sections (5 mm) were mounted on silanized slides and dried for 15 minutes at room temperature. The tissues were fixed with 4% buffered paraformaldehyde, and the sections were incubated with 0.3% H2O2/PBS for 10 minutes. For fluorescence immunohistochemistry, antibodies were diluted with antibody diluent (S3022; Dako North America) and incubated for 16 hours at 4°C. Signals were visualized with SA-488, SA-Cy5, FITC-conjugated antirat IgG, or SA-Qdot605. For GFP-signal development, tissue sections were air-dried for 10 minutes, fixed in acetone, and air-dried again for 10 minutes. The sections were then incubated in anti-GFP IgG conjugated with Alexa Fluor 488 for 2 hours. The DeltaVision RT system or the Olympus BX51 fluorescence microscope were used for observation and photography.
Flow cytometry
Cells were stained with the indicated mAbs, and flow cytometry was performed using FACSCalibur with CellQuest software (BD Biosciences, San Jose, CA). Anti-FcγIII/II receptor mAb was used to block nonspecific antibody (Ab) binding. Isotype-matched, irrelevant antibodies were used as negative staining controls in each experiment. Propidium iodide (Sigma) was used to exclude dead cells from the analysis. The acquired data were analyzed with WinMDI software (http://facs.scripps.edu/software.html).
Proliferation assay
CD35+B220+ cells and bone marrow-derived cells (BMDCs) generated from naive C57BL/6-Jcl mice were treated with Colcemid (100 ng/mL) for 2 hour. The cells (1 × 104 cells) were cocultured with BALB/c CD4+ responder T cells (1 × 104 cells/mL) in a round-bottomed 96-well plate (Corning) for 4 days. During the last 3 hours of the culture, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) solution (CellTiter 96 AQueous One Solution Cell Proliferation Assay Kit; Promega, Madison, WI) was added. Cell proliferation was assessed according to the manufacturer's instructions. All samples were assayed in quadruplicate, and results are shown as means (±SD).
Reverse transcription–polymerase chain reaction
Total RNA was extracted from CD35+B220+ cells using an RNeasy kit (QIAGEN, Valencia, CA). One microgram of total RNA was reverse-transcribed with SuperScript II (Invitrogen) and used for PCR amplification with Taq DNA polymerase (Toyobo, Osaka, Japan). Comparable quantities of cDNA were ensured by amplification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Primers: VCAM-1: forward, 5′-ccaaggaagatgcgcagtagag-3′; reverse, 5′-acttgtggaaatgtgcccga-3′; ICAM-1: forward, 5′-aggagagcacaaacagcagtgactc-3′; reverse, 5′-ttcagagtctgctgagacccctcttg-3′; MadCAM-1: forward, 5′-agatacaagaggctgagggcacaccact-3′; reverse, 5′-atttccacaggcggtaggcaaggaagac-3′; and GAPDH: forward, 5′-atcaacgaccccttcattgacc-3′; reverse, 5′-ccagtagactccacgacatactcagc-3′.
Lymphoid-follicle–like structure formation
Stromal cells (TEL-2: 0.5 × 106 cells; brain- or spleen-derived stromal cells: 1 × 106 cells) and CD35+B220+ cells (1 × 105 cells) were cocultured for 24 hours in the stromal medium and injected intradermally into C57BL/6J-Jcl mice. On day 8, 14, or 20, mice were killed and the lymphoid-follicle–like structures were recovered.
IC-binding assay
OVA and anti-OVA Ab ICs were made and incubated with CD35+B220+ cells (1 × 105 cells) isolated from the spleen at a final concentration of 1 ng/mL OVA and 6 ng/mL anti-OVA Ab in RPMI 1640 medium supplemented with 5% freshly isolated mouse serum, 5% FBS, 50 U/mL penicillin, 50 mg/mL streptomycin, and nonessential amino acids, for 12 hours at 37°C in a CO2 incubator. The IC-coupling cells were washed twice with RPMI 1640 medium and cultured while changing the medium every other day. On day 6, cells were collected and placed on silanized glass. ICs that bound to the cell surface were visualized with HRP-conjugated antirabbit IgG followed by GenPoint (Dako Japan, Kyoto, Japan) signal enhancement system and streptavidin-conjugated Qdot605. For in vitro IC-deposition assay, splenic CD35+B220+ cells were prepared from GFP-Tg mice and administered intravenously or intradermally with TEL-2 stromal cells into naive C57BL/6J-Jcl mice, and nonfixed frozen sections of the spleen or induced lymphoid-follicle–like structures, respectively, were prepared. The ICs (200 μg/mL) were preincubated with 10% fresh mouse serum for 30 minutes and applied to these sections for 1 hour at room temperature.30 The deposition of ICs was visualized with goat anti-HRP Ab followed by Cy3-conjugated antigoat IgG. Presence of the transferred GFP+ cells was detected with Alexa-488-conjugated anti-GFP antibody. CD35+ reticulum and macrophages were labeled with biotin-conjugated anti-CD35 mAb and biotin-conjugated anti-F4/80 mAb, respectively, followed by streptavidin-conjugated Cy5. ICs were visualized with goat anti-HRP Ab followed by Cy3-conjugated antigoat IgG. In another experiment, PKH26-labeled CD35+B220+ cells were incubated with ICs as described above and injected intravenously into naive C57BL/6J-Jcl mice. On day 8, frozen sections of the spleen were prepared, and ICs retained on the injected cells were visualized with HRP-conjugated antirabbit IgG followed by the GenPoint signal enhancement system and SA-488. The DeltaVision RT system was used for observation and photography.
Scanning electron microscopy (SEM)
CD35+B220+ cells or CD35+B220+ cells cocultured with isolated splenic stromal cells were fixed overnight with 2.5% glutaraldehyde in PBS at 4°C. Specimens were dehydrated with a graded series of ethanol, dried, and sputter-coated to produce a 15-nm Pt coating. Samples were examined with a Keyence VE-7800 Real Surface View scanning electron microscope (Osaka, Japan) at an accelerating voltage of 20 kV.
Statistical analysis
The statistical significance of all the assays was assessed with the 2-tailed Student t test with P less than .05 considered significant.
Results
Identification of CD35+ reticulum-forming cells in the spleen
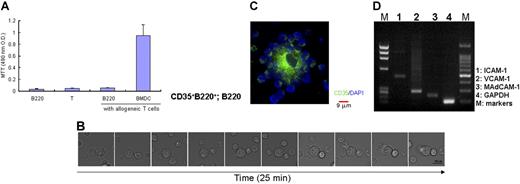
Lymphoid follicles in secondary lymphoid organs as well as ectopically formed lymph-node–like structures are inducible by immunization and/or inflammatory stimulation.11,31 It is well known that signals elicited through TNF are required for the maintenance of proper microarchitecture of the secondary lymphoid organs as well as the development of FDC networks. In addition, although the identity of such cells remains elusive, IC-bearing CD35+ cells are recognized at the early stage of splenic FDC network formation,22 implicating the involvement of such cells in this process. In our search for a putative inducer of FDC network formation, we first analyzed splenocytes derived from C57BL/6J-Jcl mice and TNF-KO mice to find a unique CD35+ cell population. To avoid contamination with CD11c+ dendritic cells and CD35-expressing B cells, such as mantle and MZ B cells, we gated out CD19+ and CD11c+ cells during the analysis. As a result, we identified a unique CD35+B220+ population in the CD19− and CD11c− cell fraction of splenocytes from C57BL/6J-Jcl mice (0.03 to 0.04% of total splenocytes; Figure 1A). Such cell population could hardly be identified in splenocytes from TNF-KO mice. These splenic CD19−CD11c−D35+B220+cells expressed moderate levels of major histocompatibility complex (MHC) class II (I-Ab) molecule and low levels of CD21/35 and B7-2 (CD86) molecules (Figure 1). Further phenotypic analysis indicated that this population was virtually devoid of lineage marker (CD11b, CD8, F4/80, CD3, and NK1.1) expression. Because we failed to obtain a sufficient number of CD19−CD11c−CD35+B220+ cells with high viability for further experiments by fluorescence-activated cell sorting, we established a protocol for the isolation of these cells from the primary culture of splenocytes by negative selection using the MACS system. Round, loosely adherent cells were obtained from the CD19−CD11c−Gr-1− fraction of splenocytes from naive C57BL/6J-Jcl mice after culture for 3 days. These adherent cells were further purified by selecting CD19-, CD3-, CD11c-, Gr-1-, CD8-, CD11b-, and NK1.1-negative phenotypes. Finally, the cells were selected for the B220+ phenotype. These B220+ cells were characterized as positive for MHC class II and CD35 (Figure 1B) and are hereafter referred to as CD35+B220+ cells. Time-lapse confocal microscopy showed an accumulation of CD35+B220+ cells having a ruffled cell membrane (Figure 1C; Figure S2). The purity of the cells shown in Figure 1B was estimated to range from 87% to 92%. Cell viability after MACS sorting was higher than 97% based on PI staining and the Trypan blue exclusion test.
Identification of CD19−CD11c−CD35+B220+ cells in mouse spleen. (A) Flow cytometric analysis of spleen cells from C57BL/6J-Jcl mice and TNF-KO mice. Whole spleen cells stained with indicated mAbs were analyzed. Gating of CD19− and CD11c− cells enabled the identification of CD35+ cells in both strains. Frequencies of CD35+B220+ cells within CD19− and CD11c− populations of splenocytes are shown at the corner of respective quadrants. (B) Isolated CD35+B220+ cells show the MHC class II+ phenotype. The phenotype of the isolated cell population, termed CD35+B220+ cells, was characterized by double color staining for MHC class II and CD35. Data are representative of 3 independent experiments. (C) Morphology of CD35+B220+ cells. Isolated CD35+B220+ cells from GFP-Tg mice were added dropwise onto a glass slide, air-dried, and fixed with 4% buffered paraformaldehyde. GFP-mediated signals were observed and photographed with a Leica confocal microscope. Photographs were taken with a 63×/1.4 NA objective.
Identification of CD19−CD11c−CD35+B220+ cells in mouse spleen. (A) Flow cytometric analysis of spleen cells from C57BL/6J-Jcl mice and TNF-KO mice. Whole spleen cells stained with indicated mAbs were analyzed. Gating of CD19− and CD11c− cells enabled the identification of CD35+ cells in both strains. Frequencies of CD35+B220+ cells within CD19− and CD11c− populations of splenocytes are shown at the corner of respective quadrants. (B) Isolated CD35+B220+ cells show the MHC class II+ phenotype. The phenotype of the isolated cell population, termed CD35+B220+ cells, was characterized by double color staining for MHC class II and CD35. Data are representative of 3 independent experiments. (C) Morphology of CD35+B220+ cells. Isolated CD35+B220+ cells from GFP-Tg mice were added dropwise onto a glass slide, air-dried, and fixed with 4% buffered paraformaldehyde. GFP-mediated signals were observed and photographed with a Leica confocal microscope. Photographs were taken with a 63×/1.4 NA objective.
CD35+B220+ cells formed B cell rosette
Because CD35+B220+ cells express MHC class II and B7-2 molecules, albeit at a low level, on their surfaces, as mentioned above, we first investigated their T-cell stimulatory activity as antigen-presenting cells (APCs) in an allogeneic mixed lymphocyte culture reaction (MLR) (Figure 2A). CD35+B220+ cells derived from C57BL/6J-Jcl splenocytes were cocultured with purified CD4+ T cells from BALB/c mice. Dendritic cells generated from C57BL/6J-Jcl BM culture showed marked T-cell stimulatory activity in allogeneic MLR, whereas CD35+B220+ cells did not support the proliferation of allogeneic T cells, suggesting that these cells were not efficient APCs (Figure 2A). We next examined the interaction between CD35+B220+ cells and B cells. DIC time-lapse observation demonstrated that CD35+B220+ cells captured B cells onto their cell membranes in a very short time (Figure 2B). Further incubation with B cells induced a morphological change of the CD35+B220+ cells, although they retained the ability to capture B cells on their well-extended plasma membranes (Figure 2C). Gene expression analysis indicated the expression of several adhesion molecules, such as VCAM-1, ICAM-1, and mucosal addressin cell adhesion molecule-1 (MAdCAM-1), on the CD35+B220+ cells (Figure 2D). Therefore, such molecules may mediate the accumulation of B cells.
Interaction of CD35+B220+ cells with B cells. (A) CD35+B220+ cells are not potent APCs. Colcemid-treated CD35+B220+ cells or BMDCs derived from C57BL/6J-Jcl mice were cultured with splenic CD4+ T cells from BALB/c mice for 4 days in quadruplicate in 96-well round-bottomed plates, and MTT reagent was added in the last 3 hours of culture. Cell proliferation was measured according to the manufacturer's instructions. Data are representative of 3 experiments yielding comparable results. The data represent the mean plus or minus SD from 3 experiments. (B) CD35+B220+ cells capture splenic B cells on their cell membrane. Splenic CD19-expressing B cells were added to CD35+B220+ cells precultured for 15 minutes on a glass-bottomed dish. DIC time-lapse images (2 minutes, 150-second gap) show dynamic changes in the shape of CD35+B220+ cells and CD19-expressing B-cell adhesion. DIC images were obtained with a DeltaVision RT system, and representatives of 3 independent experiments are shown. Photographs were taken with a 60×/oil 1.4 NA objective. (C) CD35+B220+ cells form B cell rosettes in vitro. CD35+B220+ cells and CD19-expressing B cells isolated from naive C57BL/6J-Jcl mice were cocultured for 3 days. After fixation with 4% buffered paraformaldehyde, cells were stained with anti-CD35 mAb. Merged image shows entangled CD35+ cell membrane of a CD35+B220+ cell with adhered B cells. Immunofluorescence images were obtained with a DeltaVision RT system, and a representative of 3 independent experiments is shown. Photographs were taken with a 60×/oil 1.4 NA objective. (D) mRNA expression of CD35+B220+ cells. Expression of different adhesion molecules was examined with RT-PCR.
Interaction of CD35+B220+ cells with B cells. (A) CD35+B220+ cells are not potent APCs. Colcemid-treated CD35+B220+ cells or BMDCs derived from C57BL/6J-Jcl mice were cultured with splenic CD4+ T cells from BALB/c mice for 4 days in quadruplicate in 96-well round-bottomed plates, and MTT reagent was added in the last 3 hours of culture. Cell proliferation was measured according to the manufacturer's instructions. Data are representative of 3 experiments yielding comparable results. The data represent the mean plus or minus SD from 3 experiments. (B) CD35+B220+ cells capture splenic B cells on their cell membrane. Splenic CD19-expressing B cells were added to CD35+B220+ cells precultured for 15 minutes on a glass-bottomed dish. DIC time-lapse images (2 minutes, 150-second gap) show dynamic changes in the shape of CD35+B220+ cells and CD19-expressing B-cell adhesion. DIC images were obtained with a DeltaVision RT system, and representatives of 3 independent experiments are shown. Photographs were taken with a 60×/oil 1.4 NA objective. (C) CD35+B220+ cells form B cell rosettes in vitro. CD35+B220+ cells and CD19-expressing B cells isolated from naive C57BL/6J-Jcl mice were cocultured for 3 days. After fixation with 4% buffered paraformaldehyde, cells were stained with anti-CD35 mAb. Merged image shows entangled CD35+ cell membrane of a CD35+B220+ cell with adhered B cells. Immunofluorescence images were obtained with a DeltaVision RT system, and a representative of 3 independent experiments is shown. Photographs were taken with a 60×/oil 1.4 NA objective. (D) mRNA expression of CD35+B220+ cells. Expression of different adhesion molecules was examined with RT-PCR.
CD35+B220+ cells fused with stromal cells
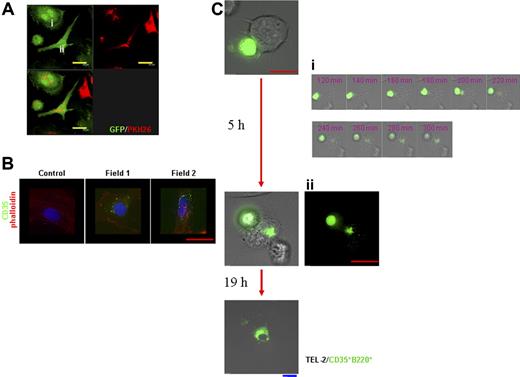
Formation of the FDC network is considered to be dependent on the stromal components as well as on the association with lymphocytes.32–34 To examine the influence of stromal cells on CD35+B220+ cells, CD35+B220+ cells were cocultured with CD45−CD35− stromal cells or TEL-2 stromal cells.35 Coculture of PKH26-labeled CD35+B220+ cells with splenic stromal cells isolated from GFP-Tg mice produced double-nuclear cells with the GFP- and PKH26-double positive phenotype (Figure 3A; Figure S3). These cells showed either round (Figure 3Ai; Figure S3A) or fibroblastic (Figure 3Aii) morphology. Some splenic stromal cells at the later stages of coculture lost organized F-actin orientation characteristic to stromal cells and became CD35+ (Figure 3B). Because splenic stromal cells used in these experiments were originally selected negative for CD35 expression, the results suggest that the interaction between stromal cells and CD35+B220+ cells modulates the characteristics of the stromal cells. A similar interaction was observed when CD35+B220+ cells from GFP-Tg mice were cocultured with TEL-2 stromal cells (Figure 3C). DIC-fluorescence time-lapse imaging showed that GFP+-CD35+B220+ cells coming in contact with TEL-2 stromal cells were captured by the stromal cells, and eventually the GFP signals were distributed in the cytosol of TEL-2 stromal cells in 5 hours (Figure 3Ci). These results indicate that the GFP+ cytosol of CD35+B220+ cells intertwined with that of TEL-2 stromal cells because of cell fusion.
CD35+B220+ cells interact with and confer CD35+ characteristic to stromal cells. (A) PKH26-labeled CD35+B220+ cells were cocultured with CD45−CD35− splenic stromal cells from GFP-Tg mice for 24 hours, and the colocalization of PKH26 signals was observed and photographed with a Leica confocal microscope. Objective magnification, 63×/1.4 NA. 3-dimensional stacked images of cells with typical morphology, either round (i) or fibroblastic (ii), are shown. Cocultured cells in separate and merged color images for each staining are shown (top right, PKH26; top left, GFP; bottom left, merged image) with scale bar. (B) CD45−CD35− splenic stromal cells cocultured with CD35+B220+ cells for 5 days were stained with anti-CD35 mAb followed by Alexa-Fluor-488-conjugated anti-mouse IgG, as described under “Materials and methods.” F-actin was visualized with Cy3-phalloidin, and cell nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI). Immunofluorescence images were obtained with a DeltaVision RT system. Objective magnification, 60×/oil 1.4 NA. Representatives of 3 independent experiments are shown. (C) TEL-2 stromal cells were cocultured with CD35+B220+ cells from GFP-Tg mice for 24 hours within the live cell imaging module. Immunofluorescence images merged or not merged with (panel Cii) DIG images were obtained with a DeltaVision RT system. DIC-fluorescence time-lapse images from 2 to 5 hours of 24-hour incubation period (15-second gap) are shown (panel Ci, corresponding time after incubation is indicated). Objective magnification, 60×/oil 1.4 NA. Representatives of 3 independent experiments are shown. Blue bar corresponds to 30 μm, yellow bar corresponds to 20 μm, and red bar corresponds to 15 μm.
CD35+B220+ cells interact with and confer CD35+ characteristic to stromal cells. (A) PKH26-labeled CD35+B220+ cells were cocultured with CD45−CD35− splenic stromal cells from GFP-Tg mice for 24 hours, and the colocalization of PKH26 signals was observed and photographed with a Leica confocal microscope. Objective magnification, 63×/1.4 NA. 3-dimensional stacked images of cells with typical morphology, either round (i) or fibroblastic (ii), are shown. Cocultured cells in separate and merged color images for each staining are shown (top right, PKH26; top left, GFP; bottom left, merged image) with scale bar. (B) CD45−CD35− splenic stromal cells cocultured with CD35+B220+ cells for 5 days were stained with anti-CD35 mAb followed by Alexa-Fluor-488-conjugated anti-mouse IgG, as described under “Materials and methods.” F-actin was visualized with Cy3-phalloidin, and cell nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI). Immunofluorescence images were obtained with a DeltaVision RT system. Objective magnification, 60×/oil 1.4 NA. Representatives of 3 independent experiments are shown. (C) TEL-2 stromal cells were cocultured with CD35+B220+ cells from GFP-Tg mice for 24 hours within the live cell imaging module. Immunofluorescence images merged or not merged with (panel Cii) DIG images were obtained with a DeltaVision RT system. DIC-fluorescence time-lapse images from 2 to 5 hours of 24-hour incubation period (15-second gap) are shown (panel Ci, corresponding time after incubation is indicated). Objective magnification, 60×/oil 1.4 NA. Representatives of 3 independent experiments are shown. Blue bar corresponds to 30 μm, yellow bar corresponds to 20 μm, and red bar corresponds to 15 μm.
CD35+B220+ cells initiated lymphoid-follicle–like structure formation
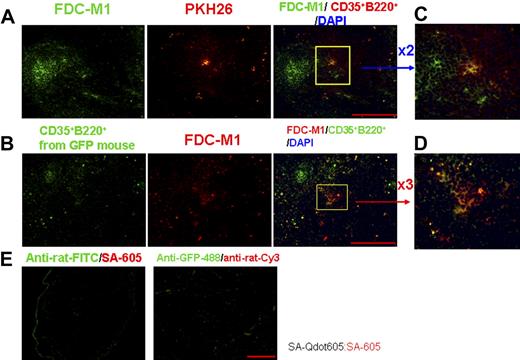
To further examine the physiological relevance of CD35+B220+ cells to FDC network formation, we generated lymphoid-follicle–like structures that would enable us to study the FDC network development process. PKH26-labeled or nonlabeled CD35+B220+ cells with or without stromal cells were injected intradermally into naive C57BL/6J-Jcl mice. When CD35+B220+ cells or stromal cells alone were injected, no visible lymphoid-follicle–like structure was formed (Table 1). It is noteworthy that such structures were detected when CD35+B220+ cells were injected together with stromal cells from different origins (Figure 4; Table 1, size: long axis × short axis: 50-250 × 40-120 μm), and such structures were maintained for at least 25 days after the injection of CD35+B220+ cells and stromal cells (data not shown). The formed lymphoid-follicle–like structures were similar in appearance to secondary lymphoid organs that contained distinct organized T-cell and B-cell areas. Of note is that cells originating from injected CD35+B220+ cells predominantly resided in B-cell follicles (Figure 4Ai), and that FDC-M1+ cells with the B220+ phenotype were identified in the cluster of FDC-M1+ cells present in the lymphoid-follicle–like structure (Figure 4B,C). These results suggest that CD35+B220+ cells play a critical role in the development of the FDC-M1+ reticulum, and the interaction between CD35+B220+ cells and stromal cells is a prerequisite for the formation of the lymphoid-follicle–like structure.
Stromal cells elicit formation of lymphoid-follicle–like structure with CD35+B220+ cells. (A) Immunostaining of lymphoid-follicle–like structures established by intradermal injection of PKH26-labeled CD35+B220+ cells (*) + splenic stromal cells (i), CD35+B220+ cells + brain-derived stromal cells (ii), or CD35+B220+ cells + TEL-2 stromal cells (iii) using mAbs for CD4, B220, and CD35. Nucleus was stained with DAPI. CD35+ reticulum in serial sections is marked by a yellow dotted line. Bars correspond to 200 μm. All images were photographed with an Olympus fluorescence microscope. (B) Lymphoid-follicle–like structures formed by CD35+B220+ cells together with brain stromal cells (i) or TEL-2 stromal cells (ii) were stained with anti-FDC-M1 mAb (green) and anti-B220 mAb (red). Lymphoid-follicle–like structures generated from B220+CD35+ cells and TEL-2 stromal cells were stained with FITC-conjugated anti-rat IgG and SA-Qdot605 as a negative control (iii). Representative of 3 independent experiments is shown with scale bar. Red bar, 200 μm; white bar, 15 μm. (C) Lymphoid-follicle–like structures generated by CD35+B220+ cells isolated from GFP-Tg mice and TEL-2 stromal cells were stained with anti-GFP mAb (green) and anti-FDC-M1 Ab (red). The serial section was stained with anti-GFP IgG conjugated with Alexa Fluor 488 and Cy3-conjugated antirat IgG as negative control. Representatives of 3 independent experiments are shown with scale bar. Green bar corresponds to 100 μm, purple bar corresponds to 30 μm, and white bar corresponds to 10 μm. Images were taken and analyzed with a DeltaVision RT system. Objective magnification, 20×/0.75 NA or 60×/oil 1.4 NA.
Stromal cells elicit formation of lymphoid-follicle–like structure with CD35+B220+ cells. (A) Immunostaining of lymphoid-follicle–like structures established by intradermal injection of PKH26-labeled CD35+B220+ cells (*) + splenic stromal cells (i), CD35+B220+ cells + brain-derived stromal cells (ii), or CD35+B220+ cells + TEL-2 stromal cells (iii) using mAbs for CD4, B220, and CD35. Nucleus was stained with DAPI. CD35+ reticulum in serial sections is marked by a yellow dotted line. Bars correspond to 200 μm. All images were photographed with an Olympus fluorescence microscope. (B) Lymphoid-follicle–like structures formed by CD35+B220+ cells together with brain stromal cells (i) or TEL-2 stromal cells (ii) were stained with anti-FDC-M1 mAb (green) and anti-B220 mAb (red). Lymphoid-follicle–like structures generated from B220+CD35+ cells and TEL-2 stromal cells were stained with FITC-conjugated anti-rat IgG and SA-Qdot605 as a negative control (iii). Representative of 3 independent experiments is shown with scale bar. Red bar, 200 μm; white bar, 15 μm. (C) Lymphoid-follicle–like structures generated by CD35+B220+ cells isolated from GFP-Tg mice and TEL-2 stromal cells were stained with anti-GFP mAb (green) and anti-FDC-M1 Ab (red). The serial section was stained with anti-GFP IgG conjugated with Alexa Fluor 488 and Cy3-conjugated antirat IgG as negative control. Representatives of 3 independent experiments are shown with scale bar. Green bar corresponds to 100 μm, purple bar corresponds to 30 μm, and white bar corresponds to 10 μm. Images were taken and analyzed with a DeltaVision RT system. Objective magnification, 20×/0.75 NA or 60×/oil 1.4 NA.
To determine whether CD35+B220+ cells can differentiate into the FDC-M1+ phenotype in vivo, lymphoid-follicle–like structures were induced by CD35+B220+ cells from GFP-Tg mice and TEL-2 stromal cells. Reticular cells double-positive for FDC-M1 and GFP were found within the lymphoid-follicle–like structures (Figure 4C). To further examine the effects of residential splenic stromal cells on the differentiation of CD35+B220+ cells in vivo, PKH26-labeled CD35+B220+ cells, or CD35+B220+ cells from GFP-Tg mice were injected intravenously into naive C57BL/6J-Jcl mice. Co-localization of the PKH26-derived signals with splenic B220+CD35+ cell clusters was observed 8 days after injection (Figure 5). However, the development of the FDC-M1+ phenotype on PKH26-labeled CD35+B220+ cells was not observed at this time point (data not shown). The presence of FDC-M1+GFP+ cells in the CD35+ reticulum in the spleen on adoptive transfer of CD35+B220+ cells from GFP-Tg mice was identified 15 days after injection (Figure 6). Taken together, these results support the idea that the association of CD35+B220+ cells with stromal cells initiates the formation of FDC networks in the lymphoid follicles.
Transferred CD35+B220+ cells are found in splenic white pulp. (A) Isolated CD35+B220+ cells were labeled with PKH26 and administered intravenously into naive C57BL/6J-Jcl mice (Live; top panels in A and B). As controls, PKH26-labeled CD35+B220+ cells were fixed with ethanol and intravenously injected into C57BL/6J-Jcl mice (Fixed; bottom panels in A). On day 8 after the adoptive transfer, cryosections of the spleen were examined by immunofluorescence staining with biotin-conjugated anti-B220, anti-CD11b, anti-IgM, and anti-CD35 mAbs or anti-MARCO polyclonal Ab. Streptavidin-conjugated Alexa Fluor 488 or anti-rat IgG conjugated with Alexa Fluor 488 was used to visualize the microarchitecture of the spleen. Samples are representatives of 3 independent experiments. All images were taken and analyzed with a DeltaVision RT system. Photographs of upper rows were taken with a 20×/0.75 NA objective. (B) High-power photographs of the area enclosed by the yellow square in panel A. Yellow bars, 100 μm; blue bars, 20 μm.
Transferred CD35+B220+ cells are found in splenic white pulp. (A) Isolated CD35+B220+ cells were labeled with PKH26 and administered intravenously into naive C57BL/6J-Jcl mice (Live; top panels in A and B). As controls, PKH26-labeled CD35+B220+ cells were fixed with ethanol and intravenously injected into C57BL/6J-Jcl mice (Fixed; bottom panels in A). On day 8 after the adoptive transfer, cryosections of the spleen were examined by immunofluorescence staining with biotin-conjugated anti-B220, anti-CD11b, anti-IgM, and anti-CD35 mAbs or anti-MARCO polyclonal Ab. Streptavidin-conjugated Alexa Fluor 488 or anti-rat IgG conjugated with Alexa Fluor 488 was used to visualize the microarchitecture of the spleen. Samples are representatives of 3 independent experiments. All images were taken and analyzed with a DeltaVision RT system. Photographs of upper rows were taken with a 20×/0.75 NA objective. (B) High-power photographs of the area enclosed by the yellow square in panel A. Yellow bars, 100 μm; blue bars, 20 μm.
CD35+B220+ cells show FDC-M1+ phenotype in splenic white pulp. Transferred CD35+B220+cells showed the FDC-M1+ phenotype. Isolated CD35+B220+ cells labeled with PKH26 (A) or CD35+B220+ cells from GFP-Tg mice (B) were administered intravenously into naive C57BL/6J-Jcl mice. On day 15 after the adoptive transfer, cryosections of the spleen were examined by immunofluorescence staining with anti-FDC-M1 Ab and Alexa-Fluor-488–conjugated anti-GFP mAb. Anti-rat IgG conjugated with FITC (panel A) or anti-rat IgG conjugated with Cy3 (panel B) was used to visualize the microarchitecture of the splenic FDC-M1+ reticulum. Samples are representative of 3 independent experiments. All images were analyzed with a fluorescence microscope and were photographed with an Olympus fluorescence microscope. Bars, 200 μm. Shown in panels C and D are high-power photographs of the area enclosed by the yellow squares in panels A and B. Samples stained with antirat IgG conjugated with FITC (panel E, left) or anti-GFP IgG conjugated with Alexa Fluor 488 and antirat IgG conjugated with Cy3 (panel E, right) were used as control. Careful examination revealed that CD35+ cells showed the FDC-M1+ phenotype in splenic white pulp on day 15 after adoptive transfer.
CD35+B220+ cells show FDC-M1+ phenotype in splenic white pulp. Transferred CD35+B220+cells showed the FDC-M1+ phenotype. Isolated CD35+B220+ cells labeled with PKH26 (A) or CD35+B220+ cells from GFP-Tg mice (B) were administered intravenously into naive C57BL/6J-Jcl mice. On day 15 after the adoptive transfer, cryosections of the spleen were examined by immunofluorescence staining with anti-FDC-M1 Ab and Alexa-Fluor-488–conjugated anti-GFP mAb. Anti-rat IgG conjugated with FITC (panel A) or anti-rat IgG conjugated with Cy3 (panel B) was used to visualize the microarchitecture of the splenic FDC-M1+ reticulum. Samples are representative of 3 independent experiments. All images were analyzed with a fluorescence microscope and were photographed with an Olympus fluorescence microscope. Bars, 200 μm. Shown in panels C and D are high-power photographs of the area enclosed by the yellow squares in panels A and B. Samples stained with antirat IgG conjugated with FITC (panel E, left) or anti-GFP IgG conjugated with Alexa Fluor 488 and antirat IgG conjugated with Cy3 (panel E, right) were used as control. Careful examination revealed that CD35+ cells showed the FDC-M1+ phenotype in splenic white pulp on day 15 after adoptive transfer.
CD35+B220+ cells retained ICs on their surface
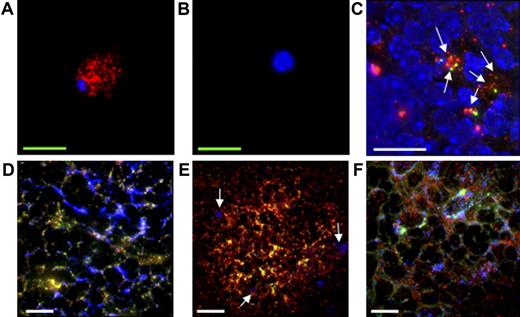
Even though CD35+B220+ cells isolated from GFP-Tg mice develop into the FDC-M1+ phenotype in the CD35+ reticulum in the splenic follicle, it is not clear whether such cells are indeed FDCs because FDC-M1 is expressed by other cells, such as tingible body macrophages and BM stromal cells. One of the unique features that can segregate other cell populations from FDCs is the ability of the latter to trap and retain ICs on their surface for a long period of time. The long-term retention of ICs on their cell surface was first tested by incubating CD35+B220+ cells with OVA-anti-OVA ICs in culture. CD35+B220+ cells incubated with ICs for 12 hours were washed twice with PBS, and the cultures were maintained for 6 days. To exclude contamination with nonbound ICs, the culture medium was replaced every other day. As shown in Figure 7A, ICs were maintained on the surface of CD35+B220+ cells after 6 days. No ICs were detected on the CD4+ T-cell surface as control (Figure 7B). To examine the retention of ICs on the surface of CD35+B220+ cells in vivo, IC-pulsed, PKH26-labeled CD35+B220+ cells were injected intravenously into naive C57BL/6J-Jcl mice, and the presence of ICs on the injected cells was assessed 8 days after injection using a deconvolution microscope. As shown in Figure 7C, ICs were colocalized with PKH26-labeled CD35+B220+ cells, indicating that the transferred CD35+B220+ cells have the capability to retain ICs on their surface in vivo for at least 8 days. The binding of ICs on the surface of reticular cells that differentiated from CD35+B220+ cells was further corroborated by assessing the binding ability of ICs by these cells in vitro. To this end, CD35+B220+ cells isolated from GFP-Tg mice were intravenously injected into naive C57BL/6J-Jcl mice, and the splenic sections were prepared 15 days after injection. ICs were specifically deposited on the CD35+ reticulum of the GC, and GFP+ reticular cells were found within this area (Figure 7D). These GFP+ reticular cells were negative for F4/80 (data not shown) and CD68 (Figure 7C), suggesting that these cells were not macrophages, including tingible body macrophages. These data, together with Figure 6, indicate that reticular cells originally differentiated from CD35+B220+ cells inherit the unique features of FDCs. Likewise, IC binding on the CD35+ reticulum in the lymphoid-follicle–like structure was examined by applying ICs onto the frozen section of ectopically formed lymphoid-follicle–like structure upon intradermal injection of CD35+B220+ cells.
Immunohistochemical labeling of CD35+B220+ cells with ICs. (A-C) CD35+B220+ cells retain ICs on their surface. CD35+B220+ cells (A) or CD4+ T cells (B) were labeled with ICs and the retained ICs (red) on cell surface were visualized with HRP-conjugated anti-rabbit IgG followed by GenPoint (Dako Japan) signal enhancement system and streptavidin-conjugated Qdot605. (C) Retention of ICs (green) on the surface of PKH26-labeled CD35+B220+ cells (red) in vivo was assessed by injecting IC-pulsed, PKH-26-labeled CD35+B220+ cells into naive C57BL/6J-Jcl mice. On day 8, the splenic sections were prepared and ICs (green) were stained with HRP-conjugated antirabbit IgG followed by GenPoint signal enhancement system and SA-488. ICs on the surface of CD35+B220+ cells are indicated by arrows. (D-F) CD35+B220+-cell–derived reticular cells retain ICs. CD35+B220+ cells isolated from GFP-Tg mice were injected intravenously into naive C57BL/6J-Jcl mice, and frozen sections of spleen were prepared 15 days later. Splenic sections were then incubated with ICs and freshly prepared mouse serum, and ICs (panels D and E, red) were visualized with goat anti-HRP Ab followed by Cy3-conjugated anti-goat IgG. CD35 (panel D, blue) or CD68 (panel E, blue) was visualized with biotin-conjugated anti-CD35 mAb or biotin-conjugated anti-CD68 mAb, respectively, followed by streptavidin-conjugated Cy5, and the GFP signal was enhanced with Alexa-488-conjugated anti-GFP antibody. CD68+ cells are indicated by arrows (panel E). In panel F, lymphoid-follicle–like structures were formed by intradermally injecting FDC-M1−CD35+B220+ cells from GFP-Tg mice together with TEL-2 stromal cells. A frozen section of lymphoid-follicle–like structure was labeled with ICs (red) and anti-CD35 mAb (blue), as described. All immunofluorescence images were obtained using a DeltaVision RT system. Red bars, 200 μm; white bars, 20 μm; green bars, 5 μm. Objective magnification, 20×/0.75 NA or 60×/oil 1.4 NA. A representative of 3 independent experiments is shown.
Immunohistochemical labeling of CD35+B220+ cells with ICs. (A-C) CD35+B220+ cells retain ICs on their surface. CD35+B220+ cells (A) or CD4+ T cells (B) were labeled with ICs and the retained ICs (red) on cell surface were visualized with HRP-conjugated anti-rabbit IgG followed by GenPoint (Dako Japan) signal enhancement system and streptavidin-conjugated Qdot605. (C) Retention of ICs (green) on the surface of PKH26-labeled CD35+B220+ cells (red) in vivo was assessed by injecting IC-pulsed, PKH-26-labeled CD35+B220+ cells into naive C57BL/6J-Jcl mice. On day 8, the splenic sections were prepared and ICs (green) were stained with HRP-conjugated antirabbit IgG followed by GenPoint signal enhancement system and SA-488. ICs on the surface of CD35+B220+ cells are indicated by arrows. (D-F) CD35+B220+-cell–derived reticular cells retain ICs. CD35+B220+ cells isolated from GFP-Tg mice were injected intravenously into naive C57BL/6J-Jcl mice, and frozen sections of spleen were prepared 15 days later. Splenic sections were then incubated with ICs and freshly prepared mouse serum, and ICs (panels D and E, red) were visualized with goat anti-HRP Ab followed by Cy3-conjugated anti-goat IgG. CD35 (panel D, blue) or CD68 (panel E, blue) was visualized with biotin-conjugated anti-CD35 mAb or biotin-conjugated anti-CD68 mAb, respectively, followed by streptavidin-conjugated Cy5, and the GFP signal was enhanced with Alexa-488-conjugated anti-GFP antibody. CD68+ cells are indicated by arrows (panel E). In panel F, lymphoid-follicle–like structures were formed by intradermally injecting FDC-M1−CD35+B220+ cells from GFP-Tg mice together with TEL-2 stromal cells. A frozen section of lymphoid-follicle–like structure was labeled with ICs (red) and anti-CD35 mAb (blue), as described. All immunofluorescence images were obtained using a DeltaVision RT system. Red bars, 200 μm; white bars, 20 μm; green bars, 5 μm. Objective magnification, 20×/0.75 NA or 60×/oil 1.4 NA. A representative of 3 independent experiments is shown.
To exclude the possible contamination of pre-existing FDCs in our CD35+B220+ cell preparation, FDC-M1−CD35+B220+ cells from GFP-Tg mice were prepared and injected intradermally together with TEL-2 stromal cells into naive C57BL/6J-Jcl mice. On day 12, lymphoid-follicle–like structures were removed and stained with ICs and anti-CD35 mAb. As shown in Figure 7F, GFP+ reticular cells identified in the CD35+ reticulum were also positive for IC deposition. Consequently, CD35+B220+ cells may be capable of transporting antigens in the form of ICs to the residential stromal cells through cell-cell interaction.
Discussion
In this study, we found that CD35+B220+ cells are a putative inducer of lymphoid-follicle–like structure and FDC network formation. FDCs are CD35+ reticular network-forming cells located in the lymphoid follicles. They retain native antigens in the form of ICs on their cell membrane for months to present these antigens to B cells during secondary immune response. Although the presence of migrating precursors of FDCs has long been hypothesized based on the findings of de novo formation of lymphoid-tissue-like structures and extranodal lymphoid follicles,22,36,37, their origin and ontogeny remain largely elusive. On the other hand, it is well known that the precursors of FDCs depend on the signals elicited through TNFp55R and LTβR for their differentiation into mature FDC networks.18 Consistent with this notion, the number of splenic CD35+B220+ cells found in TNF-KO mice was significantly decreased compared with that in wild-type mice (Figure 1), and the migration of CD35+B220+ cells into splenic B-cell follicles observed in wild-type mice (Figure 5) did not occur in TNF-KO mice (data not shown). It is likely that TNF is also essential for the differentiation of these cells. Even though CD35+B220+ cells migrated into splenic follicles within 8 days of adoptive transfer into wild-type mice (Figure 5), these cells required more than 14 days to form FDC-M1+ reticulum in the splenic white pulp (Figure 6) and in the generated lymphoid-follicle–like structures (Figure 4). Therefore, the development of the FDC-M1+ phenotype might require a substantial amount of time and/or yet unknown stimulation from in vivo microenvironment.
Among the remaining discrepancies between the features of classic FDCs and CD35+B220+ cells are the morphology and the correlation with stromal cells of the latter cells. The strong association of classic FDC/FDC-like cells with stromal cells has not been described elsewhere.27,38–40 Moreover, they possess stromal-like fibroblastic or dendritic appearance, in contrast to CD35+B220+ cells (Figure 1C; Figure S2). However, as shown in Figure 3 and Figure S3, CD35+B220+ cells and stromal cells efficiently interact with each other, resulting in the appearance of double-nuclear cells of stromal origin (ie, GFP+) that acquire the surface phenotype of CD35+B220+ cells. The most likely explanation for this observation is that these 2 cell types fuse with each other. Spontaneous cell fusion can also take place in stem cells and cancer cells in vivo and in vitro and is attributed to the possible plasticity of these cells.19,41–43 Our observation is also consistent with previous findings that freshly isolated FDCs as well as long-term cultured FDC-like cells show the double-nuclear phenotype.44,45 Nevertheless, most of the FDCs found in splenic white pulp as well as the reticular cells that differentiated from CD35+B220+ cells have a single nucleus. Thus, the present data suggest that the double-nuclear morphology may be a transient form of the fused cells, and that such process may initiate the transdifferentiation of stromal cells that function as initiators of FDC-network formation (Figures 4, 6). Furthermore, this characteristic of the CD35+B220+ cells can also explain the phenotypic diversity of FDCs17,46 and can partially account for the discrepancies mentioned above.
We also showed the de novo formation of lymphoid-follicle–like structure by CD35+B220+ cells in vivo. This was achieved only after the intradermal injection of CD35+B220+ cells and stromal cells and not after the injection of CD35+B220+ cells alone or stromal cells alone (Figure 4; Table 1). We took advantage of the intradermal injection method because we could monitor the whole sequence of lymphoid-follicle–like structure formation with this method, although we could not distinguish whether splenic follicles containing adoptively transferred CD35+B220+ cells are newly generated upon migration of these cells or the transferred cells migrate into pre-existing follicles. It should be noted that CD35+ clusters in the lymphoid-follicle–like structure contain B220+FDC-M1+ cells and GFP+FDC-M1+ cells, both of which are derived from the transferred CD35+B220+ cells (Figure 4B,C,E,F), suggesting that CD35+B220+ cells can function as an initiator of FDC network formation in the lymphoid-follicle–like structure. Furthermore, an increase in the number of CD35+ clusters was also observed 8 days after adoptive transfer of CD35+B220+ cells in the spleen (Figure S4). Collectively, the interaction between CD35+B220+ cells and stromal cells provides a niche for migrating lymphocytes to form cell clusters that, in association with the transferred cells, develop into the lymphoid-follicle–like structures.
In this study, we identified unique CD35+B220+ cells in the mouse spleen. Their IC-bearing potency and migration property suggest the antigen-transporting potential of this cell population (Figure 7), which is characteristic of antigen-transporting cells.37 It remains controversial whether antigen-transporting cells indeed differentiate into FDCs and whether MHC class II on FDCs come from MHC class II-containing microvesicles derived from B cells47 ; however, the present data clearly demonstrate the presence of IC-bearing reticular cells that differentiated from IC-loaded CD35+B220+ cells within the FDC network. Because cell fusion is implicated during this process, such property of CD35+B220+ cells and/or stromal cells may also contribute to the presence of MHC class II molecules and/or B220 molecules on the FDC-M1+ reticulum, and the transdifferentiation of these cells would induce accumulation of lymphocytes to establish the 3D microarchitecture of lymphoid follicles.
In conclusion, our findings imply that the strong interaction between CD35+B220+ cells and stromal cells initiates the formation of a niche for the accumulation of lymphocytes, which may play a critical role in the subsequent processes of lymphoid-follicle–like structure formation and FDC network development. Further study aimed at dissecting the fundamental role of stromal cells is required to understand the molecular mechanisms underlying the maturation of CD35+B220+ cells and the development of FDC networks.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are indebted to Professor E. Heinen (University of Liege, Belgium) for advice; Drs Carole Galligan (Toronto General Research Institute, ON, Canada), Mutsuo Furihata (Kochi University, Japan), Takeshi Watanabe (RIKEN Yokohama), Morihiro Watanabe and J. Oppenheim (National Cancer Institute Frederick) for critical review of the manuscript; and Dr Masaru Okabe for providing GFP-Tg mice. We thank Dr Takeshi Watanabe for providing TEL-2 stromal cells. We also thank Ms Hanae Fujimoto (RIKEN Yokohama) for fluorescence-activated cell sorting analysis, and Dr Akiko Furuno (Leica Microsystems) for confocal microscopy.
This project was supported in part by funds from the National Cancer Institute, National Institutes of Health under contract N01-CO12400. The preliminary works of this study were done at Dr Sergei Nedospasov's laboratory at National Cancer Institute Frederick.
National Institutes of Health
Authorship
Contribution: T.M. and H.O. designed the experiments; X.C., K.H., A.S., and C.N. performed the experiments; T.M. and X.C. analyzed data; T.M. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr. Hiroshi Ohno, Laboratory for Epithelial Immunology, RIKEN Research Center for Allergy and Immunology, 1-7-22 Suehiro-cho, Tsurumi-ku, Yokohama City, Kanagawa 230-0045, Japan; e-mail: ohno@rcai.riken.jp.