Abstract
Bone marrow transplants are an important therapeutic tool for treating certain types of cancer as well as genetic diseases affecting the hematopoietic system. Until the transferred stem cells differentiate and reconstitute the immune system, recipients are at increased risk from opportunistic infections. We report the rapid generation of a functional natural killer (NK) compartment in lethally irradiated mice that received bone marrow cells from a syngeneic donor by treatment with IL-2/anti–IL-2 antibody complexes. We demonstrate that IL-2 complexes specifically expand the donor but not the host NK population and discuss the implications of this finding in the context of graft-versus-host disease and tumor relapse. Finally, we show that NK cells rapidly generated by IL-2 complexes kill MHC class I–deficient cells effectively in vivo. These data underline the unique therapeutic potential of IL-2 complexes.
Introduction
Hallmark features of natural killer (NK) cells are their ability to attack cells that lack expression of appropriate MHC class I molecules or express ligands for “stress” receptors.1,2 It is well documented that NK cells are capable of lysing many virally infected and certain tumor cells,3,4 but they have also been implicated in other processes such as regulating graft-versus-host disease (GvHD).5 IL-15 has been identified as a key regulator in promoting NK-cell differentiation,6,7 survival,8–10 and activation.11 Interestingly, IL-15 is transpresented to NK cells by cells expressing the IL-15Rα.11–13 Expression of 2 components of the IL-15 receptor—the IL-2Rβ chain and the common gamma (γc) chain—on NK cells is sufficient for delivering the IL-15 signal.13
Two recent reports14,15 described the ability of IL-2 complexes (IL-2 bound to anti–IL-2 antibodies) to stimulate vigorous proliferation of several lymphocyte types in vivo, among them NK cells. Although the mechanism responsible for potentiating the action of soluble IL-2 by complexing it with an antibody is not yet understood, it is clear that these IL-2 complexes exert their effect by signaling through the IL-2Rβ chain and the γc chain. It has been speculated that these complexes deliver more than a “plain” IL-2 signal and possibly have an IL-15 signal–like character caused by the antibody mediated transpresentation of IL-2.16
Based on these previous findings, we asked whether IL-2 complex can be used to rapidly boost donor NK-cell numbers after bone marrow transplantation. Establishing a functional NK cell compartment early after transplantation has been suggested to lower susceptibility to certain opportunistic infections and improve GvHD outcome while enhancing a graft-versus-tumor (GvT) response.5,17–19
Materials and methods
Mice
B6 and B6.SJL congenic mice were purchased from the Jackson Laboratory (Bar Harbor, ME), and IL-15−/− mice from Taconic Farms (Germantown, NY). Bone marrow recipient mice were irradiated with 10 Gy on the day of transfer and 5 × 106 bone marrow cells were transferred by intravenous injection into each recipient. All groups were treated with antibiotic water (polymyxin B and neomycin) for the course of the experiment. PK136 (anti-NK1.1) antibody (500 μg) was injected intraperitoneally on day 2 after irradiation into NK-depleted control groups, as indicated.
IL-2 complex and cytokine administration
IL-2 complex was administered intraperitoneally. Each animal received a preformed complex consisting of 1.5 μg IL-2 (eBioscience, San Diego, CA) and 50 μg S4B6 (anti–IL-2 antibody) in 250 μL PBS as previously described.14,15 hIL-15 was purchased from eBioscience; 3 μg was injected intraperitoneally at the same time points as the IL-2 complex.
In vivo killing assay
B6 splenocytes (CFSE-low labeled; 5 × 106) and β2M−/− splenocytes (CFSE-high labeled; 5 × 106) were injected intravenously and in vivo killing was analyzed 48 hours after transfer.
Flow cytometry
All antibodies were from eBioscience or BD Biosciences (San Jose, CA). Samples were run on a FACSCanto (BD Biosciences) and data were analyzed with FlowJo software (TreeStar, Ashland, OR).
Results and discussion
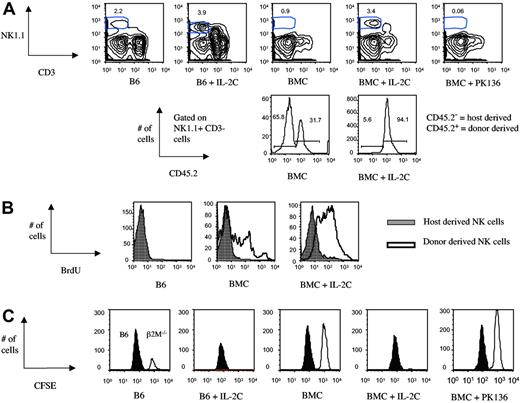
To test a potential role of IL-2 complex in generating a functional NK compartment early after bone marrow transfer, B6.SJL mice were lethally irradiated and received a congenic B6 (CD45.2) bone marrow graft shortly thereafter (day 0). IL-2 complex was injected on day 2 and day 4 after the bone marrow transfer. We analyzed spleen (Figure 1A) and bone marrow (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article) of the various experimental groups on day 8 after bone marrow transfer. As previously reported, the size of the splenic NK compartment increased in (nonirradiated) B6 mice after IL-2 complex injection compared with the untreated B6 control group. Similarly, we noticed a large increase of NK1.1+CD3− cells in the IL-2 complex–treated, bone marrow chimeric (BMC) mice compared with the untreated, BMC control group (Figure 1A), and IL-2 only– or anti–IL-2 only–treated groups (data not shown). A more pronounced difference was detected in the bone marrow compared with the spleen (about 7-fold versus 4-fold increase, respectively), suggesting that the IL-2 complex treatment has a more pronounced effect on immature NK cells or NK precursors. A substantial portion of the NK population in the untreated BMC group is host derived, with comparable ratios of donor to host cells in spleen and bone marrow. The ratio changes dramatically upon IL-2 complex injection, but not IL-2 only or anti–IL-2 only injections (Figure S2). The increase in NK1.1+CD3− cells in spleen and bone marrow was exclusively due to expansion of donor (CD45.2+) NK cells (Figure 1A and Figure S1 lower panels). Only donor-derived NK cells incorporated BrdU after bone marrow transplantation and treatment with IL-2 complex drastically increased the BrdU uptake of donor-derived NK cells, while host-derived NK cells were hardly affected (Figure 1B). The mechanism behind the unresponsiveness of host NK cells is still unclear. We believe that irradiation-damage is responsible for rendering NK cells incapable of responding to mitogenic signals. Donor NK cells promote engraftment and GvT, while suppressing GvHD in an allogeneic setting.17–19 The effect of IL-2 complex in such a setting is being investigated.
Rapid restoration of NK-cell numbers and function following irradiation and marrow grafting by IL-2 complex treatment. Irradiated bone marrow recipient mice (BMC mice) and (nonirradiated) B6 control mice were treated with IL-2 complex (IL-2C) on day 2 and day 4 after bone marrow transfer. (A) Spleen cells were analyzed on day 8 after bone marrow transfer and percentage of donor and host-derived cells, displayed in the histogram, was determined through congenic markers. (B) BrdU (2 mg) was injected intraperitoneally on day 5 after bone marrow transplantation, following treatment with IL-2 complex on day 2 and day 4. Animals were killed 10 hours after BrdU injection, and BrdU incorporation by NK1.1+CD3− cells in the spleen was determined. (C) A mix of CFSE-low B6 and CFSE-high β2M−/− splenocytes was transferred into control or B6 BMC mice and NK-mediated in vivo killing determined 48 hours later.
Rapid restoration of NK-cell numbers and function following irradiation and marrow grafting by IL-2 complex treatment. Irradiated bone marrow recipient mice (BMC mice) and (nonirradiated) B6 control mice were treated with IL-2 complex (IL-2C) on day 2 and day 4 after bone marrow transfer. (A) Spleen cells were analyzed on day 8 after bone marrow transfer and percentage of donor and host-derived cells, displayed in the histogram, was determined through congenic markers. (B) BrdU (2 mg) was injected intraperitoneally on day 5 after bone marrow transplantation, following treatment with IL-2 complex on day 2 and day 4. Animals were killed 10 hours after BrdU injection, and BrdU incorporation by NK1.1+CD3− cells in the spleen was determined. (C) A mix of CFSE-low B6 and CFSE-high β2M−/− splenocytes was transferred into control or B6 BMC mice and NK-mediated in vivo killing determined 48 hours later.
We assessed the functionality of the IL-2 complex–generated NK cells by transferring β2M−/− cells on day 6 after bone marrow transplantation and determining in vivo lysis 48 hours later. Untreated B6 mice served as the baseline readout, and BMC mice that were treated with NK-depleting antibody after bone marrow transfer served as the negative control (Figure 1C). Injection of IL-2 complex into B6 mice increased in vivo killing compared with the untreated B6 control group. Untreated BMC mice were capable of mediating some in vivo cytotoxicity (compared with the NK-depleted control group), but this was greatly increased in IL-2 complex–injected animals, which surpassed the B6 control group in NK-mediated killing. These findings demonstrate that IL-2 complex treatment induces rapid generation of functional, donor-derived NK cells after bone marrow transplantation.
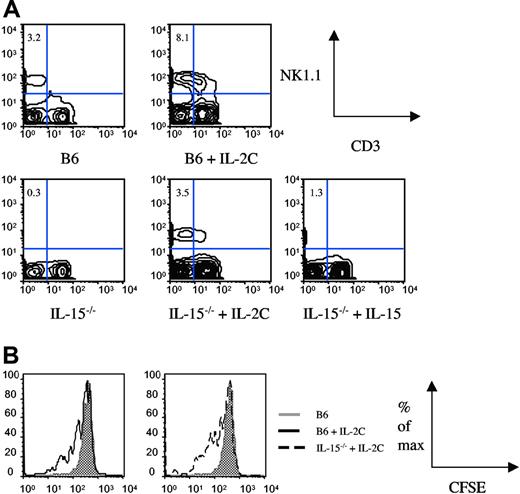
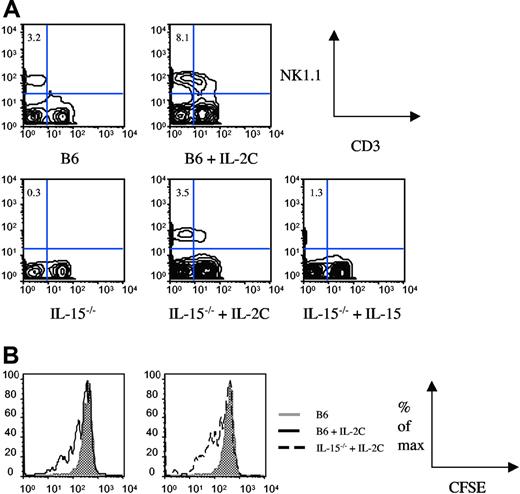
Following up on our observation that NK1.1+CD3− cells accumulate more rapidly in the bone marrow than spleen upon IL-2 complex administration (Figure 1A and Figure S1), we asked whether IL-2 complex affects NK cells once they are mature or at an earlier stage. We chose 2 different strategies to determine the stage of sensitivity to IL-2 complex treatment. First we asked whether we could generate an NK compartment in IL-15−/− mice (which lack mature NK cells6 ), similar to IL-15/IL-15Rα-Fc–mediated reconstitution of NK cells in IL-15Rα−/− mice.20 B6 mice and IL-15−/− mice were treated with either 2 injections of IL-2 complex (0 hours and 48 hours) or rat IgG control, and spleen cells were analyzed 72 hours after the first injection (Figure 2A). We confirmed the functionality of IL-2 complex in B6 mice (Figure 2A upper 2 panels). To our surprise, treatment of IL-15−/− mice with IL-2 complex generated an NK compartment comparable with (untreated) B6 mice 72 hours after the first injection (Figure 2A lower panel). This could not be achieved by injections of IL-15 (Figure 2A lower panel) or high doses of IL-2 (data not shown), underlining the unique potency of IL-2 complex.
IL-2 complex rapidly expands the NK compartment from immature precursors in IL-15−/− mice. (A) B6 or IL-15−/− mice received injections of IL-2 complex or IL-15 on day 0 and day 2 and spleens were analyzed on day 3. (B) Mice received congenically marked, NK1.1+CD3−-enriched splenocytes on day 0 and IL-2 complex injections on day 0 and day 2. Proliferation was measured by CFSE dilution on day 3.
IL-2 complex rapidly expands the NK compartment from immature precursors in IL-15−/− mice. (A) B6 or IL-15−/− mice received injections of IL-2 complex or IL-15 on day 0 and day 2 and spleens were analyzed on day 3. (B) Mice received congenically marked, NK1.1+CD3−-enriched splenocytes on day 0 and IL-2 complex injections on day 0 and day 2. Proliferation was measured by CFSE dilution on day 3.
At this point, we could not formally exclude the possibility that the few mature NK cells present in the periphery of IL-15−/− mice7 underwent massive expansion and were responsible for generating the NK compartment within 3 days. Our second strategy to address the responsiveness of mature, splenic NK cells was based on an adoptive transfer system. We transferred CFSE-labeled, NK1.1+CD3−-enriched spleen cells into B6 or IL-15−/− recipients and injected IL-2 complex shortly thereafter (day 0) and 48 hours later. Mice were killed 72 hours after transfer and donor NK-cell proliferation was determined by analysis of the CFSE profile. The data clearly show that IL-2 complex induces mature NK proliferation (Figure 2B), however not to the extent that it could account for the generation of a full NK compartment within 72 hours (Figure 2A). We therefore conclude that IL-2 complex induces proliferation and differentiation of NK-cell precursors in addition to inducing proliferation of splenic NK cells, thereby generating a functional NK compartment in a matter of days. We propose that IL-2 complex has a unique role in expanding and activating NK cells that is of great clinical interest.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Howard Hughes Medical Institute and National Institutes of Health Grant AI-19335 (M.J.B.). M.P. is a fellow of The Leukemia and Lymphoma Society.
We acknowledge B. Dere and P. Xiao for technical assistance and members of the Bevan lab for discussions.
National Institutes of Health
Authorship
Contribution: M.P. and D.K. performed research; M.P. analyzed data; and M.P. and M.J.B. designed research, interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael J. Bevan, Howard Hughes Medical Institute, University of Washington, Box 357370, Seattle, WA 98195-7370; e-mail: mbevan@u.washington.edu.