Abstract
In 1865, Armand Trousseau noted that unexpected or migratory thrombophlebitis could be a forewarning of an occult visceral malignancy. An analysis by Sack and colleagues in 1977 extended the term Trousseau's syndrome to include chronic disseminated intravascular coagulopathy associated with microangiopathy, verrucous endocarditis, and arterial emboli in patients with cancer, often occurring with mucin-positive carcinomas. In recent times the term has been ascribed to various clinical situations, ranging all the way from these classic descriptions to any kind of coagulopathy occurring in the setting of any kind of malignancy. These multiple definitions of Trousseau's syndrome are partly the consequence of multiple pathophysiologic mechanisms that apparently contribute to the hypercoagulability associated with cancer. Even the classic syndrome probably represents a spectrum of disorders, ranging from exaggerated fluid-phased thrombosis dependent on prothrombotic agents such as tissue factor to a platelet- and endotheliumum-based selectin-dependent microangiopathy associated with mucin-producing carcinomas, along with thrombin and fibrin production. Also considered here are recent hypotheses about genetic pathways within tumor cells that might trigger these thrombotic phenomena, and the reasons why therapy with heparins of various kinds remain the preferred treatment, probably because of their salutary actions on several of the proposed pathologic mechanisms.
Introduction
Trousseau's syndrome is well known to clinicians, partly because Armand Trousseau not only described it in 18651 but also diagnosed the syndrome on himself 2 years later, succumbing shortly thereafter to a gastric cancer.2,3 The association between cancer and excessive blood coagulation remains well-recognized, forming the basis of many reviews, monographs, symposia, and international conferences. Most of these begin by mentioning Trousseau and his eponymous syndrome. Trousseau made an astute clinical observation, noting that some patients who presented with unexpected, unusual, or migratory thromboses later manifested a visceral malignancy. This description was refined and extended over the years, culminating in a classic review by Sack et al,4 in which Trousseau's syndrome was reported as being frequently associated with chronic disseminated intravascular coagulopathy, platelet-rich microthrombi, microangiopathic hemolytic anemia, verrucous endocarditis, and thromboembolic problems related to these processes. In more recent times, many patients are diagnosed with Trousseau's syndrome even if they do not manifest these classic features, and the definition has included those presenting primarily with uncomplicated lower limb deep venous thrombosis. The term is sometimes even applied to patients who already have an advanced malignancy and then develop some form of thrombosis. Although similar mechanisms may be operating, such advanced cases often have other reasons for a thrombotic tendency, such as immobility, dehydration, mechanical compression of veins, infectious processes, chemotherapy, and so forth
What is Trousseau's syndrome?
Despite its frequent mention in reviews,4–17 the term Trousseau's syndrome is not in the MeSH headings of Medline or PubMed. Literature searching is confounded by the insistence of some journal editors on using “Trousseau syndrome.”18 Thus, searching Pubmed for Trousseau's syndrome and Trousseau syndrome yields mostly nonoverlapping hits. Such searches, along with review of web-based sources, yield definitions of Trousseau's syndrome ranging from “occurrence of thrombophlebitis migrans with visceral cancer” and, “spontaneous recurrent or migratory venous thromboses and/or arterial emboli caused by nonbacterial thrombotic endocarditis in a patient with malignancy” all the way to simply “carcinoma-induced coagulopathy,” “hypercoagulability syndrome associated with cancer,” “malignancy-related thromboembolism,” “idiopathic thromboembolism associated with cancer,” and “malignancy-related hypercoagulability.” On the basis of the actual history, it seems reasonable to restrict use of Trousseau's syndrome to unexplained thrombotic events that precede the diagnosis of an occult visceral malignancy or appear concomitantly with the tumor. As discussed in this review, even this restricted definition encompasses a spectrum of disorders in which multiple overlapping mechanisms are probably involved.
Early theories about mechanisms of Trousseau's syndrome
When thromboses occur in the setting of occult carcinomas, it is reasonable to speculate that tumor products entering the bloodstream are primarily responsible. The association of the syndrome with mucin-producing carcinomas initially suggested that mucins were the underlying trigger.19 Mucins are highly glycosylated secretory products of epithelial cells that become aberrantly glycosylated in carcinomas and then are inappropriately secreted into the bloodstream.20–24 However, not all cases of Trousseau's syndrome are associated with mucin-producing carcinomas.4 Furthermore, although studies of experimental mucin injection into animals were encouraging,19,25 they were probably confounded because mucins are large sticky molecules, making it difficult to free preparations from contaminating cytokines or other bioactive agents, particularly tissue factor (TF), a primary cellular initiator of fluid-phase blood coagulation.26 Meanwhile, TF itself was also considered a cause of Trousseau's syndrome27 and found at high concentrations in many malignancies,28,29 including instances when the primary tumor was extremely small and yet able to induce major systemic thromboses.26 Also described was a cysteine proteinase from carcinoma extracts, which directly activated factor X.30–32 Such findings suggested that activators of fluid-phase coagulation were the key to the pathogenesis.9–17 This review concludes that Trousseau's syndrome is actually a spectrum of disorders and considers additional molecular mechanisms that have more recently pointed to pathways for its initiation. This is done recognizing how little we still understand about this syndrome, and yet how important it is to seek a better understanding of it.
Tissue factor
In addition to cases of Trousseau's syndrome involving carcinomas rich in TF, elevated TF expression by the associated angiogenic endothelium has been reported. Activated oncogenes (K-ras, EGFR, PML-RARA, and MET) or inactivated tumor suppressors (eg, p53 or PTEN) also lead to an induction in TF levels and activity, which is postulated to promote not only hypercoagulability but also tumor aggressiveness and angiogenesis.14–16 The thrombin receptor (protease-activated receptor-1) is also up-regulated in cancer cells expressing oncogenic K-ras.33 The question arises as to how the tumor-derived TF becomes exposed to the fluid-phase coagulation system, starting with circulating factor VII. In classic studies, histologic evidence of direct contact between a TF-rich tumor and the bloodstream seem to be associated with Trousseau's syndrome,26 suggesting that the TF might be acting locally. Considering current understanding of TF biology and its membrane-associated nature,15,34,35 TF-containing membrane fragments or microvesicles produced by tumor cells would appear to be a more likely cause of distant thromboses. Although this mechanism remains speculative, earlier studies did show shed plasma membrane vesicles with procoagulant activity derived from syngeneic carcinomas cultured in vitro or grown in ascites form in vivo,36 as well as in the plasma of patients with certain types of leukemia.37 More recently, mouse models have shown that the oncogene and tumor-suppressor gene status of xenograft tumors are determinants of circulating (presumably microvesicle-associated) TF activity in the blood of the immunodeficient mice.15,33 Regardless of the originating mechanisms, it is likely that conversion of factor VII (FVII) to its active form (FVIIa) in complex with TF triggers the production of other coagulation-related proteases, particularly FXa and FIXa. Factor Xa then works with FVa to cleave circulating prothrombin and generate thrombin, which is required not only to generate fibrin and activate platelets but also to facilitate amplification through further generation of other active clotting factors such as FVIIIa and FXIa (Figure 1). Although all these mechanisms involving TF and thrombin seem reasonable, more studies are needed to directly show their role in patients with Trousseau's syndrome.
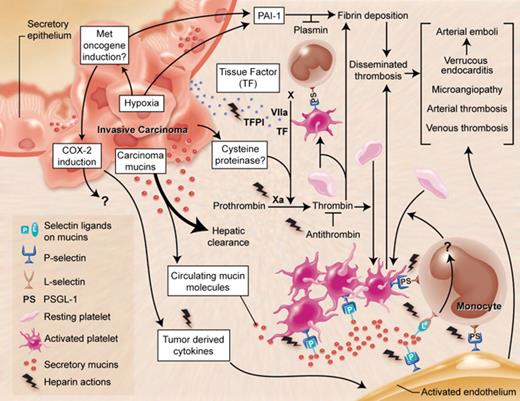
Multiple mechanisms in Trousseau's syndrome. There are multiple overlapping and interacting mechanisms that can explain the increased incidence of thrombosis in patients with malignancies. In Trousseau's syndrome, hypercoagulability manifests even before the diagnosis of the tumor and is probably the result of products arising from the tumor itself. The most common malignancies associated with this syndrome are carcinomas (cancers of epithelial origin) that are often, but not always, mucin producing. This cartoon depicts a mucin-producing carcinoma arising in a hollow organ, which secretes mucins with altered glycans inappropriately into the bloodstream. Although the bulk of these mucins are probably rapidly cleared by the liver, a small fraction are resistant to clearance and can interact with P- and L-selectins, inducing the formation of platelet-rich microthrombi by multiple pathways. Exposure of tissue factor (TF)–rich tumor cell surfaces to the bloodstream or the release of TF-rich microvesicles by the tumor is presumed to induce fibrin formation and platelet aggregation by thrombin production. There is some evidence for a cysteine proteinase secreted by carcinoma cells that can directly activate factor X to generate thrombin. Although interactions of platelet and endothelial P-selectin with P-selectin glycoprotein ligand-1 (PSGL-1) on monocytes may further contribute to these reactions, the exact mechanism by which mucins eventually generate thrombin and fibrin production is unknown. Hypoxic conditions within the tumor, the expression of the MET oncogene, or both might also enhance production of procoagulant factors such as TF and plasminogen activator inhibitor-1 (PAI-1), and tumor-derived inflammatory cytokines may serve to activate endothelial and platelet adhesion molecules. Various combinations of these mechanisms can help explain the unusual, migratory, and exaggerated thrombotic phenomena of Trousseau's syndrome. As indicated in the figure, heparin has potential salutary effects on many of the relevant processes. This may explain why heparin preparations of various kinds are the preferred agent for the management of Trousseau's syndrome.
Multiple mechanisms in Trousseau's syndrome. There are multiple overlapping and interacting mechanisms that can explain the increased incidence of thrombosis in patients with malignancies. In Trousseau's syndrome, hypercoagulability manifests even before the diagnosis of the tumor and is probably the result of products arising from the tumor itself. The most common malignancies associated with this syndrome are carcinomas (cancers of epithelial origin) that are often, but not always, mucin producing. This cartoon depicts a mucin-producing carcinoma arising in a hollow organ, which secretes mucins with altered glycans inappropriately into the bloodstream. Although the bulk of these mucins are probably rapidly cleared by the liver, a small fraction are resistant to clearance and can interact with P- and L-selectins, inducing the formation of platelet-rich microthrombi by multiple pathways. Exposure of tissue factor (TF)–rich tumor cell surfaces to the bloodstream or the release of TF-rich microvesicles by the tumor is presumed to induce fibrin formation and platelet aggregation by thrombin production. There is some evidence for a cysteine proteinase secreted by carcinoma cells that can directly activate factor X to generate thrombin. Although interactions of platelet and endothelial P-selectin with P-selectin glycoprotein ligand-1 (PSGL-1) on monocytes may further contribute to these reactions, the exact mechanism by which mucins eventually generate thrombin and fibrin production is unknown. Hypoxic conditions within the tumor, the expression of the MET oncogene, or both might also enhance production of procoagulant factors such as TF and plasminogen activator inhibitor-1 (PAI-1), and tumor-derived inflammatory cytokines may serve to activate endothelial and platelet adhesion molecules. Various combinations of these mechanisms can help explain the unusual, migratory, and exaggerated thrombotic phenomena of Trousseau's syndrome. As indicated in the figure, heparin has potential salutary effects on many of the relevant processes. This may explain why heparin preparations of various kinds are the preferred agent for the management of Trousseau's syndrome.
Tumor-associated cysteine proteinase
As mentioned above, Falanga and Gordon30 reported a cysteine protease (“cancer procoagulant” or CP) that directly activated factor X in the absence of factor VII, and this activity was later reported in many natural human tumors.38,39 However, there is some question as to whether this is due to contamination with traces of TF/VIIa complexes. A study of such preparations using anti-TF antibodies noted crossreactive proteins,32 and the researchers concluded that “it seems possible that cancer procoagulant preparations contain proteins that have some epitopes similar to the epitopes recognized in tissue factor by antitissue factor monoclonal antibodies (Mabs). However, these proteins do neither have the molecular weight nor the amino acid sequence of tissue factor.” A recent clinical study evaluated prothrombotic markers and their relation to CP concentration in the blood of patients with gastrointestinal adenocarcinomas with or without metastatic disease.40 The data suggested that CP is only a minor risk factor for deep venous thrombosis in such patients. However, more studies of this matter are needed.
Mechanisms involving tumor hypoxia
In 2001, Denko and Giaccia8 proposed that tumor hypoxia represented the “physiologic link between Trousseau's syndrome and tumor metastasis.” Although unaccompanied by direct experimental support, the researchers made a strong case that tumor cells under microenvironmental stress are likely to produce procoagulant and angiogenic factors. They specifically suggested that hypoxia (decreased oxygenation) could increase the expression of genes that facilitate coagulation, including tissue factor and plasminogen activator inhibitor type 1 (PAI-1). These researchers also proposed a link between these prothrombotic processes and metastatic disease, revisiting the well-known observation that the two phenomena share much in common.
Carcinomas mucins
Early in vivo studies of carcinoma mucins19,25 were probably confounded by contamination with other bioactive factors, including TF. Carcinoma mucins are large, heavily glycosylated molecules20–24 and are often carriers of sialylated, fucosylated, sulfated glycans that can act as ligands for the selectins.41,42 Such selectin-mucin interactions seem to be involved in the hematogenous phase of tumor metastasis.42–45 Mixtures of these abnormal carcinoma mucins (or their proteolytic fragments or both) are also shed by tumors and can be found in the bloodstream of patients with cancer,46–53 where their levels are sometimes measured as prognostic markers. Thus, it remained reasonable to suggest that mucins are involved in Trousseau's syndrome.
One way to eliminate contamination is to take advantage of the fact that tumor mucins contain heavily glycosylated segments that are resistant to denaturation, boiling, and even proteases. Thus, when human colon carcinoma xenograft extracts were treated with multiple enzymes (including broad-spectrum proteases) followed by boiling and denaturation, the only surviving macromolecules were fragments of heavily glycosylated mucins.18 These preparations were free of TF or endotoxin and were incapable of accelerating clotting in vitro. However, they rapidly induced disseminated platelet-rich microthrombi when injected intravenously into normal mice. These occurred even when animals were pretreated with amounts of hirudin sufficient to block in vivo thrombin formation (although as expected, the associated fibrin deposition did not occur). Moreover, this platelet aggregation was dependent on P-selectin and L-selectin, as determined using genetically deficient mice.18 This is consistent with prior findings that carcinoma mucins have binding sites for both P- and L-selectins.42 Further studies indicated a stepwise process in which the mucins initially activate leukocytes by L-selectin ligation, generating an as yet unknown mediator, which then cooperates with the mucins interacting with P-selectin on endothelium or platelets or both, ultimately generating the platelet-rich microthrombi (Figure 1), including fibrin deposition, by an as yet unknown mechanism. Interestingly, although the formation of the platelet clumps themselves could not be abrogated by thrombin inhibitors such as hirudin, it was efficiently blocked by heparin. This is consistent with the finding that heparin (at concentrations acceptable in clinical practice) is a potent inhibitor of P- and L-selectin interactions with natural or carcinoma ligands.42,54,55
The great majority of such injected carcinoma mucins were rapidly cleared by multiple glycan receptors in the mouse liver.56 The small amounts surviving clearance were heavily sialylated, and presumably represented the fraction mediating the pathologic reactions with platelets and leukocytes. Thus, patients with carcinomas probably secrete large quantities of mucins into circulation, which are rapidly cleared, leaving behind only the proverbial “tip of the iceberg” in the bloodstream. The pathologic processes triggered by such carcinoma mucins require not only altered glycosylation but also inappropriate secretion into the vascular compartment. Thus, mucins might also enter the bloodstream in nonmalignant processes involving mucin-secreting organs. Could this help explain the high frequency of venous thrombosis in inflammatory bowel disease?57 Elevated levels of serum mucin antigens are also associated with hepatocellular disease and liver cirrhosis. Because the liver itself does not produce mucins, this may represent failure of the mucin clearance system to remove the low levels of mucins that normally spill into the blood from hollow organs. This is also a potential explanation for the “false positive” tumor marker studies58 and the unexplained coagulopathies seen in patients with chronic liver disease.59
Mechanisms involving oncogene activation
In 2005, Boccaccio et al60 stated that Trousseau's syndrome had “so far resisted a mechanistic explanation.” Although this claim was at odds with prior literature, the researchers did present a truly novel mechanism, using a mouse model in which targeting an activated human MET oncogene to adult mouse liver caused slowly progressing hepatocarcinogenesis, which was preceded and accompanied by a syndrome “manifesting first with blood hypercoagulation (venous thromboses), and then evolving toward fatal internal hemorrhages.” Following up on these observations, these investigators showed that the pathogenesis of this model syndrome was driven by the transcriptional response to the MET oncogene, especially involving up-regulation of PAI-1 and cyclooxygenase-2 (COX-2) genes. Because both molecules can theoretically support a thrombohemorrhagic phenotype, they concluded that they had found “direct genetic evidence for the long-sought-after link between oncogene activation and hemostasis.” They later went on to point out that hypoxia could induce Met oncogene transcription, suggesting that this provided “a clinically important perspective on malignant invasion and metastasis.”61 However, as with all earlier mechanisms described above, this one also requires validation in the natural clinical setting of patients with Trousseau's syndrome.
Spectrum of overlapping mechanisms in Trousseau's syndrome
It is reasonable to suggest that all these proposed mechanisms represent a range of overlapping pathways that contribute to various extents toward the thromboembolic diathesis in patients with tumors (Figure 1.). Thus, Trousseau's syndrome is better considered a spectrum of disorders, ranging at one extreme with thrombosis induced primarily by the production of TF by tumor cells, all the way to a platelet-rich microthrombotic process triggered by carcinoma mucins and involving P- and L-selectins. Other mechanisms proposed about hypoxia, oncogene activation, and so forth, can fit into this spectrum of pathways, as shown in Figure 1, all eventually resulting in thrombin generation and fibrin deposition. An additional possibility not explored is activation of endothelium by tumor-derived inflammatory cytokines, which could induce expression of various adhesive molecules such as V-CAM and E-selectin.
Management of Trousseau's syndrome
Regardless of underlying mechanisms, the primary approach to treating Trousseau's syndrome is to eliminate the causative tumor, if possible. Although this is often not feasible, the one recurring theme in the literature is that heparin is the preferred treatment, and that specifically blocking factor Xa or thrombin is insufficient in many instances.4,62–66 Indeed, there are many reports of marked and even catastrophic acceleration of thrombosis on discontinuation of heparin in Trousseau's syndrome.4,26,62,67 Taken together with the relative inactivity of vitamin K antagonists or direct thrombin inhibitors in some cases, it is reasonable to suggest that this heparin activity is mediated by more than just its ability to inactivate thrombin by enhancement of antithrombin.
Why are heparins the preferred treatment in Trousseau's syndrome?
Although originally isolated and approved for clinical use as an anticoagulant, unfractionated heparin is actually a complex and heterodisperse mixture of glycosaminoglycans extracted from certain animal sources.68–71 The serpin antithrombin binds to a specific modified pentasaccharide sequence that is distributed sporadically within long heparin chains,70 markedly enhancing its ability to irreversibly inactivate both activated factor Xa and thrombin, thereby interrupting fluid-phase thrombosis, as well as secondary platelet activation. However, as shown in Figure 1, heparin preparations have a variety of other biologic activities that can help explain its beneficial effects in Trousseau's syndrome. Perhaps this is why heparins have remained the preferred treatment for Trousseau's syndrome. In this regard, it is worth noting that modern therapeutic approaches have tended to become more and more specific, being highly targeted toward defined molecular mechanisms. In fact, complex clinical situations such as Trousseau's syndrome may be better managed by drugs such as heparin, which have multiple nonoverlapping moderating actions.
Heparin-mediated interactions that are potentially beneficial in Trousseau's syndrome
In addition to antithrombin, heparins also activate two other serpins involved in blood coagulation, Heparin cofactor II72 and protein C inhibitor.73 Another independent heparin action is to block the binding of L- and P-selectins to their natural and pathologic ligands.54,74–76 This interrupts carcinoma-mucin–dependent adhesion phenomena mediated by these selectins (Figure 1) as well as signaling pathways triggered by their interactions with natural ligands (eg, induction of tissue factor or platelet-activating factor synthesis in monocytes by P-selectin engagement of PSGL-1),77,78 which is one possible explanation for the subsequent generation of thrombin and fibrin. Meanwhile, tissue factor pathway inhibitor (TFPI) is released from the vascular endothelium by heparin, perhaps delivering higher concentrations of this potent anticoagulant to the sites of ongoing thrombosis.79–83 The effects of heparin on fibrinolysis are more complex, because both activators and inhibitors can be affected.84,85 Heparin can also bind and potentially neutralize a wide variety of cytokines and chemokines.86–88 Some of these inflammatory cytokines could potentially aggravate Trousseau's syndrome by activating endothelial cells, causing enhanced expression of adhesion molecules, including P-selectin.
Alternative approaches to the management of Trousseau's syndrome
In recent years low molecular weight heparins (LMWHs) have become popular, in part because of their improved pharmacokinetics, the ability to give single daily dosages, and the reduced incidence of heparin-induced thrombocytopenia.89–91 Moreover, LMWHs selectively inhibit factor Xa without affecting thrombin and may be less likely to deplete TFPI pools over time.79,80,82,92,93 Thus, there have been several reports in which LMWHs have been successfully substituted for unfractionated heparin in managing Trousseau's syndrome.64,94 However, it should be noted that the ability of some LMWHs to mediate some of the heparin actions indicated in Figure 1 may not be equivalent. For example, some LMWHs of shorter length are not as effective at blocking P- and L-selectins, even at comparable levels of anti-Xa activity.55,95 Therefore, although switching to LMWHs may be convenient and even beneficial,96–98 further studies are needed to assure that any useful effects are not being lost when using some of them. An interesting prediction arising from recent work is that the synthetic pentasaccharide fondaparinux (Arixtra) might be least effective, even if given at doses that achieve equivalent anti–factor Xa activity.55 This needs to be studied further. Of course, one can envisage an alternate approach, using a combination of modern anticoagulant agents that would block many of the processes thought to be involved in Trousseau's syndrome.99 However, as with any form of combination therapy, there is an increased probability of side effects.
Risk of an occult cancer in a patient with unexplained thrombosis
By its original definition, the diagnosis of Trousseau's syndrome is made in retrospect, when the occult malignancy is found. Thus, one can ask whether there is any practical value in making a diagnosis of this syndrome. The risk of an occult carcinoma being found after an episode of otherwise unexplained venous thrombosis has ranged widely in various retrospective series. In a prospective analysis,100 apparently cancer-free patients with acute idiopathic venous thromboembolism were randomly assigned to have either extensive screening for occult cancer or no further testing. In the former group, approximately 13% of the patients were shown to have an occult cancer. A similar number of cancers emerged in the control group during a 2-year period. As expected, malignancies found in the extensive screening group were at an earlier stage. However, cancer-related mortality during the 2-year follow-up period was not markedly different. A subsequent decision analysis of these data suggested that screening for cancer with an abdominal or pelvic computed tomography scan with or without mammography or sputum cytology appeared potentially useful for cancer screening in patients with unexplained thromboses.101 Overall, the conclusions were that “the cost-effectiveness analysis of this strategy needs confirmation in a large trial” and that although “Data from these studies do not conclusively demonstrate that earlier diagnosis ultimately prolongs life… the collective observation makes such a beneficial effect likely.”102
There are many features in common between the pathogenesis of Trousseau's syndrome and factors that appear to facilitate tumor metastases, including roles for TF, selectins, platelets, endothelium, and fibrin.5–8,42,60,103–105 Thus, it is likely that the thrombotic processes involved in Trousseau's syndrome also facilitate the spread of tumors. Together with the fact that advanced visceral carcinomas are mostly incurable, this may explain why the search for occult malignancies has not had a large impact on the final outcome of cancer survival.
On a related note, others have reported that middle-aged men free of malignancy who showed persistent activation of the coagulant pathway (prothrombin fragments 1 + 2 and fibrinopeptide A concentrations exceeding the upper quartiles of the population distribution in 2 consecutive annual examinations) showed a higher mortality,106 which was largely due to cancers of the digestive tract. It is unclear whether this persistent activation of the coagulant pathway is the consequence of an occult malignancy (ie, a “preclinical Trousseau's syndrome”), or whether the hypercoagulability arising from some other cause “awakens” dormant tumor cells in the host.107 Another interesting suggestion is that toxicity from elevated ambient body iron levels might explain this association between persistent coagulation activation and occult cancer.108 The contribution of increased iron to tumor initiation and promotion is suggested to collaborate with the effects of iron-induced oxidative damage on lipids, which can cause increased TF expression, while down-regulating the activity of TFPI and the expression of thrombomodulin.108
Conclusions and future prospects
Regarding how exactly to define Trousseau's syndrome, we should recognize that it is a somewhat semantic issue. However, as articulated well by others,3 if ever there was a case when an eponym was deserved for a clinical syndrome it is that of Trousseau, who not only made an astute clinical observation that has survived the test of time but also diagnosed it accurately on himself. Thus, it is suggested that the term Trousseau's syndrome be reserved for situations in which thrombotic problems that cannot be explained by any other obvious factor(s) occur in the setting of either an occult or a recently diagnosed carcinoma. This suggestion is made with the recognition that even in its classic form Trousseau's syndrome is probably mediated by multiple mechanisms, and that any given patient may have different combinations of overlapping and interacting mechanisms as a cause of their prothrombotic condition. In the final analysis there is still much to be learned about this syndrome and its optimal management.
Acknowledgments
I thank Kenneth Kaushansky, Philip Majerus, George Broze, Nissi Varki, and two anonymous reviewers for helpful comments and suggestions.
This work was supported by the National Institutes of Health (grants HL057345 and CA38701).
National Institutes of Health
Authorship
Conflict-of-interest disclosure: A.V. is cofounder and current consultant to Noble Molecules, a company seeking to bring the non–anticoagulant therapeutic benefits of heparins to the bedside.
Correspondence: Ajit Varki, University of California, San Diego, 9500 Gilman Dr MC 0687, La Jolla, CA 92093-0687; e-mail: a1varki@ucsd.edu.