Abstract
In the immune system, extracellular ATP functions as a “natural adjuvant” that exhibits multiple proinflammatory effects. It is released by damaged cells as an indicator of trauma and cell death but can be inactivated by CD39 (nucleoside triphosphate diphosphohydrolase-1 [NTPDase 1]), an ectoenzyme that degrades ATP to AMP. Here, we show that CD39 is expressed primarily by immune-suppressive Foxp3+ regulatory T (Treg) cells. In mice, the enzyme is present on virtually all CD4+CD25+ cells. CD39 expression is driven by the Treg-specific transcription factor Foxp3 and its catalytic activity is strongly enhanced by T-cell receptor (TCR) ligation. Activated Treg cells are therefore able to abrogate ATP-related effects such as P2 receptor-mediated cell toxicity and ATP-driven maturation of dendritic cells. Also, human Treg cells express CD39. In contrast to mice, CD39 expression in man is restricted to a subset of Foxp3+ regulatory effector/memory-like T (TREM) cells. Notably, patients with the remitting/relapsing form of multiple sclerosis (MS) have strikingly reduced numbers of CD39+ Treg cells in the blood. Thus, in humans CD39 is a marker of a Treg subset likely involved in the control of the inflammatory autoimmune disease.
Introduction
In the central nervous system (CNS), extracellular ATP has been recognized long ago as an important signaling molecule.1 It acts as a neurotransmitter and modulator of neuronal excitability that can exhibit toxic as well as trophic effects. Although ATP as a signaling molecule is mostly associated with the CNS, it plays equally important roles in other tissues. A number of studies revealed that it is linked to diverse phenomena such as chemosensory signaling, secretion, vasodilatation, blood clotting, male fertility, pain, and embryonic development.2,3
In the immune system, extracellular ATP functions as indicator of tissue destruction. The intracellular ATP concentration is rather high (approximately 3-5 mM)4 so that significant amounts of the nucleotide are released upon cell damage. The presence of extracellular ATP can be sensed by purinergic P2 receptors.5 These receptors are either cation-selective receptor channels (P2X) or metabotropic G protein-coupled receptors (P2Y). They are highly expressed in immune and epithelial cells,5,6 in which the exposure to ATP induces mostly proinflammatory responses. On monocytes, stimulation with ATP leads to the release of IL-1β, also known as “endogenous pyrogen,” which mediates the panoply of host reactions collectively summarized as “acute phase” response.7,8 On dendritic cells (DCs), exposure to extracellular ATP triggers chemotaxis9 and induces maturation, although the release of certain proinflammatory cytokines seems to be blocked by ATP.4 Thus, extracellular ATP acts as a “natural adjuvant”10 or “danger signal”11 that activates the immune system.12–15
The pool of extracellular nucleoside triphosphates (NTPs) is controlled by ectonucleoside triphosphate diphosphohydrolases (E-NTPDases).16,17 Members of this enzyme family are expressed on the surface of various cells and remove NTPs by degrading them to NMP (nucleoside monophosphate). Of this family, CD39 is the dominant ectoenzyme in the immune system.18 It hydrolyzes ATP or ADP to AMP and is expressed by B cells, DCs, and subsets of T cells. Although the impact of extracellular ATP on the immune system is well established,19 the importance of CD39 for immune regulation is not yet clear. Besides removing a proinflammatory stimulus, it may also act in concert with CD73, another ectonucleotidase present on the surface of lymphocytes,20 to produce adenosine. This nucleoside exhibits mostly inhibitory and antiproliferative effects,19,21 so the overall effect of CD39 activity should be mainly immune suppressive.
CD39 was initially described as an activation marker of lymphoid cells.22 Here we show, however, that the ectonucleotidase is constitutively expressed by regulatory Foxp3+ T (Treg) cells. Treg cells represent a population of suppressor cells that contain autoreactive and overshooting inflammatory immune responses by active suppression. The mechanism of suppression is largely based on bystander reactions allowing the inhibition and neutralization of activated effector cells. Multiple potentially redundant mechanisms have been reported, such as direct cell-cell contact, depletion of IL-2, or the release of soluble inhibitory factors like IL-10 or TGFβ.23 Here, we suggest that catalytic inactivation and conversion of extracellular ATP by CD39 is another anti-inflammatory key mechanism of Treg cells with intriguing implications in the immune suppression of human autoimmune diseases, such as multiple sclerosis (MS).
Patients, materials, and methods
Patients
A total of 26 patients with relapsing-remitting MS (8 men and 18 women; age, 36.3 ± 8.2 years) and 74 sex- and age-matched healthy volunteers with no previous history of neurologic diseases (23 men and 51 women; age, 35.2 ± 11.3 years) were enrolled in the study. All patients were in the stable phase of the disease, as judged by clinical assessment, and had not undergone immune-modulating therapy for the 3 months preceding entry in the study. They had relatively mild neurologic disability (expanded disability status scale < 4). Approval by the ethics committee of Santa Lucia, Rome, Italy, and written informed consent in accordance with the Declaration of Helsinki from all participants were obtained before study initiation. Comparisons between pairs of data sets were performed by means of Student t tests for unrelated samples.
Antibodies and reagents
The following antibodies were used: mCD4 (GK1.5), mCD25 (PC61; 7D4), mCD73 (TY/23), hCD3 (HIT3a), hCD4 (RPA-T4), hCD25 (2A3), hCD45RO (UCHL1), hCCR6 (11A9), hCD49d (9F10), HLA-DR (L243), and hCD39 (TÜ66) (all from BD Biosciences, San Jose, CA); hFoxp3 (PCH101) and mFoxp3 (FJK-16s) were from eBioscience (San Diego, CA); mCD11c (N418) was from Caltag/Invitrogen (Carlsbad, CA); polyclonal rabbit anti-mouse mCD3924 and mCD39L125 was provided by J. Sevigny and S. C. Robson (Harvard Medical School, Boston, MA); mCD3 (145.2C11), mCD28 (37.51), mCD86 (GL1), HLA-DP (B7/21), and HLA-DQ (SPVL3) were all produced at the MDC. CFSE (5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester) was obtained from Invitrogen (Carlsbad, CA); and ATP, LPS (L-2654), DAPI, and ARL67156 were obtained from Sigma-Aldrich (St Louis, MO).
Flow cytometry and cell preparation
Fluorescence-activated cell sorting (FACS) analysis was carried out on a FACSCalibur, FACSCanto, or LSR II (BD Biosciences). Cell sorting was carried out by magnetic-activated cell sorting (MACS; Miltenyi Biotech, Bergisch Gladbach, Germany): mCD4 isolation kit followed by α-mCD25 depletion (mCD4+CD25−); mCD25 isolation kits (mCD4+CD25+); hCD25 isolation kits (hCD4+CD25+ and hCD4+CD25−). Human mononuclear cells were isolated by Ficoll gradient centrifugation (Pharmacia, Uppsala, Sweden). Human cells were sorted with a MoFlo high speed cell sorter (Dako, Glostrup, Denmark). Dead cells were excluded by propidium iodide (PI); purity of sorted cells was always above 90%. Data were analyzed using CellQuest and FACSDiva (BD Biosciences) and FlowJo software (Treestar, Ashland, OR).
Transduction of mouse T cells with Foxp3
CD4+CD25− mouse cells isolated from lymph nodes and spleens of naive C57/Bl6 mice were infected with retroviral constructs as described.26 Foxp3/MIGR1 and MIGR1 vectors were provided by S. Sakaguchi (Kyoto University, Japan).
Activation of Treg cells
CD4+CD25+ T cells were activated for 3 days with 10 μg/mL plate bound α-CD3, 2 μg/mL soluble α-CD28, and 100 U/mL IL-2 (Roche Diagnostics, Mannheim, Germany).
ATP hydrolysis assay
CD4+CD25+ cells were incubated in 96-well round-bottom plates with medium containing 50 μM ATP. ATP concentration was determined in a Wallac Victor V reader using the CellTiter-Glo Luminescent Cell Viability Assay (Promega, Madison, WI). Cells were used either freshly or were activated for 3 days. ATP consumption (μM/cell) was determined by a hyperbola regression [y = y0 + ax / (b + x)] using the starting slope (a/b) calculated by the Sigmaplot software (Systat Software, San Jose, CA). Specific activity (fmol/second/cell) was calculated by dividing this value by the time of incubation (10-20 minutes) and the incubation volume (50 μL).
Impact of ATP on viability and proliferative capacity of Treg cells
A total of 50 000 CD4+CD25+ cells/well were activated for 3 days in the presence of ATP (50 or 100 μM) and/or ARL67156 (250 μM) as indicated (no change of medium during incubation). Prior to the incubation, cells were labeled with 0.5 μM CFSE. Viability was determined by FACS after staining with PI. Proliferation was analyzed by determining the CFSE fluorescence of the living population gated on PI-negative cells.
Maturation of mouse DCs
DCs were isolated from the bone marrow of BALB/c mice and were cultured in the presence of 10 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF; R&D Systems, Minneapolis, MN). After 6 days, maturation was triggered by incubating 25 000 DCs/well with 100, 200, or 400 μM ATP or 10 ng/mL LPS. In some experiments, the ATP-containing medium was preincubated for 25 minutes with 400 000 freshly isolated CD4+CD25− or with CD4+CD25+ T cells either freshly isolated or activated for 3 days.
Confocal microscopy
Human CD25+ cells were isolated from the peripheral blood mononuclear cells (PBMCs) of healthy donors by MACS sorting (Miltenyi Biotech). Cells were stained for CD39 (MCA1268; Serotec, Kidlington, United Kingdom), followed by goat anti-mouse Alexa 546 (Invitrogen). Cells were plated on polylysinated slides, fixed, and stained intracellularly for Foxp3 and DAPI. Cells were analyzed on a Zeiss LSM510 confocal microscope (Oberkochen, Germany).
Suppression assay
In vitro suppression assays were carried out in RPMI/10% FCS in 96-well V-bottom plates (Costar, Corning, NY) with 2.5 × 104 CD4+CD25− responder cells, titrated amounts of FACS-sorted CD4+CD39+ and CD4+CD39− cells and 5 × 104 irradiated (3000 rad) antigen-presenting cells (APCs) that were T-cell-depleted with α-CD3 (T3D). Stimulation was carried out with plate-bound α-CD3 (UCTH1; 10 μg/mL). After 72 hours at 37°C, 0.037 MBq/well (1 μCi/well) 3H-thymidine was added for an additional 6 to 12 hours. Proliferation was determined using a beta-plate reader (Wallac, Gaithersburg, MD). Percentage of inhibition was calculated by [1 − (proliferation (responder and suppressor)/proliferation(responder only)] × 100.
Results
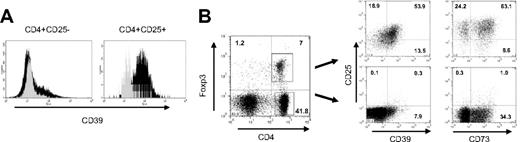
CD25, the α-chain of the IL-2 receptor, has recently gained enormous interest as a marker molecule. On CD4+ non-Treg cells it is induced only upon activation, but it is constitutively expressed by Treg cells. When analyzing mouse lymphocytes isolated from naive mice, we noticed, however, that the same applies also for the ectoenzyme CD39. Only a small fraction of CD4+CD25− T cells expressed CD39, while virtually all CD25+ T cells stained positive for the marker (Figure 1A). Thus, similar to CD25, also CD39 may serve as specific marker for mouse Treg cells.
Expression of CD39 on mouse Treg cells. (A) CD39 is constitutively expressed on CD4+CD25+ T cells. Lymphocytes isolated from naive mice were analyzed by FACS. CD39 expression is shown for CD4+CD25− (left panel) and CD4+CD25+ subsets (right panel). Gray histogram represents isotype control (α-CD39L1, not expressed by lymphocytes). (B) CD39 is expressed by CD4+Foxp3+ cells. Mouse lymphocytes were stained for intracellular Foxp3 and gated for CD4+Foxp3− and CD4+Foxp3+ cells (left panel). CD39 and CD73 expression for the 2 subsets are shown in costaining with α-CD25 (right panels). Numbers in end quadrant indicate percentage of total cells.
Expression of CD39 on mouse Treg cells. (A) CD39 is constitutively expressed on CD4+CD25+ T cells. Lymphocytes isolated from naive mice were analyzed by FACS. CD39 expression is shown for CD4+CD25− (left panel) and CD4+CD25+ subsets (right panel). Gray histogram represents isotype control (α-CD39L1, not expressed by lymphocytes). (B) CD39 is expressed by CD4+Foxp3+ cells. Mouse lymphocytes were stained for intracellular Foxp3 and gated for CD4+Foxp3− and CD4+Foxp3+ cells (left panel). CD39 and CD73 expression for the 2 subsets are shown in costaining with α-CD25 (right panels). Numbers in end quadrant indicate percentage of total cells.
Foxp3 is a key transcription factor that largely controls the phenotype and function of Treg cells.26 Expression is restricted only to the CD4+ suppressor cell subset, so that the costaining of mouse lymphocytes with Foxp3 and CD4 clearly separates Foxp3+ Treg cells from CD4+ non-Treg cells (Figure 1B, left panel). Gating on the Foxp3+ subset confirmed that it is indeed the Treg population that expresses CD39 (Figure 1B, right panel). FACS analysis further revealed that most of the Treg cells coexpress the ecto-5′-nucleotidase CD73, which converts AMP, the product of CD39-mediated hydrolysis, further to adenosine, a nucleoside that exhibits direct immunosuppressive effects. Compared with CD39, however, association of CD73 with Foxp3 was not as strict: approximately 60% of Treg cells and 30% of CD4+ non-Treg cells expressed CD73.
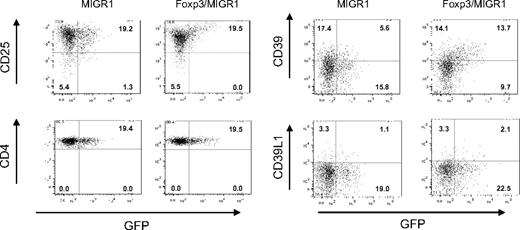
Foxp3 is known to drive the expression of Treg-associated markers such as CD25, CTLA-4, and GITR.26 The apparent cosegregation of CD39 with CD25 suggested that Foxp3 is also responsible for the constitutive expression of the ectonucleotidase on mouse Treg cells. Retroviral transduction of CD4+CD25− lymphocytes with Foxp3 confirmed that the transcription factor indeed induces the expression of CD39 (Figure 2). In line with a previous publication,26 up-regulation was observed for CD25 but not for CD4 when using constructs in which Foxp3 expression was linked to green fluorescent protein (GFP) by an IRES sequence (Figure 2, left panel). Up-regulation was also observed for CD39 (Figure 2, right panel). The induction correlated in an almost linear fashion to the expression of Foxp3, while no changes in the level of CD73 were observed (O.R., unpublished data, August 2006). No signal was detected with control antibodies directed against CD39L1, a member of the CD39 family not expressed by lymphocytes, demonstrating specificity of the CD39 staining (Figure 2, right panel).
Induction of CD39 by Foxp3. CD4+CD25− mouse lymphocytes were infected with a retroviral construct encoding for Foxp3 and GFP (Foxp3/MIGR1) or a control construct encoding only GFP (MIGR1). At 7 days after infection, cells were stained with α-CD25, α-CD4, α-CD39, and α-CD39L1. Staining is shown versus GFP fluorescence, indicating infected cells. Numbers in end quadrant indicate percentage of total cells.
Induction of CD39 by Foxp3. CD4+CD25− mouse lymphocytes were infected with a retroviral construct encoding for Foxp3 and GFP (Foxp3/MIGR1) or a control construct encoding only GFP (MIGR1). At 7 days after infection, cells were stained with α-CD25, α-CD4, α-CD39, and α-CD39L1. Staining is shown versus GFP fluorescence, indicating infected cells. Numbers in end quadrant indicate percentage of total cells.
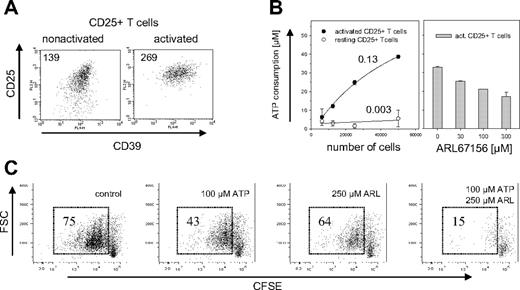
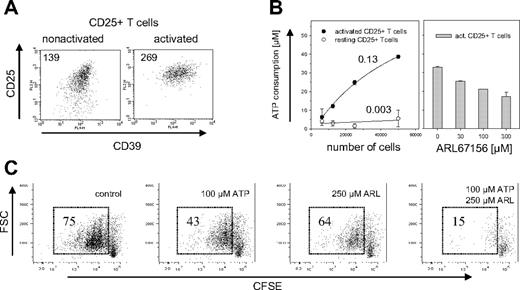
CD4+CD25− T cells usually do not express CD39 on their surface, but up-regulate its expression upon TCR ligation (A.S., unpublished data, November 2005). Mouse Treg cells express CD39 constitutively, so that activation has only a limited effect on CD39 expression levels (Figure 3A). Compared with nonactivated Treg cells, activated Treg cells increased their CD39 expression by less than a factor of 2. Nevertheless, freshly isolated Treg cells showed almost no hydrolysis of extracellular ATP (0.003 fmol/second/cell), whereas activated Treg cells consumed 0.13 fmol/second ATP per cell, a more than 40-fold increase compared with nonactivated cells (Figure 3B, left panel). ATP hydrolysis was dose-dependently inhibited by the ecto-ATPase inhibitor ARL67156.27,28
ATPase activity of mouse CD39+ Treg cells. (A) Expression of CD39 on activated and nonactivated Treg cells. Cell surface expression of CD39 is shown for CD4+CD25+ cells that were freshly isolated (left panel) or had been activated for 3d with α-CD3 and α-CD28 (right panel). Staining of CD39 is shown versus α-CD25, the mean fluorescence intensity (MFI) of CD39 is indicated. (B) ATP hydrolysis by activated and nonactivated CD39+ Treg cells. The indicated number of activated (●) or nonactivated (resting) CD25+CD4+ cells (○) were incubated for 10 minutes with 50 μM ATP (left panel). Numbers indicate the ATP consumption in fmol per second per cell and were calculated by determining the starting slope using a hyperbola regression. The experiment on the right panel was carried out in the same way except that 50 000 activated CD4+CD25+ T cells were incubated with ATP in the presence of indicated amounts of the ecto-ATPase inhibitor ARL67156. The ATP consumption was determined by a luminometric assay. Error bars indicate standard deviation of data points. (C) Abrogation of ATP-induced inhibition of proliferation. CD4+CD25+ T cells were labeled with CFSE and activated for 3d in the presence of 100 μM ATP, 250 μM ARL67156, 100 μM ATP/250 μM ARL67156, or without these reagents. Proliferation of the cells was determined by FACS analysis after gating on the PI-negative cells; numbers indicate percentage of total cells.
ATPase activity of mouse CD39+ Treg cells. (A) Expression of CD39 on activated and nonactivated Treg cells. Cell surface expression of CD39 is shown for CD4+CD25+ cells that were freshly isolated (left panel) or had been activated for 3d with α-CD3 and α-CD28 (right panel). Staining of CD39 is shown versus α-CD25, the mean fluorescence intensity (MFI) of CD39 is indicated. (B) ATP hydrolysis by activated and nonactivated CD39+ Treg cells. The indicated number of activated (●) or nonactivated (resting) CD25+CD4+ cells (○) were incubated for 10 minutes with 50 μM ATP (left panel). Numbers indicate the ATP consumption in fmol per second per cell and were calculated by determining the starting slope using a hyperbola regression. The experiment on the right panel was carried out in the same way except that 50 000 activated CD4+CD25+ T cells were incubated with ATP in the presence of indicated amounts of the ecto-ATPase inhibitor ARL67156. The ATP consumption was determined by a luminometric assay. Error bars indicate standard deviation of data points. (C) Abrogation of ATP-induced inhibition of proliferation. CD4+CD25+ T cells were labeled with CFSE and activated for 3d in the presence of 100 μM ATP, 250 μM ARL67156, 100 μM ATP/250 μM ARL67156, or without these reagents. Proliferation of the cells was determined by FACS analysis after gating on the PI-negative cells; numbers indicate percentage of total cells.
To investigate the functional role of CD39, murine Treg cells were exposed to elevated concentrations of extracellular ATP. High doses of ATP are toxic for most cells, where it seems to induce both necrosis as well as apoptosis.29 Treg cells are particularly sensitive to ATP.30 To determine whether the catalytic activity of CD39 decreases ATP sensitivity, the influence of ATP on the proliferative response of Treg cells was determined in the absence and in the presence of ARL67156. FACS analysis of CD25+ T cells labeled with CFSE prior to activation revealed that the addition of ARL67156 indeed dramatically decreased their proliferative capacity (Figure 3C). In addition to the apparent cytostatic effect, blockade of ectonucleotidases slightly increased the cytotoxic effect of ATP, indicated by an increase in the number of PI positive cells (Figure S1, available at the Blood website; see the Supplemental Materials link at the top of the online article). Thus, the ATPase activity of CD39 was required to lower the extracellular ATP concentration below the toxic threshold.
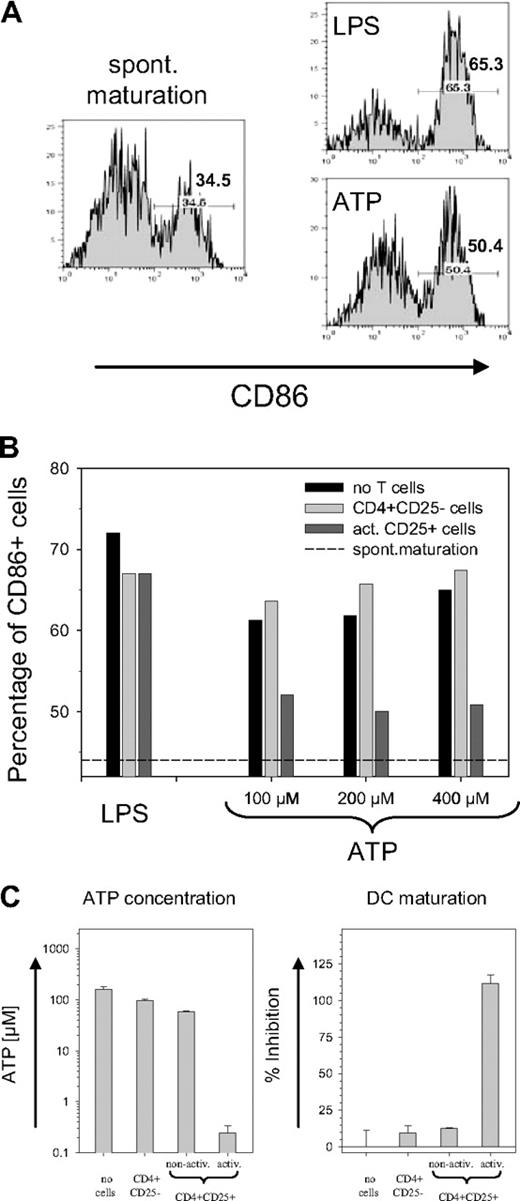
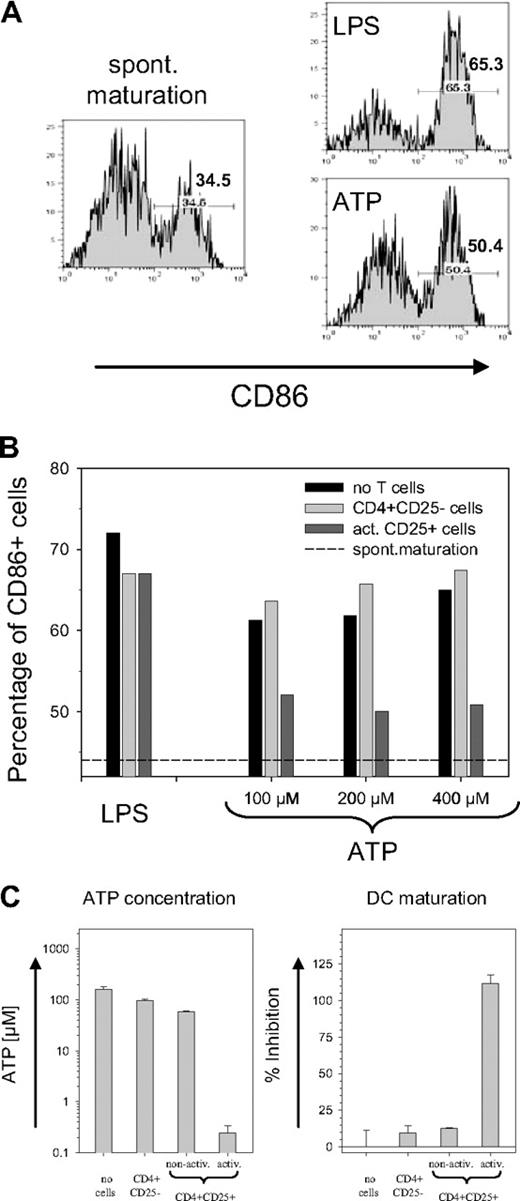
Although the activity of CD39 potentially allows Treg cells to tolerate high ATP levels, the removal of ATP by Treg cells should also have a bystander effect on other cells. Immature DCs exposed to ATP have been reported to up-regulate the maturation marker CD86.4 As shown in Figure 4A, an increase of CD86 was observed in response to both LPS and ATP. To establish a functional link with the CD39 ectoenzyme on the Treg surface, the ATP-containing medium was briefly exposed to CD4+CD25− T cells or to activated CD39-expressing Treg cells prior to the incubation with DCs. While pre-exposure to CD25− T cells resulted in a slight increase in the number of CD86+ DCs, preexposure to activated Treg cells strongly reduced the ATP-driven DC maturation (Figure 4B). No difference was observed with LPS-containing medium, confirming that the removal of extracellular ATP prevented the maturation of DCs. Notably, the effect was evident only with activated and not with nonactivated Treg cells (Figure 4C). Freshly isolated CD4+CD25+ cells removed only slightly more ATP than nonactivated CD4+CD25− cells, which mostly lack the CD39 ectoenzyme. Activated CD4+CD25+ cells, in contrast, hydrolyzed most of the ATP during exposure, and the ATP concentration decreased from 200 μM to less than 0.3 μM (Figure 4C, left panel). As a consequence, activated CD4+CD25+ cells effectively prevented the generation of CD86+ DCs, while only a slight inhibition of ATP-driven DC maturation was detected with nonactivated CD4+CD25+ cells (Figure 4C, right panel).
Inhibition on ATP-driven maturation of mouse DCs. (A) ATP-driven DC maturation. Immature bone marrow-derived mouse DCs were incubated for 24 hours without any activator (left panel) or with 10 ng/mL LPS or 200 μM ATP (right panels). Histograms are shown for the maturation marker CD86 of cells gated on CD11c; numbers indicate the percentage of CD86+ cells. (B) Prevention of ATP-driven maturation of mouse DCs by CD39+ Treg cells. LPS or ATP containing medium was used either directly (■) or after 20 minutes of pre-exposure to freshly isolated CD4+CD25− cells (▒) or to activated CD4+CD25+ cells (▓) to incubate DC cultures. Bars represent the percentage of CD86+ cells (gated on CD11c); dashed line represents percentage of CD86+ cells obtained by spontaneous maturation. (C) Treg activation is required for inhibition of ATP-induced maturation. The experiment was carried out as in Figure 4B except that in addition to freshly isolated CD4+CD25− cells and activated CD4+CD25+ cells, nonactivated CD4+CD25+ cells were also used. Left panel shows the ATP concentration remaining after 25 minutes of exposure of the ATP containing medium (200 μM) to the T cells; the right panel shows the inhibition of the ATP-driven DC maturation. Inhibition of maturation is expressed as a percentage and is determined after subtracting the spontaneous maturation in reference to the maximal increase of CD86+ cells after ATP exposure. Error bars indicate standard deviation of data points.
Inhibition on ATP-driven maturation of mouse DCs. (A) ATP-driven DC maturation. Immature bone marrow-derived mouse DCs were incubated for 24 hours without any activator (left panel) or with 10 ng/mL LPS or 200 μM ATP (right panels). Histograms are shown for the maturation marker CD86 of cells gated on CD11c; numbers indicate the percentage of CD86+ cells. (B) Prevention of ATP-driven maturation of mouse DCs by CD39+ Treg cells. LPS or ATP containing medium was used either directly (■) or after 20 minutes of pre-exposure to freshly isolated CD4+CD25− cells (▒) or to activated CD4+CD25+ cells (▓) to incubate DC cultures. Bars represent the percentage of CD86+ cells (gated on CD11c); dashed line represents percentage of CD86+ cells obtained by spontaneous maturation. (C) Treg activation is required for inhibition of ATP-induced maturation. The experiment was carried out as in Figure 4B except that in addition to freshly isolated CD4+CD25− cells and activated CD4+CD25+ cells, nonactivated CD4+CD25+ cells were also used. Left panel shows the ATP concentration remaining after 25 minutes of exposure of the ATP containing medium (200 μM) to the T cells; the right panel shows the inhibition of the ATP-driven DC maturation. Inhibition of maturation is expressed as a percentage and is determined after subtracting the spontaneous maturation in reference to the maximal increase of CD86+ cells after ATP exposure. Error bars indicate standard deviation of data points.
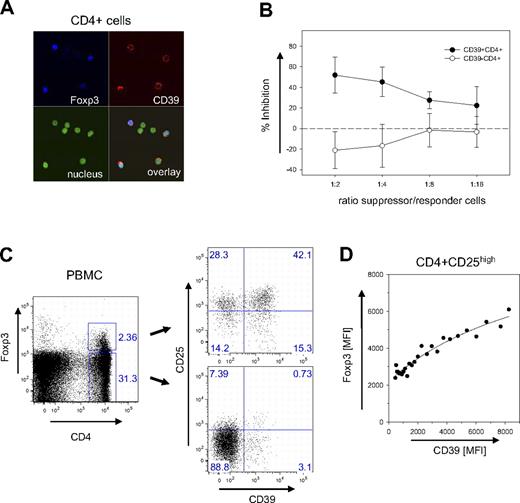
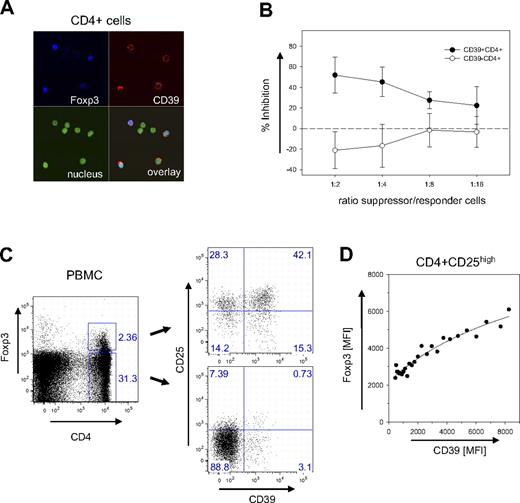
While mouse and human Treg cells share most of their features, some differences exist regarding subset specificities and marker molecules. Confocal microscopy of human CD4+ cells indicated, however, that also in human CD39 is a surface marker expressed almost exclusively by Foxp3+ cells (Figure 5A). Suppression assays confirmed that CD4+ cells sorted only based on CD39 expression are potent inhibitors of cell proliferation (Figure 5B), efficiently preventing the expansion of CD4+CD25− responder cells. In contrast, CD4+CD39− cells proliferated extensively along with the responder cells. These data strongly suggest that CD39 is a key marker also of human Treg cells.
Expression of CD39 on human Treg cells. (A) Confocal image of CD39+Foxp3+cells. CD4+ cells of human PBMCs were stained for CD39 and Foxp3 and analyzed by laser confocal microscopy (see “Patients, materials and methods; Confocal microscopy” for image acquisition details). Nuclear staining was carried out with DAPI. (B) Suppressive capacity of CD4+CD39+ cells. The inhibitory activity of FACS-sorted populations of CD4+CD39+ (●) and CD4+CD39− (○) was determined with CD4+CD25− responder cells in a 3H-thymidine incorporation assay. Inhibition of proliferation is expressed as a percentage; the ratio between suppressor and responder cells is indicated. Error bars indicate standard deviation of data points. (C) CD39+ and CD39− subpopulations within the Foxp3+ subset. PBMCs were gated as indicated into CD4+Foxp3+ and CD4+Foxp3− subsets (left panel). The CD39 expression of the 2 subsets is shown in a double-staining with α-CD25 (right panel). (D) Correlation between CD39 and Foxp3 expression. The mean fluorescence intensities of CD39 and Foxp3 are shown for the subset of CD4+CD25high cells. MFI values of data points were determined from the FACS data of a single donor by scanning the CD39 expression with a narrow gate for Foxp3 using the FlowJo analysis software. Line represents a hyperbola regression calculated by the Sigmaplot software package.
Expression of CD39 on human Treg cells. (A) Confocal image of CD39+Foxp3+cells. CD4+ cells of human PBMCs were stained for CD39 and Foxp3 and analyzed by laser confocal microscopy (see “Patients, materials and methods; Confocal microscopy” for image acquisition details). Nuclear staining was carried out with DAPI. (B) Suppressive capacity of CD4+CD39+ cells. The inhibitory activity of FACS-sorted populations of CD4+CD39+ (●) and CD4+CD39− (○) was determined with CD4+CD25− responder cells in a 3H-thymidine incorporation assay. Inhibition of proliferation is expressed as a percentage; the ratio between suppressor and responder cells is indicated. Error bars indicate standard deviation of data points. (C) CD39+ and CD39− subpopulations within the Foxp3+ subset. PBMCs were gated as indicated into CD4+Foxp3+ and CD4+Foxp3− subsets (left panel). The CD39 expression of the 2 subsets is shown in a double-staining with α-CD25 (right panel). (D) Correlation between CD39 and Foxp3 expression. The mean fluorescence intensities of CD39 and Foxp3 are shown for the subset of CD4+CD25high cells. MFI values of data points were determined from the FACS data of a single donor by scanning the CD39 expression with a narrow gate for Foxp3 using the FlowJo analysis software. Line represents a hyperbola regression calculated by the Sigmaplot software package.
FACS analysis confirmed that CD39 is expressed mostly by Foxp3+ cells. Less than 4% of the Foxp3− cells expressed the marker (Figure 5C). In contrast to mice, however, not all Foxp3+ cells are also positive for CD39. In the example shown in Figure 5C, about 40% of the human Foxp3+ cells did not express the marker. CD39+ cells, however, expressed high levels of Foxp3. Although in CD39− Treg cells the mean fluorescence intensity (MFI) for Foxp3 was below 2500, it increased to more than 6000 on cells stained brightly for CD39+. A detailed analysis revealed an almost linear correlation between the expression levels of the 2 markers (Figure 5D).
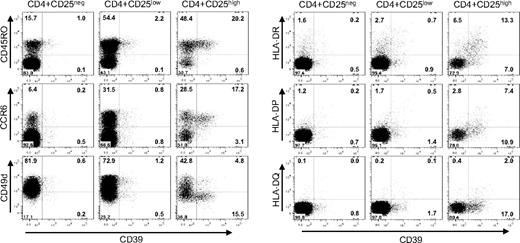
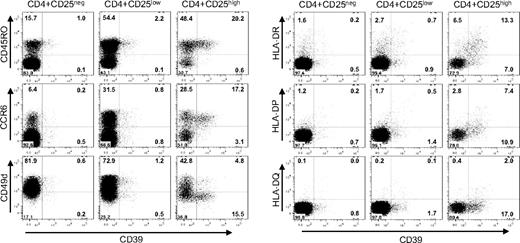
To further characterize the distribution of CD39+ cells among the CD4+ population, a detailed phenotypic analysis was performed (Figure 6). In humans, only cells characterized by a high expression of CD25 (CD25high) are endowed with immunosuppressive abilities,31 while naive CD4+ cells do not express CD25, and effector and memory CD4+ cells express low amounts of the marker (CD25low). FACS analysis of the CD4+ CD25high cells revealed that in humans CD39 expression is confined to a subset of TREM cells.32 CD39+ cells coexpress CD45RO,33 a human memory marker, and CCR6, a chemokine receptor associated with antigen-experienced effector/memory cells.32,34 Moreover, CD39+ Treg cells lack expression of the α-chain of the VLA-4 integrin (CD49d) and express class II MHC molecules (HLA-DR, HLA-DP, and, to a lesser extent, also HLA-DQ). HLA-DR has recently been described as a marker of a mature Treg population acting through early contact-dependent inhibition,35 and the absence of CD49d is indicative of a Treg subset with enhanced suppressive capacity (G.B. et al, submitted manuscript). In contrast to mice, where CD39 is ubiquitously expressed among Treg cells, in humans the expression of CD39 is confined to a subset of TREM cells representing activated effector/memory-like suppressor cells.
CD39+ is expressed by a subset of CD45RO+CCR6+ TREM cells. Cell-surface expression is shown for PBMCs gated for CD4+CD25high, CD4+CD25low and CD4+CD25neg cells. Cells were stained with α-CD39 and counterstained with α-CD45RO, α-CCR6, α-CD49d, and with antibodies directed against the class II MHC molecules HLA-DR, HLA-DQ, and HLA-DP. Numbers in each quadrant indicate percentage of total cells.
CD39+ is expressed by a subset of CD45RO+CCR6+ TREM cells. Cell-surface expression is shown for PBMCs gated for CD4+CD25high, CD4+CD25low and CD4+CD25neg cells. Cells were stained with α-CD39 and counterstained with α-CD45RO, α-CCR6, α-CD49d, and with antibodies directed against the class II MHC molecules HLA-DR, HLA-DQ, and HLA-DP. Numbers in each quadrant indicate percentage of total cells.
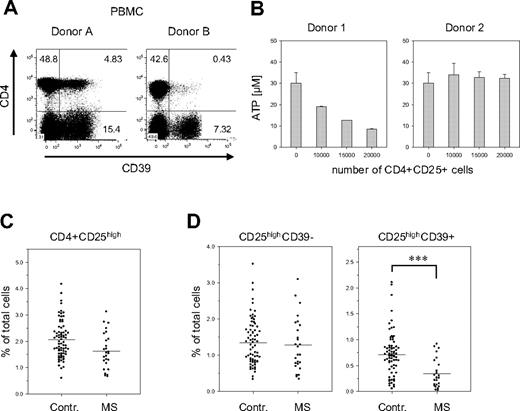
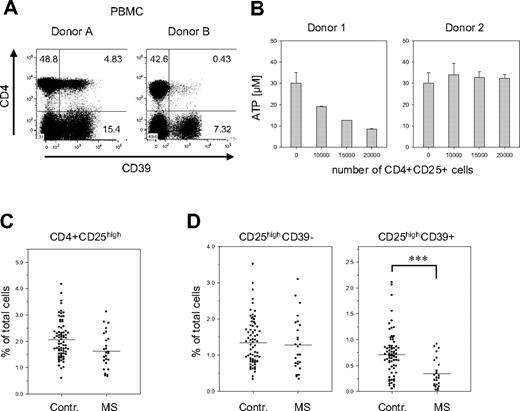
When analyzing the PBMCs of healthy donors, we noticed that the number of CD39+ cells greatly varied between donors. These variations were particularly striking in the subset of CD4+ Treg cells: their fraction ranged from less than 2% to almost 60% of the CD4+ CD25high population. In the examples shown in Figure 7A, donor B has less than 10% of the number of CD4+CD39+ Treg than donor 1, but still about 50% of CD4−CD39+ cells. These differences were also reflected in the capacity to hydrolyze extracellular ATP. Treg cells isolated from donors containing high numbers of CD39+ Treg cells efficiently hydrolyzed ATP, while Treg cells obtained from donors with low numbers of CD39+ cells showed reduced activity (Figure 7B).
Reduced numbers of CD39+ Treg cells in PBMCs of patients with MS. (A) Donor-specific variations in the number of the CD39+ Tregs. PBMCs of 2 healthy donors were stained for CD39 and CD4 expression. (B) Donor-specific ATP-hydrolysis capacity. CD4+CD25high cells isolated from individuals containing either high numbers of CD39+ Tregs (approximately 40%; donor 1) or low numbers of CD39+ Tregs (< 3%; donor 2) were isolated and incubated with ATP as described in Figure 3B. (C) Fraction of CD25high Treg cells in PBMCs of patients with MS. PBMCs of 74 healthy controls and 26 patients with MS were analyzed by FACS for the expression of CD4 and CD25. The relative fraction of CD4+CD25high cells on total PBMCs is shown as a scatterplot. (D) Reduced numbers of CD39+ Treg cells in patients with MS. The cells shown in panel C were also analyzed for CD39 expression. The relative fraction on total PBMCs is shown for the CD4+CD25highCD39− (left panel) and the CD4+CD25highCD39+ subsets (right panel). Asterisks indicate P < .001.
Reduced numbers of CD39+ Treg cells in PBMCs of patients with MS. (A) Donor-specific variations in the number of the CD39+ Tregs. PBMCs of 2 healthy donors were stained for CD39 and CD4 expression. (B) Donor-specific ATP-hydrolysis capacity. CD4+CD25high cells isolated from individuals containing either high numbers of CD39+ Tregs (approximately 40%; donor 1) or low numbers of CD39+ Tregs (< 3%; donor 2) were isolated and incubated with ATP as described in Figure 3B. (C) Fraction of CD25high Treg cells in PBMCs of patients with MS. PBMCs of 74 healthy controls and 26 patients with MS were analyzed by FACS for the expression of CD4 and CD25. The relative fraction of CD4+CD25high cells on total PBMCs is shown as a scatterplot. (D) Reduced numbers of CD39+ Treg cells in patients with MS. The cells shown in panel C were also analyzed for CD39 expression. The relative fraction on total PBMCs is shown for the CD4+CD25highCD39− (left panel) and the CD4+CD25highCD39+ subsets (right panel). Asterisks indicate P < .001.
The functional status of Treg cells has recently been implicated in the control of autoimmune diseases. In particular, patients affected by MS show a significant decrease in the suppressive capacity of CD4+CD25high cells,31 although most studies did not reveal any significant changes in their total number.31,36,37 In line with these reports, we observed only a slight reduction of the fraction of CD4+CD25high T cells in the blood of patients affected by the remitting/relapsing form of MS (Figure 7C). The striking fluctuations in the amount of CD39 expressing Treg cells among individuals, however, suggested that CD39 in combination with CD25 may be a more appropriate marker than CD25 alone. A much clearer picture, in fact, emerged when the CD25high Treg cells were further discriminated based on CD39 expression (Figure 7D). In patients with MS, the number of CD39+CD25high cells was less than half of the number in the control: their fraction decreased from 0.71% (control) to 0.34% (MS) (P < .001). Notably, no significant differences were observed for CD39− Treg cells (control, 1.4%; MS, 1.3%), indicating that the shifts are solely confined to the CD39+ Treg subset. This reduction was dependent on the clinical status of the patient, since it was evident only in untreated patients in the stable phase of the disease. Patients treated with immunomodulatory therapies or patients undergoing clinical relapses show numbers of CD39+ Treg cells comparable with those of healthy donors (D.D.M., A.D., O.R., K.F., G.B., L.B., manuscript in preparation).
Discussion
Extracellular ATP functions as a danger signal released by damaged cells as chemoattractant for lymphocytes, activator of proinflammatory responses, and inducer of local pain.19 The ectoenzyme CD39 removes the nucleotide by hydrolytic cleavage and therefore exhibits immediate immunomodulatory influences on its proximal environment. In mice we could establish that its expression is driven directly by Foxp3. It is therefore expressed on virtually all Foxp3+ suppressor cells, representing a novel cell surface marker of Treg cells.
Although CD39 is ubiquitously expressed by mouse Treg cells, their ATPase activity strictly depends on the activation status. Although ARL67156 inhibits CD39 as well as other e-NTPDases such as NTPDase3,27,28 the increase on activated cells is likely to be linked to changes in the catalytic activity of CD39. It is known that posttranslational modifications of CD39 determine targeting to caveolae,38 recruitment into cholesterol-rich microdomains39 and the association with the regulatory Ran-binding protein RanBPM.40 On mouse Treg cells, hydrolysis of ATP was observed only upon TCR activation, while on nonactivated Treg cells, the enzyme was inactive. In the context of other cell systems such as endothelial cells, CD39 recruitment into lipid rafts was found to strongly influence the activity of the enzyme.39 On mouse Treg cells, these mechanisms seem to synchronize ATP hydrolysis with Treg activation. TCR ligation is known to “arm” Treg cells, evident in a significantly increased suppressive capacity that is detectable even after fixing the preactivated Treg cells with paraformaldehyde.41 Only in an activated state should Tregs act as suppressors, and only in this situation should the hydrolytic activity of the ectoenzyme be required. Accordingly, an inhibition of ATP-driven DC maturation was evident with activated but not with nonactivated Treg cells. To what extent this applies also to human Treg cells, where CD39 expression is confined to a subset of activated TREM cells, remains to be determined.
Removal of extracellular ATP by CD39 may allow the Treg cell to enter inflamed regions. Leakage of ATP from damaged cells in this area could produce elevated levels that otherwise would induce P2 receptor-mediated death of the Treg cell. Even more important, however, may be the quenching of ATP-driven proinflammatory processes on other cells. A previous study42 has shown that the expression of CD39 can modulate the ATP-induced release of the endogenous pyrogen of IL-1β on endothelial cells. As shown here, also the maturation of DCs can be mediated by CD39 expressed by Treg cells. Strength and direction of an immune response largely depends on the quality of the APCs triggering the T-cell response. By adjusting the extracellular ATP levels, Treg cells can influence the functional state of the DCs so that CD39 may represent another important element involved in immune regulation.
While the removal of ATP by CD39 per se has obvious anti-inflammatory effects, these might be further amplified by the generation of adenosine. The nucleoside exhibits multiple suppressive and antiproliferative activities21 and can be generated by CD39 in concert with the 5′-ecto-nucleotidase CD73, which dephosphorylates the CD39 product AMP. CD4+ cells isolated from mice that lack the adenosine-specific A2A receptor cannot be controlled to prevent colitis.43 Moreover, CD73 on Treg cells has been shown to suppress proliferation and cytokine secretion of T helper 1 (Th1) and Th2 cells.44 Hence, the activity of CD39 has apparently 2 synergistic anti-inflammatory effects: the removal of ATP and the generation of the immunosuppressive nucleoside adenosine.
In contrast to mice, CD39 expression in man is restricted to a subset of activated TREM cells. Nevertheless, in humans also the conversion of extracellular ATP by CD39 seems to play an important role in the balance of immune reactions. In mice, we have previously shown that CCR6+ TREM cells represent a population of Treg cells equipped for the control of immune reactions in inflamed tissues.32 In the same study, we reported a redistribution of this specific Treg subset between blood and the inflamed CNS during the course of experimental autoimmune encephalomyelitis (EAE), evident in reduced numbers of TREM cells in the blood of diseased mice. Using CD39 as marker of activated human TREM cells, the same phenomenon is apparently also evident in MS.
As shown here, CD39 expression is highly correlated with elevated levels of Foxp3. Moreover, human CD49d− TREM cells, a subset largely overlapping with the CD39 Treg population, are particularly potent immunosuppressors in vitro (G.B. et al, submitted manuscript). Patients with MS are characterized by a reduced suppressive capacity of their Treg cells,31 and while apparently no major shifts in the total CD25high Treg populations have been observed, decreased Foxp3 levels in patients with MS have been reported.45,46 The reduced number of CD39+ Treg cells in patients with MS reported in this study might well account for both observations. Decreased Foxp3 levels combined with a lack of suppressive capacity of Treg cells was also reported for patients suffering of active systemic lupus erythematosus (SLE).47 Also, here the total number of CD4+CD25high cells remained unchanged, suggesting that the characteristic shift in the CD39+ Treg subset composition may not be restricted to MS.
The striking reduction in the number of CD39+ Treg cells observed in the blood of MS patients may be of diagnostic value, as it also suggests a specific role of this subset in the control of the disease. Whether CD39 is directly involved is not yet clear. Preliminary data indicate that CD39+ Treg cells from patients with MS efficiently hydrolyze ATP, so that reduced numbers of these cells should result in lower hydrolytic activities in MS patients. This correlation, however, needs to be established in a large cohort of patients and in different phases of the disease. Moreover, CD39−/− mice18 should be used in EAE studies to determine the immediate impact of the ectoenzyme on disease progression.
MS is a T-cell-mediated autoimmune disease, and patients with the relapsing-remitting form of the disease face recurrent activation of autoreactive T cells that leads to clinical relapses. CD39+ Treg cells are present at higher frequencies in the blood of healthy individuals; therefore, in the common occurrence of infections and of minor tissue damage, extracellular ATP in excess may be hydrolyzed, preventing DC maturation and proliferation of autoreactive T cells, thus tempering unwanted immune responses. Analog to murine EAE, in human MS a specific Treg subset rather than the global population of CD25high cells is also affected. It remains to be determined, though, whether a direct genetic link or a tissue redistribution of the CD39+ Treg subset account for the reduction of these cells in patients with MS.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank S. Giering for help in preparing the manuscript, S. Sakaguchi for providing retroviral vectors, and J. Sevigny and S.C. Robson for antibodies.
This work was supported by SFB 650 network grant of the Deutsche Forschungsgemeinschaft (DFG) (O.R. and K.F.). A.S. was supported by a stipend awarded by a graduate student program GRK 1258/1 of the DFG. S.H. was supported by a grant awarded by a PhD stipend program of the Max-Delbrück-Center. G.B. was supported by a grant from the Italian MS Society (FISM). L.B. and D.C. were supported by the Italian Ministry of Research (FIRB) and by the Italian Ministry of Health (Progetto Finalizzato).
Authorship
Contribution: O.R. and K.F. designed research and wrote the paper; G.B. designed and performed research; L.B. designed research; M.K., D.D.M., A.S., A.D., R.G., and S.H. performed research; D.C., G.B., M.L.D.A., and P.M.R. provided essential material.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kirsten Falk or Olaf Rötzschke, Max-Delbrück-Center for Molecular Medicine (MDC), Robert-Rössle-Str. 10, D-13125 Berlin, Germany; e-mail: falk@mdc-berlin.de or roetzsch@mdc-berlin.de.