Abstract
Several cytoplasmic proteins, such as GTPases of the Ras family, containing a C-terminal CAAX motif are prenylated by farnesyltransferase to facilitate localization to cellular membranes where activation occurs. Farnesyltransferase inhibitors (FTIs) interfere with this farnesylation process, thereby preventing proper membrane localization and rendering the proteins unavailable for activation. Currently, FTIs are being explored as antineoplastic agents for the treatment of several malignancies. However, since farnesylated proteins like Ras are also involved in intracellular signaling in lymphocytes, FTIs might interfere with T-cell activation. Based on this hypothesis we examined the effect of several FTIs on cytokine production in response to anti-CD3 + anti-CD28 monoclonal antibodies or PMA + ionomycin. Murine Th1 and Th2 clones, stimulated in the presence of FTIs, showed a dose-dependent reduction of lineage-specific cytokine secretion (IFN-γ, IL-2, IL-4, IL-5). However, no inhibition of ERK or JNK MAP kinases was observed, nor was induction of cytokine mRNA affected. Rather, intracellular cytokine protein synthesis was blocked. Inhibition of human T-cell INF-γ production also was observed, correlating with reduced phosphorylation of p70S6K. These results indicate that FTIs inhibit T-cell activation at the posttranscriptional level and also suggest that they may have potential as novel immunosuppressive agents.
Introduction
Small GTPases of the Ras family such as Ras, Rho, and Rac are important regulators of growth factor receptor–induced activation events in a variety of cellular systems. A hallmark of GTPases like N-, K-, and H-Ras is that several posttranslational modifications of the synthesized protein must occur before localization to distinct cell membranes is achieved, a necessary prerequisite for functional activity.1 Prenylation catalyzed by 1 of 3 intracellular enzymes, farnesyltransferase (FTase) or geranylgeranyltransferases (GGTase I and II), is the first critical modification step. Physiologically, prenylation in posttranslational processing of Ras proteins in mammalian cells is predominantly achieved by FTase. However, in cells where FTase activity is blocked, alternative prenylation of K-Ras, N-Ras, and RhoB by GGTase I has been described.2 The FTase substrate in all Ras family proteins is the common COOH-terminal CAAX tetrapeptide sequence. FTase catalyzes the transfer of a 15-carbon farnesyl group from farnesyldiphosphate, a product of the cholesterol biosynthesis pathway, to the CAAX cysteine residue.3 Besides members of the Ras family and a variety of additional molecules such as HDJ-2 and Lamin A and B, several retinal and centromere-associated proteins are also known substrates of cellular FTase.4–6 Further posttranslational modification for membrane targeting of Ras proteins after prenylation includes proteolysis of the AAX motif followed by alpha-carboxymethylation of the farnesylated cysteine residue. In addition, H-Ras and N-Ras are subsequently palmitoylated.7
Since posttranslational isoprenoid modification is regarded as the critical event in localization of Ras proteins to cellular membranes, preventing the synthesis of the farnesyl precursor mevalonate by blocking of HMG-CoA reductase results in the depletion of intracellular farnesyl and accumulation of nonprocessed cytosolic Ras.8 Furthermore, statins, which are HMG-CoA inhibitors widely used as cholesterol-lowering agents, have recently been attributed clinically relevant immunomodulatory properties.9
Farnesyltransferase inhibitors (FTIs) are a class of drugs initially generated to interfere with the farnesylation of oncogenic Ras, thereby retaining it in the cytosol and preventing its activity. The development of several structurally different FTIs as anticancer agents was based on the initial observation that the phenotype of oncogenic Ras-transformed fibroblasts could be reversed by FTI treatment10 followed by a variety of data regarding the antineoplastic activity of FTIs in several in vitro and in vivo tumor models.5 Cancer cell lines treated with FTI show inhibition of proliferation,11 induction of apoptosis,12 or disturbed cell-cycle progression13 in vitro. In particular, pediatric T-cell acute lymphoblastic leukemia (ALL) and French-American-British (FAB) M5 acute myeloid leukemia (AML) have been proven very sensitive to FTI-mediated cytotoxicity.14 Inhibition of malignant cell growth could also be demonstrated in vivo11,13,15 via a mechanism that might be mediated in part by an antiangiogenic effect.16 Interestingly, FTI inhibition of malignant cell growth is apparently not solely dependent on Ras mutation status,11,14 raising questions regarding the true mechanism of action of these agents. In clinical phase 1/2 studies, FTI showed promising activity in the treatment of myeloid malignancies,17–20 although optimal dosing schedules and putative combined treatment approaches still need to be established.
In T cells it has been demonstrated that Ras is activated by T-cell receptor (TCR) ligation21,22 and contributes to cytokine gene induction.23 Reduced Ras activity correlates with a status of functional unresponsiveness of T cells termed “anergy.”24 Since many data considering the role of Ras in T cells were generated in T-cell tumor lines, molecular aspects of Ras activity in normal peripheral T cells are still relatively poorly defined.25
Based on the hypothesis that FTIs would block Ras farnesylation, thereby resulting in interference with Ras-dependent signals triggered by TCR ligation in T cells, we examined the effect of FTIs on signaling events and cytokine production by Th1 and Th2 murine T-cell clones and human T cells in response to a variety of stimuli. Unexpectedly, we found evidence that FTIs do block T-cell activation but without affecting several defined Ras effectors and without inhibiting cytokine mRNA induction. Rather, inhibition of cytokine production appeared to occur at the posttranscriptional level and was associated with inhibition of p70S6 kinase phosphorylation. These results have potential implications for the mechanism of action of FTIs as antitumor agents and also raise the possibility of the use of FTIs as immunosuppressive compounds.
Materials and methods
T-cell clones
Ovalbumin (OVA)–specific murine CD4+ Th1 (PGL10) and Th2 (PL104) T-cell clones were cultured in DMEM (Life Technologies, Gaithersburg, MD) supplemented with 5% FCS and incubated at 37°C in an 8% CO2 atmosphere. They were maintained by weekly stimulation with irradiated DBA/2 splenocytes (20 Gy), OVA (200 μg/mL; Sigma, St Louis, MO), and human rIL-2 (12.5 U/mL; Chiron, Emeryville, CA), as previously reported.26
Farnesyltransferase inhibitors
The FTI CP 390392 was a generous gift from Pfizer (Groton, CT). The FTI SCH 66336 was a generous gift from Schering-Plough (Kenilworth, NJ). For confirmatory experiments, the additional FTIs R11577, 179626, and L-744832 (Sigma) were used. All FTIs were dissolved in DMSO and kept at minus 20°C. Working solutions were obtained by dilution with T-cell culture medium.
T-cell activation and cytokine ELISA
T-cell clones were preincubated with varying concentrations of FTI at 37°C for 24 hours (or less where indicated) before stimulation. T cells were usually activated using beads (Dynal, Oslo, Norway) coated with varying concentrations of anti-CD3 monoclonal antibody (mAb; 145-2C11) in the presence or absence of anti-CD28 mAb (PV1), as previously described.27 Alternatively, stimulation with PMA (50 ng/mL) plus ionomycin (1 μM) was used where indicated. FTI was present during the whole stimulation period. Cytokine production was determined using 105 T cells stimulated with anti-CD3/anti-CD28 mAb or PMA/ionomycin in 96-well microtiter plates for 18 hours at 37°C. Supernatants were collected and analyzed by enzyme-linked immunosorbent assay (ELISA) for IL-2, IFN-γ, IL-4, or IL-5 content using Ab pairs obtained from BD PharMingen (San Diego, CA). Living cells were determined after FTI incubation and stimulation using trypan blue exclusion.
Assessment of FTI effect on human T-cell activation
For the experiments with human T cells, peripheral blood mononuclear cells (PBMCs) or buffy coats from volunteer donors were used. Donor PBMCs were seeded at 2 × 105 cells per well in triplicate in 96-well plates, along with staphylococcal enterotoxin A (SEA) (final concentration 0.1 μg/mL) with or without R115777 (20 μM). Supernatants were collected at 20 hours and assessed for IFN-γ content by ELISA (R&D Systems, Minneapolis, MN). For biochemical analyses, CD4+ T cells were enriched by labeling of buffy coats with anti-CD4 mAb-coated beads together with column separation systems (Miltenyi, Bergisch-Gladbach, Germany) according to the manufacturer's instructions. Stimulation of enriched CD4+ T cells was achieved by beads coated with anti-CD2/anti-CD3/anti-CD28 antibodies (T-cell activation Kit; Miltenyi) followed by Western blotting
Intracellular cytokine staining
Intracellular detection of IFN-γ was performed as described.28 Th1 T cells were preincubated with FTIs and stimulated with PMA (50 ng/mL) and ionomycin (1 μM) for up to 25 hours in the presence of FTIs. Brefeldin A (1 μg/mL) was added for the last 2 hours of stimulation. Cells were washed with PBS/2% FCS and stained with FITC–anti-CD3 mAb (Pharmingen, San Diego, CA). After washing, cells were fixed by incubation with paraformaldehyde (4%) for 10 minutes, followed by another wash with PBS/FCS. After another wash with permeabilization buffer (PBS/2% FCS/0.1% Saponin), cells were stained by incubation with IFN-γ–PE antibodies (2 μg/mL; Pharmingen) in permeabilization buffer. Stained cells were washed with permeabilization buffer and PBS and analyzed by flow cytometry. The percentages of positive cells were determined at the indicated time points on 10 000 gated events.
Western blotting
Cells were lysed in 0.5% Triton X-100 lysis buffer (50 mM Tris, pH 7.6; 150 mM NaCl 0.9%; 0.5% Triton X-100; 1 mM trypsin inhibitor; 5 mM EDTA; 1 mM benzamidine; 1 mM sodium orthovanadate [Na3VO4]; 10 μg/mL aprotinin; 25 μM p-nitrophenyl-p'-guanidinobenzoate; 1 mM NaF; 1 mM phenylmethylsulfonyl fluoride); cleared lysates were boiled in 5 × reducing sample buffer as described.27 Protein separation was achieved using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; 10% or 12% gels) and transfer onto PVDF membranes (Millipore, Bedford, MA), and Western immunoblotting was performed as described.22 Antibodies against the following proteins were obtained as indicated: HDJ-2 (NeoMarkers, Fremont, CA); β-actin (Santa Cruz Biotechnology, Santa Cruz, CA); IFN-γ (Pharmingen); phospho-ERK, phospho-JNK (Promega, Madison, WI); phospho-p70S6K (Cell Signaling, Danvers, MA); ERK (Zymed, San Francisco, CA); or JNK1 (BD PharMingen).
RNAse protection assays
Stimulated T cells were lysed in TRIZOL reagent (Invitrogen, Carlsbad, CA) to obtain total RNA. RNAse protection assays for cytokine mRNA were performed using a RiboQuant in vitro transcription and RPA Kit (BD Biosciences, Bedford, MA) according to the manufacturer's instructions. The mCK-1 murine cytokine set (BD Biosciences) was used as a cDNA template for cytokine RNA transcription. For kinetic studies of IFN-γ and IL-5 mRNA transcripts, protection assays were performed at indicated time intervals after stimulation. After hybridization, the intensity of the labeled specific mRNA was determined by analysis of film using UN-SCAN-IT (Silk Scientific, Orem, UT) image analyzer software. The relative amount of specific cytokine RNA was determined as a ratio of cytokine RNA signal to control RNA (L32/GAPDH) signal.
Results
The FTI CP 390392 prevents HDJ-2 prenylation in T cells
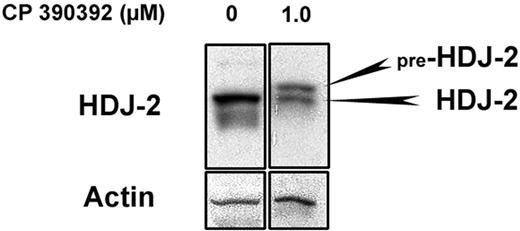
The chaperone protein HDJ-2 undergoes farnesylation-dependent processing, resulting in a mobility shift when FTase is inhibited.4,17,20 Since unfarnesylated HDJ-2 runs with a higher molecular weight on SDS-PAGE gels, it is more straightforward to detect inhibition of FTase by gel mobility shift of HDJ-2 than for small GTPases. Therefore, to confirm that the FTI CP 390392 suppresses farnesylation in murine T-cell clones, Th1 cells were incubated for 24 hours with increasing concentrations of CP 390392, and HDJ-2 mobility shift was determined by Western-blot analysis. The indicated incubation time was chosen in most of the T-cell experiments in order to achieve a substantial accumulation of unfarnesylated protein species based on the turnover of typical substrates (eg, the half-life of Ras protein is estimated to be at least 20 hours).29 As shown in Figure 1, as low as 1.0 μM of drug resulted in the detection of unprocessed, mobility-shifted pre–HDJ-2 in T cells, indicating at least partial inhibition of FTase activity.
Farnesyltransferase inhibitor CP 390392 prevents HDJ-2 prenylation. Th1 T cells were incubated for 24 hours in FTI CP 390392 at the indicated concentrations. Cells (2.5 × 106/lane) were lysed and loaded onto a 10% SDS-PAGE gel. Blotting with HDJ-2 antibodies at an FTI concentration of 1.0 μM revealed a second higher-weight band representing unfarnesylated pre–HDJ-2 protein. Blotting for β-actin was performed as a loading control.
Farnesyltransferase inhibitor CP 390392 prevents HDJ-2 prenylation. Th1 T cells were incubated for 24 hours in FTI CP 390392 at the indicated concentrations. Cells (2.5 × 106/lane) were lysed and loaded onto a 10% SDS-PAGE gel. Blotting with HDJ-2 antibodies at an FTI concentration of 1.0 μM revealed a second higher-weight band representing unfarnesylated pre–HDJ-2 protein. Blotting for β-actin was performed as a loading control.
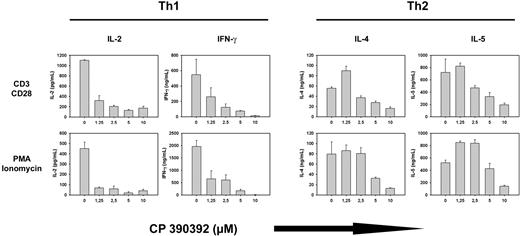
FTase inhibition suppresses T-cell cytokine production
Based on the hypothesis that FTIs would inhibit Ras-dependent intracellular signaling pathways, we examined whether pretreatment of T cells with FTI CP390392 for 24 hours would interfere with T-cell activation and effector function triggered by TCR/CD28 ligation or with addition of PMA/ionomycin. As shown in Figure 2, this was indeed the case. In both Th1 and Th2 clones, production of the appropriate lineage-specific cytokines (IL-2, IFN-γ, IL-4, and IL-5) in response to either anti-CD3/anti-CD28 mAb or PMA/ionomycin was inhibited in the presence of FTI in a dose-dependent manner (Figure 2). A significant decrease of IL-2 and IFN-γ production could already be observed at an FTI concentration of 1.25 μM, which correlated with reduced FTase activity according to mobility-shifted pre–HDJ-2 at the same concentration (Figure 1). Similar results were obtained using the additional FTIs R11577, 179626, and L-744832 (data not shown).
FTI treatment suppresses T-cell lineage–specific cytokine production. Th1 (A) and Th2 (B) T cells were preincubated with varying concentrations (0-10 μM) of FTI CP 390392 for 24 hours followed by stimulation with beads coated with anti-CD3 (2C11, 1 μg/mL) plus anti-CD28 (PV1, 1 μg/mL) antibodies or PMA/ionomycin. Cell activation took place in the presence of indicated concentrations of FTI during the whole culture period. After an additional 18 hours, culture supernatants were analyzed by ELISA for lineage-specific cytokine content (IL-2, IL-4, IL-5, IFN-γ). Stimulations at distinct concentrations were performed in triplicate. Error bars are SD. Cytokine levels were undetectable in the absence of stimulation (data not shown).
FTI treatment suppresses T-cell lineage–specific cytokine production. Th1 (A) and Th2 (B) T cells were preincubated with varying concentrations (0-10 μM) of FTI CP 390392 for 24 hours followed by stimulation with beads coated with anti-CD3 (2C11, 1 μg/mL) plus anti-CD28 (PV1, 1 μg/mL) antibodies or PMA/ionomycin. Cell activation took place in the presence of indicated concentrations of FTI during the whole culture period. After an additional 18 hours, culture supernatants were analyzed by ELISA for lineage-specific cytokine content (IL-2, IL-4, IL-5, IFN-γ). Stimulations at distinct concentrations were performed in triplicate. Error bars are SD. Cytokine levels were undetectable in the absence of stimulation (data not shown).
Reduced production of IL-2 in the presence of FTIs was also observed with naive murine CD4+ T cells stimulated with PMA/ionomycin (data not shown). Besides inhibition of activation-induced cytokine production, proliferation of T cells in response to anti-CD3/anti-CD28 was also inhibited by FTIs in a dose-dependent manner, which could not be overcome by exogenous IL-2 (data not shown). Collectively, these results indicate that farnesyltransferase inhibition suppresses T-cell cytokine production and activation in the absence of appreciable cell death.
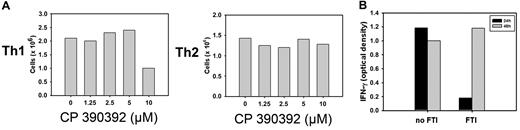
FTI concentrations at which suppression of cytokines occur are not toxic to T cells
An important consideration with the observed suppressive effect of FTIs on cytokine production by activated T cells is that nonspecific toxicity of the FTI compound might interfere with the viability of examined T cells. Therefore, the viability of treated Th1 and Th2 cells was determined after 24 hours of FTI treatment. As shown in Figure 3A, recovery of incubated T cells was impaired only at concentrations much higher than necessary for the inhibition of cytokine production in Th1 and Th2 cells. In addition, removal of the compound from FTI-treated cells resulted in full recovery of cytokine production after TCR/CD28 ligation (Figure 3B). These results make it unlikely that nonspecific toxicity of FTI was responsible for the observed reduction in cytokine production.
FTI-mediated suppression of cytokine production is reversible and not caused by diminished viability of T cells. (A) Th1 (3 × 106) and Th2 (2 × 106) cells were incubated with varying concentrations of FTI. Survival of T cells after 24-hour culture was determined by trypan blue exclusion. Data are representative of 3 independent experiments. (B) Th1 cells were preincubated with 10 μM FTI or medium for 24 hours and stimulated with anti-CD3 + anti-CD28 antibodies. IFN-γ production was determined by ELISA of stimulated FTI- or medium-treated cells. After the first 24 hours of incubation, Th1 cells initially cultured with medium alone were kept in medium for an additional 24 hours. Initially FTI-treated Th1 cells were washed and kept in medium alone for an additional 24 hours. Both populations were stimulated with anti-CD3 plus anti-CD28 antibodies after total culture of 48 hours, and IFN-γ production was determined by ELISA.
FTI-mediated suppression of cytokine production is reversible and not caused by diminished viability of T cells. (A) Th1 (3 × 106) and Th2 (2 × 106) cells were incubated with varying concentrations of FTI. Survival of T cells after 24-hour culture was determined by trypan blue exclusion. Data are representative of 3 independent experiments. (B) Th1 cells were preincubated with 10 μM FTI or medium for 24 hours and stimulated with anti-CD3 + anti-CD28 antibodies. IFN-γ production was determined by ELISA of stimulated FTI- or medium-treated cells. After the first 24 hours of incubation, Th1 cells initially cultured with medium alone were kept in medium for an additional 24 hours. Initially FTI-treated Th1 cells were washed and kept in medium alone for an additional 24 hours. Both populations were stimulated with anti-CD3 plus anti-CD28 antibodies after total culture of 48 hours, and IFN-γ production was determined by ELISA.
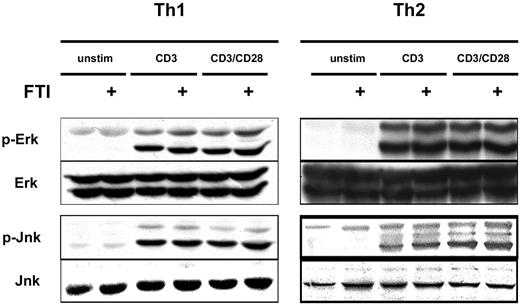
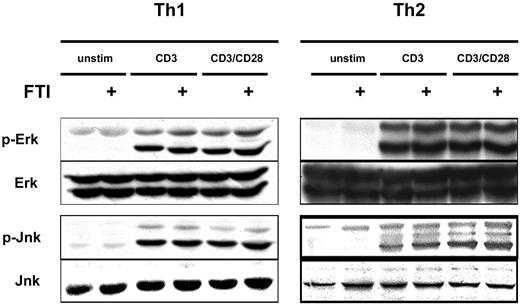
FTI treatment does not interfere with CD3/CD28-induced MAP kinase activation
Given the inhibition of cytokine production by FTIs, we investigated whether the expected inhibition of Ras-MAP kinase signaling had occurred. To examine this question we stimulated Th1 and Th2 cells with anti-CD3 and anti-CD28 mAb, with or without overnight preincubation with CP390392, and examined phosphorylation of ERK and JNK by Western-blot analysis. Surprisingly, comparable rapid activation of ERK and JNK was observed following stimulation whether or not cells were pretreated with the FTI (Figure 4). These results suggest that inhibition of farnesyltransferase is not suppressing T-cell cytokine production by inhibiting TCR/CD28-mediated Ras-MAP kinase activation. The fact that cytokine production in response to PMA plus ionomycin was also inhibited suggests that FTIs can block T-cell activation without participation of proximal tyrosine kinases and downstream from regulated calcium entry.
FTI treatment does not interfere with CD3/CD28-induced MAP kinase activation. Th1 and Th2 T cells were preincubated with 10 μM of FTI CP 390392 or culture medium for 24 hours, followed by stimulation with beads coated with anti-CD3 (2C11, 1 μg/mL) with or without anti-CD28 (PV1, 1 μg/mL) antibodies. The FTI was present during the whole culture period. 2.5 × 106 cells were used in each experimental condition. After 20 minutes of stimulation, T-cell activation was stopped by placing the cells on ice, and cell lysates were analyzed by SDS-PAGE for the presence of phospho-ERK and phospho-JNK. Total ERK and JNK were assessed as a loading control.
FTI treatment does not interfere with CD3/CD28-induced MAP kinase activation. Th1 and Th2 T cells were preincubated with 10 μM of FTI CP 390392 or culture medium for 24 hours, followed by stimulation with beads coated with anti-CD3 (2C11, 1 μg/mL) with or without anti-CD28 (PV1, 1 μg/mL) antibodies. The FTI was present during the whole culture period. 2.5 × 106 cells were used in each experimental condition. After 20 minutes of stimulation, T-cell activation was stopped by placing the cells on ice, and cell lysates were analyzed by SDS-PAGE for the presence of phospho-ERK and phospho-JNK. Total ERK and JNK were assessed as a loading control.
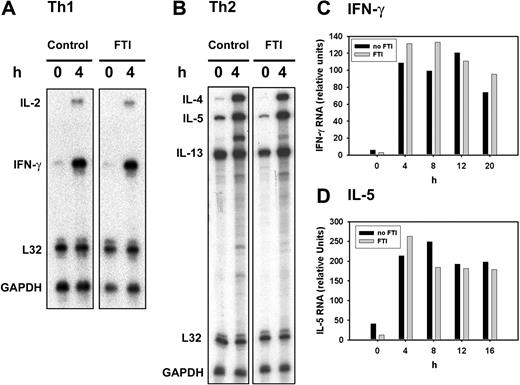
Farnesyltransferase inhibition can inhibit cytokine production without suppressing cytokine mRNA
Inasmuch as MAP kinase signaling did not seem to be affected by FTI treatment, it was of interest to determine whether inhibition occurred at the level of cytokine gene expression. To address this question, T cells were stimulated and cytokine transcripts were analyzed by RNAse protection assays. As shown in Figure 5A-B, after 4 hours of activation, induced transcripts for Th1 (IL-2, IFN-γ) and Th2 (IL-4, IL-5, IL-13) cytokines were not inhibited by FTI exposure. Cytokine release as assessed by ELISA analyzed in parallel was inhibited as expected (data not shown). Because cytokine content in supernatants is measured after 20 hours of stimulation, a kinetic analysis of mRNA levels was performed and IFN-γ and IL-5 transcript levels were quantified as representative Th1 and Th2 cytokines. In fact, IFN-γ and IL-5 transcripts were not decreased by FTI treatment during the duration of 20 hours of stimulation (Figure 5C-D). These results suggest that farnesyltransferase inhibition can suppress cytokine production without significantly inhibiting cytokine gene expression, implicating a posttranscriptional mechanism of effect.
FTI treatment can suppress cytokine production without significantly inhibiting cytokine mRNA induction. (A,B) Th1 (A) and Th2 (B) T cells were preincubated with 10 μM of FTI CP 390392 or culture medium for 24 hours followed by stimulation with PMA/ionomycin at 37°C for the indicated time periods (0-20 hours). FTI was included during the whole culture period. After indicated intervals, stimulation was stopped by placing the cells on ice followed by cell lyses with TRIZOL. After adjustment of total mRNA content, cytokine mRNA were detected after in vitro transcription and hybridization using the mCK-1 murine cytokine set (BD Biosciences, Bedford, MA) and RiboQuant and RPA Kit (BD Biosciences, Bedford, MA). (C,D) Following a kinetics analysis, intensity of radiolabeled signals on the exposed films was analyzed using UN-SCAN-IT (Silk Scientific, Orem, UT) image analyzer software. IFN-γ and IL-5 mRNA content were quantified as relative units, comparing the mRNA cytokine signal intensity to the intensity of housekeeping gene mRNA (L32, GAPDH).
FTI treatment can suppress cytokine production without significantly inhibiting cytokine mRNA induction. (A,B) Th1 (A) and Th2 (B) T cells were preincubated with 10 μM of FTI CP 390392 or culture medium for 24 hours followed by stimulation with PMA/ionomycin at 37°C for the indicated time periods (0-20 hours). FTI was included during the whole culture period. After indicated intervals, stimulation was stopped by placing the cells on ice followed by cell lyses with TRIZOL. After adjustment of total mRNA content, cytokine mRNA were detected after in vitro transcription and hybridization using the mCK-1 murine cytokine set (BD Biosciences, Bedford, MA) and RiboQuant and RPA Kit (BD Biosciences, Bedford, MA). (C,D) Following a kinetics analysis, intensity of radiolabeled signals on the exposed films was analyzed using UN-SCAN-IT (Silk Scientific, Orem, UT) image analyzer software. IFN-γ and IL-5 mRNA content were quantified as relative units, comparing the mRNA cytokine signal intensity to the intensity of housekeeping gene mRNA (L32, GAPDH).
Diminished IFN-γ production of T cells by FTIs is correlated with a reduction in intracellular IFN-γ protein
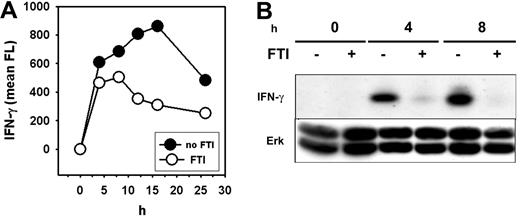
Decreased cytokine secretion without diminished mRNA induction suggested inhibition of T-cell activation at the posttranscriptional level. This could be explained by decreased secretion of cytokine protein into the culture medium or decreased synthesis of protein from cytokine transcripts. To distinguish between these possibilities, expression of intracellular cytokine protein was assessed using flow cytometry on permeabilized cells and Western blotting on whole-cell lysates. Preliminary experiments were performed to determine which cytokine-specific mAbs would detect each specific protein using Western blotting, and IFN-γ was found to be easily detected using this method, whereas other Abs failed. Therefore, attention was focused on this cytokine for these experiments.
Flow-cytometric analysis revealed that FTI pretreatment of Th1 cells stimulated with PMA/ionomycin resulted in a reduced induction and more rapid decline of intracellular IFN-γ protein over the course of a 25-hour stimulation period (Figure 6A). In addition, Western-blot analysis confirmed a substantial decrease in total cellular IFN-γ protein levels as a consequence of farnesyltransferase inhibition (Figure 6B). Total ERK levels were constant (Figure 6B), indicating that global protein synthesis was not affected. Consistent with this observation, uptake of tritiated leucine by activated T cells was not inhibited by FTI exposure (data not shown). These results suggest that FTIs likely block IFN-γ production and other cytokines relatively selectively at the level of protein synthesis.
FTI treatment inhibits generation of IFN-γ protein. Th1 (pGL10) cells were preincubated with 10 μM of FTI CP 390392 or culture medium for 24 hours followed by stimulation with PMA/ionomycin at 37°C for the indicated time periods (0–25 hours). (A) After indicated intervals, cells were permeabilized and IFN-γ protein was detected by intracellular antibody staining. Fluorescence intensity was determined by fluorescence-activated cell sorter (FACS) analysis. (B) In parallel, stimulated cells were lysed, separated on SDS-PAGE, and analyzed by Western blotting using anti–IFN-γ Abs. Anti-ERK was used as a loading control.
FTI treatment inhibits generation of IFN-γ protein. Th1 (pGL10) cells were preincubated with 10 μM of FTI CP 390392 or culture medium for 24 hours followed by stimulation with PMA/ionomycin at 37°C for the indicated time periods (0–25 hours). (A) After indicated intervals, cells were permeabilized and IFN-γ protein was detected by intracellular antibody staining. Fluorescence intensity was determined by fluorescence-activated cell sorter (FACS) analysis. (B) In parallel, stimulated cells were lysed, separated on SDS-PAGE, and analyzed by Western blotting using anti–IFN-γ Abs. Anti-ERK was used as a loading control.
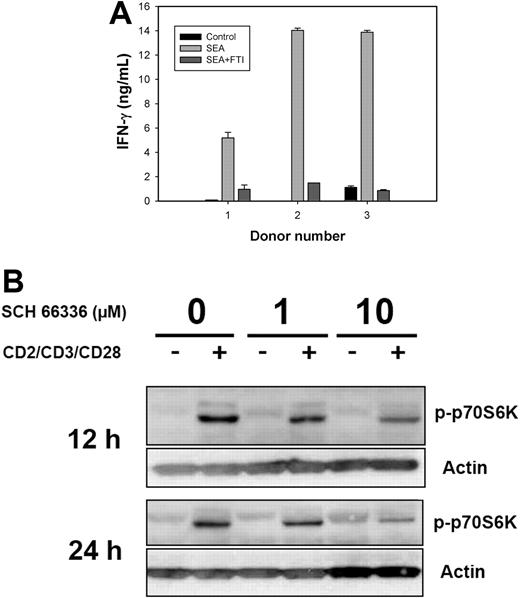
Human T cells show inhibition of both IFN-γ production and phosphorylation of p70S6K in the presence of FTIs
To prove relevance of the observed findings to application of FTIs in humans, we tested the effect of FTIs that are in established clinical trials (R11577, SCH 66336) on the activation of human T cells. First, peripheral blood T cells were stimulated with SEA in the presence or absence of the FTI R115777. T-cell activation by SEA for 20 hours resulted in strong IFN-γ production that was nearly completely blocked by the FTI (Figure 7A). These data confirm our findings obtained in murine T-cell clones.
Reduced IFN-γ production correlates with suppressed TCR-induced phosphorylation of p70S6K in activated human T cells in the presence of FTI. (A) Donor PBMCs were seeded at 200 000 cells per well in triplicate in 96-well plates, along with SEA (final concentration 0.1 μg/mL) with or without R11577 (20 μM). For control, PBMCs remained unstimulated. Supernatants were collected at 20 hours and assessed for IFN-γ content by ELISA (R&D Systems). Results represent the mean (± SD) of triplicate cultures. Similar results were seen with 3 additional human donors. (B) Human CD4+ T cells were enriched from buffy coats using anti-CD4 mAb-coated beats together with column separation systems. Stimulation of enriched CD4+ T cells was achieved for the indicated time points by beads coated with anti-CD2/anti-CD3/anti-CD28 antibodies. In FTI treatment, cells were preincubated for 3 hours with SCH 66336 at indicated concentrations and FTI was present during the entire stimulation. After the indicated time points, stimulation of human T cells was stopped by placing the cells on ice, and cell lysates were analyzed by SDS-PAGE for the presence of phospho-p70S6K. Blotting for actin was used as a loading control. The data are representative of 3 independent experiments.
Reduced IFN-γ production correlates with suppressed TCR-induced phosphorylation of p70S6K in activated human T cells in the presence of FTI. (A) Donor PBMCs were seeded at 200 000 cells per well in triplicate in 96-well plates, along with SEA (final concentration 0.1 μg/mL) with or without R11577 (20 μM). For control, PBMCs remained unstimulated. Supernatants were collected at 20 hours and assessed for IFN-γ content by ELISA (R&D Systems). Results represent the mean (± SD) of triplicate cultures. Similar results were seen with 3 additional human donors. (B) Human CD4+ T cells were enriched from buffy coats using anti-CD4 mAb-coated beats together with column separation systems. Stimulation of enriched CD4+ T cells was achieved for the indicated time points by beads coated with anti-CD2/anti-CD3/anti-CD28 antibodies. In FTI treatment, cells were preincubated for 3 hours with SCH 66336 at indicated concentrations and FTI was present during the entire stimulation. After the indicated time points, stimulation of human T cells was stopped by placing the cells on ice, and cell lysates were analyzed by SDS-PAGE for the presence of phospho-p70S6K. Blotting for actin was used as a loading control. The data are representative of 3 independent experiments.
In an additional experiment, CD4+ human T cells were stimulated with beads coated with anti-CD2/anti-CD3/anti-CD28 antibodies in the presence of SCH 66336. Since our previous data suggested an effect of FTIs on a posttranscriptional level, activation statuses of proteins involved in translation initiation were examined. We observed that inducible phosphorylation of p70S6K as detected by Western blotting was significantly inhibited by the presence of SCH 66336 (Figure 7B). This reduction of p70S6K phosphorylation at Thr389 was detectable at 12 and 24 hours after TCR simulation, well beyond early signaling that is regarded to be involved in cytokine gene transcription. Therefore, these kinetics are in accordance with the observed suppression in IFN-γ protein synthesis.
Discussion
Our present data demonstrate that inhibition of farnesyltransferase by specific inhibitors suppresses TCR/CD28-mediated T-cell activation and cytokine production in vitro. Inhibition was reversible and occurred in the absence of apoptosis. In addition, suppression of cytokine production occurred without blockade of MAP kinase activation and without diminution in cytokine mRNA levels but rather appeared to reduce the level of cytokine protein synthesis. Modification of regulatory elements at the posttranscriptional level points to a new mechanism of action of FTIs and also suggests the possibility of using these drugs as immunosuppressive agents.
Two biochemical features of FTI treatment of T cells were surprising in our current study. First, it was unexpected that FTI exposure did not diminish MAP kinase activation in response to CD3/CD28 ligation. Although physiologically all Ras proteins are farnesylated by FTase to achieve full functionality, some Ras proteins (K- and N-Ras) can also be prenylated by GGTase when FTase is pharmacologically inhibited.2 Thus, it is possible that geranylgeranylated Ras accumulated in our cells and retained function. It is also conceivable that small residual amounts of functional Ras remained and were sufficient for T-cell activation. These possibilities remain to be addressed. Of note, our results in T cells are consistent with those of Law et al30 who observed rapid FTI-induced cell growth arrest in mouse keratinocyte cell lines stimulated with several mitogens. FTI pretreatment of 30 minutes was sufficient for 60% reduction in the DNA-synthesis rate, yet EGF-induced ERK activation was completely unaffected even by prolonged (up to 72 hours) FTI pretreatment.31
Second, despite lack of inhibition of MAP kinase signaling, cytokine production was potently suppressed at a posttranscriptional level. Our data revealed diminished phosphorylation of p70S6K at Thr389, although effects on additional components of the translational machinery are also conceivable. Phosphorylation of Thr389 is critical for kinase function and most closely reflects p70S6 kinase activity in vivo.32 In a model of mitogen-stimulated murine keratinocyte cell lines, Law et al30 also observed reduced p70S6K activation in the presence of an FTI. These data suggest that activation of p70S6K by mitogens may depend upon a farnesylated protein. Of note, our results were not due to a global blockade of protein translation, inasmuch as tritiated leucine uptake was not blocked by FTI treatment (data not shown).
Our T-cell data suggest that alternative mechanisms of action of FTIs besides inhibition of Ras-based signaling may also be operational in tumor cells. Indeed, the well-established antineoplastic activity of FTIs remains incompletely understood on a molecular level. Several distinct mechanisms appear to be involved depending on the examined tumor model.33 However, in a variety of in vitro and in vivo tumor cell systems, Ras mutational status does not predict sensitivity to FTI treatment.14,33 Potential targets for FTIs include the small GTPases Rac1 and CDC42, as well as Rho family members. For example, it has been suggested that inhibition of RhoB explains the antiproliferative effect of FTIs in some tumor cells in vitro.34 However, these molecules are thought to be involved early in TCR signaling as is Ras and are unlikely to explain the ability of FTIs to suppress cytokine production in response to PMA plus ionomycin, which bypasses early receptor-mediated events. Similarly, NF-κB signaling has been reported to be sensitive to FTIs,35 but IFN-γ transcription is known to be NF-κB dependent, and the lack of effect of FTIs on IFN-γ mRNA induction in our current work argues against this playing a role in this system. Other candidate farnesylated signaling proteins include Rheb; the phosphatases PRL-1, -2, and -3; and the centromeric proteins CENP-E and CEN-F.5 Finally, as our mechanistic experiments focused more on IFN-γ due to the ability of the anti–IFN-γ Ab to work well by Western blotting, it is formally possible that the precise way in which FTIs inhibit production of each individual cytokine is at least partially distinct.
Regarding our data implying that farnesylated proteins might regulate cytokine production at the posttranscriptional level, the inhibition of Rheb by FTIs, which could be demonstrated in various tumor cell lines, needs to be considered.36 Rheb is a GTPase with ubiquitous tissue expression involved in mTOR (mammalian target of rapamycin) signaling and therefore a putative regulator of protein biosynthesis.37 In the human breast cancer cell line MCF-7, lack of Rheb farnesylation correlated with reduced phosphorylation of S6 ribosomal protein, indicating reduced p70S6 kinase activity in FTI-treated cells.36 Inactivation of the eukaryotic translation elongation factor 2 (eEF2) has also been observed in FTI-treated tumor cells.38 Another study suggested that VEGF production was inhibited posttranscriptionally by FTI treatment of 2 distinct cell lines in an epidermal tumor model,39 indicating that a posttranscriptional effect in protein expression might not be unique to T cells. Regardless of the precise mechanism of action of FTIs, our data demonstrate that inhibition of protein farnesylation suppresses functional T-cell activation, at least in part at a posttranscriptional level. In addition, interference with protein prenylation has also been observed through FTase substrate starvation by statin-mediated depletion of intracellular L-mevalonate.9 While the latter effect results in reduced in vitro T-cell proliferation,40 in vivo use of statins has been shown to attenuate disease progression in murine models of autoimmunity.9 Similarly, our results suggest that FTIs may have theoretic utility as immunosuppressive agents with a unique mechanism of action. Recent in vivo data demonstrating delayed development of acute allograft rejection by FTIs in a cardiac transplantation model41 support this hypothesis. It will be of interest to examine the effect of FTIs in murine models of allograft rejection, autoimmunity, and allergy for potential immunosuppressive activity in vivo.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Pfizer, Inc; Johnson & Johnson, Inc; and Schering-Plough, Inc for providing the FTIs used in these studies. We also thank Wolfgang Melchinger for technical support and Fabiola Rivas and Candace Cham for helpful suggestions.
This work was supported in part by R01 AI47919 from the National Institutes of Health (NIH), a grant from the Arthritis Foundation, and a grant from the Deutsche Krebshilfe (AZ107274). R.E.M. was supported by a grant from the Deutsche Forschungsgemeinschaft.
National Institutes of Health
Authorship
Contribution: R.E.M. designed and performed research, analyzed data, and wrote the paper; A.W.H., C.R., T.K. and S.B. performed research; and T.F.G. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Reinhard Marks, MD, University Hospital Freiburg, Department of Hematology/Oncology, Hugstetter Str 55, 79106 Freiburg, Germany; e-mail: reinhard.marks@uniklinik-freiburg.de.