Abstract
Definitive erythropoiesis occurs in islands composed of a central macrophage in contact with differentiating erythroblasts. Erythroid maturation including enucleation can also occur in the absence of macrophages both in vivo and in vitro. We reported previously that loss of Rb induces cell-autonomous defects in red cell maturation under stress conditions, while other reports have suggested that the failure of Rb-null erythroblasts to enucleate is due to defects in associated macrophages. Here we show that erythropoietic islands are disrupted by hypoxic stress, such as occurs in the Rb-null fetal liver, that Rb−/− macrophages are competent for erythropoietic island formation in the absence of exogenous stress and that enucleation defects persist in Rb-null erythroblasts irrespective of macrophage function.
Introduction
Erythropoietic islands consist of a central macrophage surrounded by erythroblasts at various stages of development.1–3 Proposed roles for macrophages in erythroid differentiation include a simple scaffolding function,4 promotion of red cell proliferation,5 provision of concentrated EPO, direct transfer of iron or iron-regulating factors,6 inhibition of apoptosis, and more recently clear evidence has emerged testifying to the importance of macrophages in the engulfment of nuclei extruded by mature red cells.7
Mice carrying a targeted deletion of the Rb tumor suppressor gene in their germ line die at E13.5 to E14.5 of gestation, exhibiting extensive cell death in multiple tissues, including the fetal liver, and this has been attributed to systemic ischemia due to defective placental function and anemia.8,9 The production of enucleated mature red blood cells appeared normal in mice derived from germ-line Rb-null chimeras10 and in conditionally targeted E15.5 embryos in which Rb was not deleted in the placenta.8 However, analysis of erythroblast maturation in vitro11,12 or in Rb-null chimeric mice that had been challenged in vivo with phenylhydrazine to induce stress erythropoiesis recapitulated the erythroid maturation defects seen previously in the developing Rb-null fetal liver, including the failure to enucleate and up-regulate TER119.12 Thus, we proposed that pRb was required in a cell-intrinsic manner to regulate erythropoiesis, specifically under conditions of oxidative or proliferative stress,13 such as occurs in the Rb-null embryo with a defective placenta,8 or in Rb-null chimeric mice with hemolytic anemia.12
An additional layer of complexity was added to our understanding of the erythroid defect in Rb-null mice with work identifying a defect in macrophage maturation and island formation at E13.5, coincident with embryonic lethality.14 Since E13.5 Rb-null mice exhibit extensive cell death in the liver and other tissues,15,16 we questioned what effect hypoxia and cell death in the Rb-null fetal liver environment might have on erythropoietic island formation, since both ischemia and dying cells are known to act extrinsically on macrophages to promote their proliferation and migration.17,18 We examined the number and activity of macrophages and erythroblast islands in wild-type and Rb-null fetal liver in vivo as a function of the extent of hypoxia and cell death, and determined how hypoxia affected erythroblast island integrity in vitro as a function of constitutive or conditional deletion of Rb. We have concluded that chronic hypoxia disrupts erythroblastic islands, Rb-deficient macrophages are competent for island formation in the absence of exogenous stress, and Rb-null erythroblasts are defective for enucleation irrespective of macrophage function.
Materials and methods
Mice
Timed matings of Rb heterozygous and Rbflox/flox;Meox2-Cre mice were carried out as described previously.12 “E13.5 early” embryos were defined as those dissected before noon on the 13th day of gestation that showed mild or no edema. “E13.5 late” embryos used in this study were dissected after 5pm on the 13th day of gestation and exhibited severe edema.
Immunohistochemistry
Flow cytometry
Flow cytometry was carried out on disaggregated fetal liver cells as described previously12 using a PE conjugated anti-F4/80 antibody (Caltag, Burlingame, CA). The percentage of enucleated cells was determined by flow cytometry as a function of Draq5 uptake by cells gated for side scatter.
Cell culture, colony assays, and erythroblast island reconstitution experiments
Macrophage-enriched stroma was generated by culturing wild-type whole bone marrow in coculture expansion media (IMDM media, with 15% FCS, 2 U/mL Epo, 50 ng/mL stem cell factor, 10 ng/mL interleukin-3, 140 mM monothioglycerol, and 1 μM dexamethasone). Following 48 hours in culture, cells were trypsinized and plates were washed to remove trypsin-sensitive cells (adhesion of erythroblasts to macrophages is trypsin sensitive, while adhesion of macrophages to the dish is trypsin resistant). Macrophage cultures were then returned to coculture media and cultured for at least 48 hours. These cultures continued to expand for at least 2 weeks. “Purified” native and reconstituted islands were obtained following rigorous washing of macrophage-erythroid clusters in PBS/2.5 μM CaCl2 to remove nonadherent cells. Liquid culture of primary erythroblasts and colony-forming unit erythroid (CFU-E) colony assays was carried out as described previously.12 colony-forming units-granulocyte, erythrocyte, monocyte (CFU-GEMMs) were isolated and cytospun following 12 days of culture in methyl cellulose (M3434; StemCell Technologies, Vancouver, BC). Suspension cultures matched to macrophage cocultures were obtained by releasing macrophage-bound erythroid progenitors from “purified” erythroblastic islands in 0.5 mM EDTA/PBSA. For growth under hypoxic conditions, cultures were grown for approximately 16 hours in a specialized, humidified chamber equilibrated with air at 1.0% oxygen/94% nitrogen/5% carbon dioxide.
Reconstitution of macrophage islands was carried out by replacing macrophage expansion media with differentiation media (StemSpan SFEM media [Stem Cell Technologies] with 15% FCS, 2 U/mL erythropoietin, 10 ng/mL interleukin-3), containing 106 fetal liver cells per milliliter precleared of adherent cells by 2 hours in vitro culture. Enucleation cocultures were maintained at high density on macrophage-enriched stroma and cells were isolated prior to flow cytometry by washing in 0.5 mM EDTA/PBSA. Macrophage-depleted suspension cultures were generated by plating into wells lacking macrophage-enriched stroma and then serially transferring nonadherent cells to fresh wells 2 and 16 hours following addition of differentiation media.
Fluorescence microscopy and island counts
Native islands were isolated by gentle disaggregation of E12.5 and E13.5 fetal livers using a 23-gauge needle and equivalent cell numbers were plated in 3-cm tissue culture wells for 2 hours. Plates were gently washed in media and cultured for an additional 2 hours before a final wash and incubated for an additional 12 hours prior to fixation. Erythropoietic islands were fixed in CSK buffer (100 mM NaCl, 300 mM sucrose, 10 mM PIPES [pH 6.8], 3 mM MgCl2, 1 mM EGTA) with 4% paraformaldehyde for 5 minutes followed by 3:1 methanol-acetone. Cells were labeled in PBS containing 2% fetal calf serum with PE-conjugated F4/80 antibodies (Caltag) and biotin-conjugated rat antimouse TER119 antibodies (BD Pharmingen), followed by streptavidin-conjugated FITC (BD Pharmingen) and visualized on an Axiovert 200 epifluorescent microscope (Carl Zeiss, Heidelberg, Germany). Islands were recorded as the number of islands (1 adherent macrophage + ≥ 5 attached erythroblasts = 1 island) in 20 randomly selected 100× microscope fields, and the number of erythroblasts per macrophage was counted on the first 20 randomly encountered F4/80-positive cells bearing 1 or more associated TER119-positive cells on their surface. Light microscopic images were obtained using a DMLB microscope (Leica Microsystems, Frankfurt, Germany) using Leica HC PL fluotar objectives at 10×/0.30 NA, 20×/0.50 NA, or 40×/0.75 magnification. Digital images were captured using an Insight QE digital camera and acquired images were analyzed using associated SPOT imaging software version 4.0.2 (Diagnostic Instruments, Sterling Heights, MI) and Adobe Photoshop version 7.0 (Adobe, San Jose, CA).
Electron microscopy
Fetal livers were fixed in 2.5% glutaraldehyde, and sections analyzed using a Philips CM120 transmission electron microscope (New York, NY) at 120 kV.
Microarray data and quantitative real-time PCR
Wild-type and Rb-null fetal liver microarray data have been deposited in NCBI's Gene Expression Omnibus (GEO)20 and are accessible through GEO Series accession number GSE6787 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc = GSE6787), including detailed preparation, analysis, and statistical methods. Briefly, microarray data were analyzed using dChip software and “invariant set normalization.” Differential expression of genes was determined using the Anova software's (DNA Chip Analyzer (dChip), Harvard University, Cambridge, MA) parametric test assuming unequal variance. Uncorrected P values were used to identify differentially expressed genes of interest in order to avoid exclusion of potentially interesting candidates, and differential expression of candidate genes was confirmed by Northern blot analysis or quantitative real-time polymerase chain reaction (QRT-PCR). Relative quantitation of real-time PCR product was performed using QuantiTect SYBR Green PCR (Qiagen, Valencia, CA) and the Applied Biosystems 7900 Fast Real-Time PCR System (Foster City, CA), and analyzed using the associated SDS 2.3 software (Applied Biosystems). Samples were amplified in triplicate and normalized by subtracting CT values for 18S rRNA.
Results
Erythroblast island defects in Rb-null fetal liver are associated with increased cell death
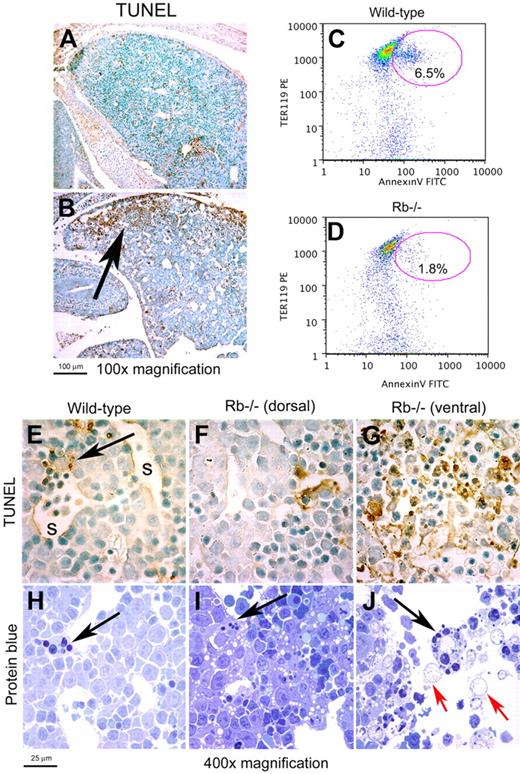
The majority of Rb-null embryos die in utero between E13.5 and E14.5 of gestation,21 and when we examined the fetal liver of Rb-null embryos at E13.5 prior to death of the embryo, we observed large regions of TUNEL positivity (Figure 1B) that were not present in the fetal liver of wild-type littermate control embryos (Figure 1A), and that were not evident in E12.5 fetal liver of either genotype (data not shown). TUNEL positivity in E13.5 Rb-null fetal liver was largely localized to the ventral tips that are most distant from the hepatic vasculature (Figure 1B arrow). Despite prominent, localized TUNEL staining, Rb-null fetal livers showed only low levels of annexin-V staining when analyzed by flow cytometry (Figure 1D). Since TUNEL labeling techniques detect DNA fragmentation, which occurs in both apoptotic and necrotic cell death,22 and given the placental defects and systemic anemia in Rb-null embryos, we set out to determine whether cell death at the distal tips of Rb-null fetal liver was in fact necrotic. High-power light microscopy of TUNEL-stained (Figure 1G) and protein blue-stained Rb-null fetal liver (Figure 1J) showed extensive extracellular debris, loss of defined cellular morphology, and the presence of distended, remnant nuclei (Figure 1J red arrows), which are all typical features of necrosis,23 but few identifiable pyknotic nuclei (Figure 1G). Thus, our data indicate increased necrotic cell death in poorly vascularized regions of the Rb-null fetal liver, consistent with ischemia induced by placental and red blood cell defects.8,12
Localized necrotic cell death in Rb-null fetal liver. (A-B) TUNEL immunohistochemistry on sagittal sections of wild-type and Rb-null E13.5 fetal liver. (C-D) Representative flow cytometric analysis of annexin-V and TER119 staining on disaggregated whole E13.5 fetal liver from wild-type (C, 7.1% ± 0.6%, P < .002) and Rb-null (D, 2.8% ± 1.0%, P < .002) embryos to detect phosphotidyl serine externalization. (E-G) TUNEL staining for DNA fragmentation of wild-type (E) and Rb-null (F-G) E13.5 fetal livers (1000× magnification). The arrow in panel E identifies a phagocytic macrophage and s indicates a liver sinusoid. The dorsal region of the Rb-null fetal liver (F) shows markedly less TUNEL positivity than the ventral end of the liver that is distant from the hepatic vasculature. (H-J) Protein blue staining of sections of E13.5 wild-type fetal liver (H), more dorsal, healthy regions of the Rb-null fetal liver (I), and dying regions in the ventral end of the Rb−/− fetal liver (J) with black arrows indicating the presence of phagocytic macrophages and red arrows indicating distended, remnant nuclei. See ″Fluorescence microscopy and island counts″ for image acquisition information.
Localized necrotic cell death in Rb-null fetal liver. (A-B) TUNEL immunohistochemistry on sagittal sections of wild-type and Rb-null E13.5 fetal liver. (C-D) Representative flow cytometric analysis of annexin-V and TER119 staining on disaggregated whole E13.5 fetal liver from wild-type (C, 7.1% ± 0.6%, P < .002) and Rb-null (D, 2.8% ± 1.0%, P < .002) embryos to detect phosphotidyl serine externalization. (E-G) TUNEL staining for DNA fragmentation of wild-type (E) and Rb-null (F-G) E13.5 fetal livers (1000× magnification). The arrow in panel E identifies a phagocytic macrophage and s indicates a liver sinusoid. The dorsal region of the Rb-null fetal liver (F) shows markedly less TUNEL positivity than the ventral end of the liver that is distant from the hepatic vasculature. (H-J) Protein blue staining of sections of E13.5 wild-type fetal liver (H), more dorsal, healthy regions of the Rb-null fetal liver (I), and dying regions in the ventral end of the Rb−/− fetal liver (J) with black arrows indicating the presence of phagocytic macrophages and red arrows indicating distended, remnant nuclei. See ″Fluorescence microscopy and island counts″ for image acquisition information.
Interestingly, wild-type embryos exhibited occasional TUNEL-positive foci evenly distributed throughout the fetal liver (Figure 1A) with individual foci localized primarily adjacent to fetal liver sinusoids (Figure 1E arrow), where phagocytic macrophages were easily identifiable in protein blue-stained sections (Figure 1H arrow). Red cell enucleation is accompanied by externalization of phosphotidyl serine,7 and this may explain the increased level of annexin-V staining in wild-type fetal liver compared with Rb-null fetal liver that fails to exhibit red cell enucleation. Thus, TUNEL-positive foci in wild-type fetal liver, consistent with low levels of Ter119/annexin-V double-positive staining (Figure 1C), likely represent phagocytosed erythroid nuclei similar to those reported following phagocytosis of thymocyte nuclei.24 Consistent with this hypothesis and the failure of Rb-null erythroblasts to enucleate, Rb-null fetal livers had many fewer TUNEL-positive foci in the main body of the liver, which is more extensively vascularized (Figure 1B,F). By contrast, the necrotic regions of the Rb-null fetal liver contained numerous phagocytic macrophages (Figure 1J black arrow).
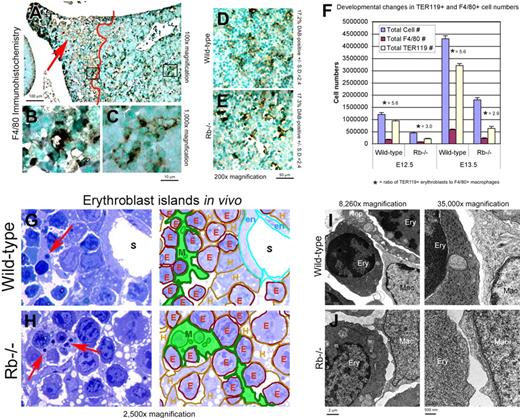
Given that both hypoxia and dying cells induce macrophages to proliferate, migrate, and aggregate,17,18 we postulated that defects in macrophage function and erythroblast island formation in E13.5 Rb-null fetal livers14 were indirect consequences of ischemic cell death in distal hepatic lobes. When we examined expression of F4/80 (a macrophage-specific marker) in situ in the fetal liver of E13.5 Rb-null mice, we observed staining consistent with the reticulate nature of erythroblastic island macrophages but only in those parts of the Rb-null fetal liver that were healthy and distant from regions of ischemia and cell death (Figure 2A right side; 2C). By contrast, the F4/80-positive macrophages that appeared to accumulate in the TUNEL-positive regions of the Rb-null fetal liver (Figure 2A left side, red arrow; 2D) did not form a reticulate structure around erythroblasts (Figure 2B), indicating that erythroblast islands were disrupted. We noted that while erythroblast island-associated macrophages were dispersed throughout wild-type fetal liver, phagocytic macrophages were most prominent around liver sinusoids (Figure 1H arrow). Similarly, in healthy regions of the Rb-null liver, phagocytes were detected adjacent to liver sinusoids (Figure 1I arrow). However, in dying regions of the Rb-null liver, phagocytes were more numerous away from defined ductal structures and were packed with dying cells and debris (Figure 1J black arrow). These results suggest that macrophages in the Rb-null fetal liver may be diverted from their role in erythropoietic island formation to promote clearance of dying cells.
Rb-null macrophages are competent for erythroblast interactions in vivo. (A-C) Immunohistochemical staining for F4/80 on Rb-null fetal liver showing the increased numbers of F4/80+ macrophages accumulating in the distal tip of the liver (red arrow, B), and the difference in staining of F4/80 in healthy regions of the liver to the right of the red line (D) compared with dying and ischemic regions of the liver to the left of the red line (C). (D-E) F4/80 immunohistochemistry on intact Rb-null (F) and wild-type (E) fetal liver with F4/80-positive surface area determined by optical densitometry for DAB positivity. (F) Graphic representation of total fetal liver cell number, number of F4/80-positive macrophages, number of TER119-positive erythroblasts, and ratio of TER119-positive erythroblasts to F4/80-positive macrophages in wild-type and Rb-null fetal livers at E12.5 and E13.5. At E12.5, F4/80-positive macrophages represented 13.2% (± 2.7%) and 15.4% (± 0.9%) of total cell number for wild type and Rb null, respectively, and 13.4% (± 4.0%) for wild-type and 12.2% (± 0.4%) for Rb null at E13.5. (G-H) Protein blue-stained E13.5 wild-type (H) and Rb-null (I) fetal livers showing erythroblastic islands in situ (left) with a color-coded key (right) to indicate distinct cell types, including macrophages (M, green), erythroblasts (E, red), hepatocytes (H, brown), endothelial cell (en, turquoise), and liver sinusoids (S). (I-J) Electron microscopy of macrophage-erythroblast contacts in E13.5 wild-type (J) and Rb-null (K) fetal liver. Erythroblasts (Ery), macrophages (Mac), and hepatocytes (Hep) were distinguished on the basis of mitochondrial size and numbers (hepatocytes have larger, more numerous mitochondria), nuclear and cytoplasmic electron density, and smaller cell size (erythroblasts) and larger cell size, cytoplasmic projections, and inclusion bodies (macrophages). See ″Fluorescence microscopy and island counts″ for image acquisition information.
Rb-null macrophages are competent for erythroblast interactions in vivo. (A-C) Immunohistochemical staining for F4/80 on Rb-null fetal liver showing the increased numbers of F4/80+ macrophages accumulating in the distal tip of the liver (red arrow, B), and the difference in staining of F4/80 in healthy regions of the liver to the right of the red line (D) compared with dying and ischemic regions of the liver to the left of the red line (C). (D-E) F4/80 immunohistochemistry on intact Rb-null (F) and wild-type (E) fetal liver with F4/80-positive surface area determined by optical densitometry for DAB positivity. (F) Graphic representation of total fetal liver cell number, number of F4/80-positive macrophages, number of TER119-positive erythroblasts, and ratio of TER119-positive erythroblasts to F4/80-positive macrophages in wild-type and Rb-null fetal livers at E12.5 and E13.5. At E12.5, F4/80-positive macrophages represented 13.2% (± 2.7%) and 15.4% (± 0.9%) of total cell number for wild type and Rb null, respectively, and 13.4% (± 4.0%) for wild-type and 12.2% (± 0.4%) for Rb null at E13.5. (G-H) Protein blue-stained E13.5 wild-type (H) and Rb-null (I) fetal livers showing erythroblastic islands in situ (left) with a color-coded key (right) to indicate distinct cell types, including macrophages (M, green), erythroblasts (E, red), hepatocytes (H, brown), endothelial cell (en, turquoise), and liver sinusoids (S). (I-J) Electron microscopy of macrophage-erythroblast contacts in E13.5 wild-type (J) and Rb-null (K) fetal liver. Erythroblasts (Ery), macrophages (Mac), and hepatocytes (Hep) were distinguished on the basis of mitochondrial size and numbers (hepatocytes have larger, more numerous mitochondria), nuclear and cytoplasmic electron density, and smaller cell size (erythroblasts) and larger cell size, cytoplasmic projections, and inclusion bodies (macrophages). See ″Fluorescence microscopy and island counts″ for image acquisition information.
Macrophages are neither sufficient nor required for red cell enucleation
Rb-null fetal livers are 2- to 3-fold smaller than wild-type in terms of total cell number at both E12.5 and at E13.5 (Figure 2F). However, the number of F4/80-positive cells relative to total cell number was similar between wild type and Rb null at both gestational ages (Figure 2F; E12.5: 13.2% ± 2.7% for wild-type, 15.4% ± 0.9% for Rb null; E13.5: 13.4% ± 4.0% for wild-type, 12.2% ± 0.4% for Rb null). Importantly, there were proportionately more macrophages per erythroblast in the Rb-null fetal liver than in the wild-type (Figure 2F) with approximately 5.6 erythroblasts for every F4/80-positive macrophage in the wild-type fetal liver and approximately 3.0 erythroblasts to every macrophage in the Rb-null fetal liver. This argues against there being insufficient macrophages to bind developing erythroblasts in the Rb-null fetal liver. The absence of any reduction in proportionate representation of F4/80-positive macrophages between wild type and Rb null is consistent with data showing that the quality of erythroblastic islands in the intact proximal regions of the Rb-null fetal liver is similar to wild type. In these healthy regions of the Rb-null fetal liver, F4/80 staining appeared normal with a comparable surface area per section in Rb-null fetal liver (17.3% DAB positive, Figure 2E) compared with wild type (17.2% DAB positive, Figure 2D).
When we examined the interaction of macrophages with erythroblasts in wild-type and in healthy regions of the Rb-null fetal liver by high-power light microscopy and electron microscopy, we observed well-formed erythroblastic islands with differentiating erythroblasts surrounding macrophages in both genetic contexts (Figure 2G,H). Both wild-type and Rb-null island macrophages also contained phagocytic particles that could represent nuclei or other cellular debris (Figure 2G,H red arrows). In transmission electron micrographs, we were able to discern direct interactions between Rb-null macrophages and erythroblasts in vivo (Figure 2J). The cell-cell contacts made between Rb-null macrophages and erythroblasts (Figure 2J right panel) were indistinguishable from those observed in wild-type fetal liver (Figure 2I right panel). These data indicate that Rb-null macrophages bind erythroblasts in vivo and carry out phagocytosis, consistent with a mature macrophage function for Rb-null macrophages in erythropoietic island formation.
When we plated native erythroblast islands from disaggregated E12.5 fetal livers in media that support their attachment to tissue culture dishes and expansion of associated erythroblasts, and grew these cultures for 16 hours in vitro, we were able to identify native erythroblastic islands and observed that Rb-null macrophages (Figure 3B red) and wild-type macrophages (Figure 3A red) were both competent to bind erythroblasts (green). Similarly, when we examined the development of erythroblasts cultured in vitro in close contact with macrophages in the context of CFU-GEMM colonies derived from E12.5 fetal liver, we observed that Rb-null erythroblasts bound to macrophages (Figure 3D arrow). Nevertheless, red cell enucleation was not observed despite the presence of many benzidine-positive erythroid cells in Rb-null CFU-GEMM colonies (Figure 3D), in contrast to wild-type CFU-GEMM colonies where many enucleated red cells were seen by day 12 in methylcellulose culture (Figure 3C). The lack of enucleated cells in Rb-null CFU-GEMM colonies despite cell-cell contacts suggests that macrophage-erythroid interactions alone are insufficient to restore enucleation capacity to Rb-null erythroblasts in vitro. To further assess how Rb deficiency affected erythropoietic island integrity, we quantified the effect of Rb loss on both the number of islands recovered in vitro (Figure 3F) and the number of erythroblasts per macrophage (Figure 3E) as a function of gestational age. We observed that both the number and size of native erythroblastic islands recovered after 16 hours in culture were indistinguishable between wild-type and Rb-null cultures when E12.5 or early E13.5 fetal liver was used as a source of cells (Figure 3E-F). However, when fetal liver from late E13.5 Rb-null mice, which has extensive amounts of cell death (Figure 1B,G), is used as a source of cells, we observe a significant reduction in the number of islands and the number of erythroblasts per macrophage recovered either at 4 or 16 hours in culture (Figure 3E-F) relative to littermate wild-type controls. These results indicate that defects in erythroblast island formation correlate more strongly with the extent of cell death in the fetal liver than with Rb status.
Macrophages are not required for erythroid enucleation. (A,B) Native erythroblast islands from disaggregated wild-type (left) or Rb−/− (right) E12.5 fetal liver were examined 16 hours after culture, using anti-F4/80 to stain macrophages (red) and anti-TER119 (green) to stain erythroblasts, and Hoechst 33342 for DNA, indicating no difference in island formation between wild-type and Rb-null-derived cells. (C,D) High-magnification analysis of the interaction between red cells (benzidine-stained brown cells) and macrophages (large “foamy” cells) is examined in the context of 12-day CFU-GEMM colony differentiation derived from wild-type (C) or Rb-null (D) fetal liver progenitors. We noted that Rb-null erythroblasts (D) failed to enucleate irrespective of macrophage contacts (arrow) and demonstrated increased size and abnormal chromatin structure as reported previously.11,12 (E,F) Native erythroblast islands cultured in vitro from mice with the indicated Rb genotypes and at early or later stages of embryonic development were enumerated (F). Only definitive islands in which 5 or more erythroblasts were bound were counted as islands. Numbers of erythroblasts per macrophage were also counted (E). Experiments were carried out in triplicate. Error bars are standard deviations. Significant differences were observed for the number of erythroblasts per macrophage in “late” E13.5 wild-type and Rb-null fetal liver after 4 hours (P = .001) or 16 hours (P = .001) in culture (E). Student t test. (G) Quantitation of enucleation by flow cytometric analysis of all erythroid cells (floating and attached) was carried out after 60 hours in culture, either in the presence of macrophages and erythroblast island formation or in suspension culture. The percentage of enucleated Rb-null cells was significantly reduced relative to wild type in suspension culture (P < .009) and in macrophage coculture (P < .003). (H) Purified erythroblast progenitors from E12.5 fetal livers were cultured for 48 hours in adherence with macrophages or in suspension culture, and then harvested for CFU-E assay in methylcellulose. Viable cells (250) were plated in triplicate per sample. Numbers of CFU-Es formed were compared with cultures seeded with erythroblasts recovered after only 90 minutes in culture (day 0). See ″Fluorescence microscopy and island counts″ for image acquisition information.
Macrophages are not required for erythroid enucleation. (A,B) Native erythroblast islands from disaggregated wild-type (left) or Rb−/− (right) E12.5 fetal liver were examined 16 hours after culture, using anti-F4/80 to stain macrophages (red) and anti-TER119 (green) to stain erythroblasts, and Hoechst 33342 for DNA, indicating no difference in island formation between wild-type and Rb-null-derived cells. (C,D) High-magnification analysis of the interaction between red cells (benzidine-stained brown cells) and macrophages (large “foamy” cells) is examined in the context of 12-day CFU-GEMM colony differentiation derived from wild-type (C) or Rb-null (D) fetal liver progenitors. We noted that Rb-null erythroblasts (D) failed to enucleate irrespective of macrophage contacts (arrow) and demonstrated increased size and abnormal chromatin structure as reported previously.11,12 (E,F) Native erythroblast islands cultured in vitro from mice with the indicated Rb genotypes and at early or later stages of embryonic development were enumerated (F). Only definitive islands in which 5 or more erythroblasts were bound were counted as islands. Numbers of erythroblasts per macrophage were also counted (E). Experiments were carried out in triplicate. Error bars are standard deviations. Significant differences were observed for the number of erythroblasts per macrophage in “late” E13.5 wild-type and Rb-null fetal liver after 4 hours (P = .001) or 16 hours (P = .001) in culture (E). Student t test. (G) Quantitation of enucleation by flow cytometric analysis of all erythroid cells (floating and attached) was carried out after 60 hours in culture, either in the presence of macrophages and erythroblast island formation or in suspension culture. The percentage of enucleated Rb-null cells was significantly reduced relative to wild type in suspension culture (P < .009) and in macrophage coculture (P < .003). (H) Purified erythroblast progenitors from E12.5 fetal livers were cultured for 48 hours in adherence with macrophages or in suspension culture, and then harvested for CFU-E assay in methylcellulose. Viable cells (250) were plated in triplicate per sample. Numbers of CFU-Es formed were compared with cultures seeded with erythroblasts recovered after only 90 minutes in culture (day 0). See ″Fluorescence microscopy and island counts″ for image acquisition information.
To quantify the effect of macrophages on erythroid enucleation, we plated fetal liver erythroblasts (precleared of adherent cells) at high density in the presence or absence of a wild-type macrophage-enriched stroma and cultured for 60 hours. Red cell enucleation was measured by flow cytometric analysis of Draq5 uptake (a vital dye that binds nuclear DNA). We failed to detect any significant effect of macrophage coculture on the percentage of enucleated cells in either wild-type or Rb-null cultures (Figure 3G), indicating that wild-type macrophages were unable to restore enucleation potential to Rb-null erythroblasts in vitro.
To address alternative roles for macrophages in regulating erythropoiesis, we examined the effect of macrophages on the number of erythroid colony-forming units (CFU-Es) recovered from cocultures after 2 days. Islands were allowed to form for 90 minutes on macrophage-enriched stroma and were then washed in the presence of calcium revealing many distinct erythroblastic islands. Erythroblasts were released from islands by washing in the absence of calcium, either immediately (day 0 and suspension culture) or after 48 hours (macrophage coculture). After growth in suspension culture for 2 days, the number of progenitors that gave rise to CFU-E colonies (when equal numbers of cells were plated in methylcellulose with erythropoietin) was dramatically reduced (Figure 3H suspension culture) compared to that obtained with cells obtained directly following release from islands formed after 90 minutes (Figure 3H day 0). Interestingly, culturing either wild-type or Rb-null fetal liver erythroblasts on macrophages prevented depletion of CFU-Es in 48-hour cultures (Figure 3H macrophage coculture). These results indicate that while macrophages do not appear to promote red cell enucleation, they can promote retention of CFU-E capacity among erythroid progenitors.
Hypoxia disrupts erythroblast island formation independent of Rb status
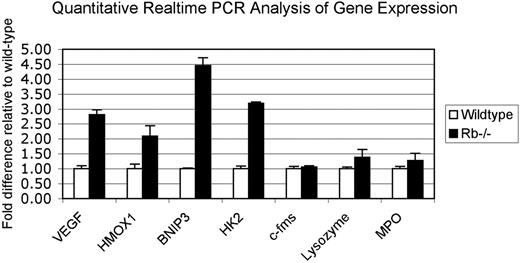
To gain molecular insight into the erythroblastic island defect in Rb-null fetal liver, we performed differential gene expression array analysis on E12.5 wild-type and Rb-null fetal liver (Table 1, NCBI GEO Series accession number GSE6787). This analysis revealed a marked induction of known hypoxia-inducible genes in the Rb-null fetal liver compared with wild-type littermate controls (Table 1). For example, there was a statistically significant induction of VEGF (vascular endothelial growth factor), heme oxygenase-1, BNip3, hexokinase II, and PGK-1 in Rb-null fetal liver relative to wild type (Table 1). Induction of these genes in the Rb-null fetal liver, relative to wild-type control liver, was confirmed by real-time PCR (Figure 4). The induction of known HIF target genes in the E12.5 Rb-null fetal liver is consistent with ischemia (due to placental defects and anemia) being the cause, as opposed to the consequence, of the cell death that is first detected in E13.5 Rb-null fetal liver.8,9,12
Up-regulation of hypoxia-inducible genes in Rb-null fetal liver without changes in macrophage-specific or PU1 target genes. Quantitative real-time PCR using wild-type and Rb-null total E13.5 fetal liver cDNA was used to validate and extend data from differential microarray analysis of gene expression. Values are the mean value obtained from 3 separate data sets for each genotype with the wild-type values normalized to 1.0 and values for Rb null samples expressed relative to wild-type. Error bars represent the standard deviation from the mean for each genotype.
Up-regulation of hypoxia-inducible genes in Rb-null fetal liver without changes in macrophage-specific or PU1 target genes. Quantitative real-time PCR using wild-type and Rb-null total E13.5 fetal liver cDNA was used to validate and extend data from differential microarray analysis of gene expression. Values are the mean value obtained from 3 separate data sets for each genotype with the wild-type values normalized to 1.0 and values for Rb null samples expressed relative to wild-type. Error bars represent the standard deviation from the mean for each genotype.
We also used the microarray data and quantitative real-time PCR to examine how loss of Rb affected expression of macrophage-specific genes that had been previously implicated in the erythroblast island defect in Rb-null fetal liver,14 including c-Fms, myeloperoxidase (MPO), cathepsin S, complement components, and lysozyme. In contrast to the effect of Rb loss on hypoxia-inducible gene expression, we failed to detect any significant change in the expression levels of most macrophage-specific genes in Rb-null fetal liver relative to wild-type at E12.5 (Figure 4). Expression of c-Fms (a PU1 target gene) was previously shown to be deregulated in Rb-null MEFs and, together with data implicating deregulated PU1 in erythroblast island defects in Rb-null fetal liver, was taken as evidence that macrophages were not functioning normally in Rb-null fetal liver.14 However, we were unable to detect any reduction in c-Fms levels by quantitative real-time PCR (Figure 4) in Rb-null fetal liver relative to wild type and, together with other reports showing that Id2 overexpression is insufficient to suppress c-Fms expression,25 is more consistent with ischemia, as opposed to macrophage defects, being the major cause of erythroblastic island defects and cell death in the Rb-null fetal liver.
Given the correlation between erythroblast island disruption, hypoxia-inducible gene expression, and ischemic cell death in the Rb-null fetal liver, we speculated that erythroblast island formation was defective in late-stage Rb-null embryos due to hypoxia. To test the hypothesis that hypoxia affects erythroblast island stability, we counted the total number of erythroblast islands and the number of erythroblasts per macrophage in cultures of native islands grown at either 21% oxygen (regular tissue culture conditions) or 1.0% oxygen (hypoxia). For these assays, we used fetal liver from control mice (Rbflox/flox) and conditionally targeted Rbflox/flox;Meox2-Cre mice in which Rb is deleted in the embryo but not in the placenta8 to control for differential effects of ischemia experienced in vivo. At 21% oxygen, we failed to observe any significant difference in numbers of islands or in erythroblasts per macrophage formed with Rbflox/flox;Meox2-Cre fetal liver cells relative to control Rbflox/flox fetal liver (Figure 5A,B). These observations are consistent with data obtained previously showing no difference in numbers or size of erythroblast islands between wild-type and Rb-null fetal livers at either E12.5 or early E13.5 (Figure 3E,F), prior to overt fetal liver deterioration. However, when we cultured fetal liver from Rbflox/flox controls or from Rbflox/flox;Meox2-Cre mice under hypoxic conditions (1.0% oxygen), we observed a dramatic reduction in both the number of islands and the number of erythroblasts per macrophage (Figure 5A,B) suggesting that hypoxia, as opposed to Rb gene status, was instrumental in disrupting erythroblast islands. Indeed, the number of islands and erythroblasts per macrophage under hypoxic conditions was similar to that observed for late E13.5 Rb-null fetal liver (Figure 3E,F). Cytologic analysis of erythroblast islands revealed that in contrast to growth at 21% oxygen (Figure 5C,D), growth at 1% oxygen (hypoxia) induced most erythroblasts to detach from macrophages (Figure 5E,F) such that most erythroblasts were found in suspension (Figure 5G,H). These results demonstrate that hypoxia disrupts erythroblast islands in vitro and, together with data showing that the incidence of hypoxia in the Rb-null fetal liver coincides with disruption of erythroblast islands in vivo, strongly support our hypothesis that erythroblast island formation in late-stage Rb-null embryos is defective due to secondary consequences of placental malfunction and anemia.
Hypoxia disrupts erythropoietic islands. (A,B) The effect of hypoxia on erythropoietic island numbers and numbers of erythroblasts per macrophage was quantified as described previously (Figure 3E,F). Hypoxia had a greater effect on both island integrity and numbers of erythroblasts per macrophage than did the Rb status of the cells. Differences in numbers of erythroblasts per macrophage as a function of oxygen tension (21% vs 1% O2) were significant in the absence of Cre expression (P < .001) and in the presence of Cre expression (P < .001). The numbers of islands formed at 21% compared with 1% O2 were also significant (P < .04 for Cre− and P < .02 for Cre+). Error bars represent standard deviation from the mean value for numbers of erythroblasts per island (A) and numbers of islands (B). (C-H) The effect of hypoxia on erythroblast island integrity was visualized by staining of adherent (C-F) and floating (G,H) cells from purified island cultures exposed to 21% oxygen for 36 hours (C,D) or to 21% oxygen for 18 hours and subsequently to 1.0% oxygen for 18 hours (E-H). Significant differences were observed for the number of erythroblasts per macrophage in E13.5 wild-type and Rb-null fetal liver after 4 hours (P = .001) or 16 hours (P = .001) in culture at 21% oxygen and between Rb-positive and Rb-deficient cultures derived from Rbflox mice when cultured at 1% oxygen versus 21% oxygen for 12 hours (P = .001 for Cre+; P = .001 for Cre−) but not between wild type and Rb null earlier in gestation or possessing a wild-type placenta (Rbflox;Cre+ vs Cre−). See ″Fluorescence microscopy and island counts″ for image acquisition information. (B) Island numbers were reduced in E13.5 Rb-null fetal liver compared with wild type (P = .01) and in conditionally targeted fetal livers following 16 hours at 1% oxygen when compared with normal tissue culture (P = .02 and P = .04, respectively) Notably, placental rescue increased Rb-deficient island number (P = .03; Student t test).
Hypoxia disrupts erythropoietic islands. (A,B) The effect of hypoxia on erythropoietic island numbers and numbers of erythroblasts per macrophage was quantified as described previously (Figure 3E,F). Hypoxia had a greater effect on both island integrity and numbers of erythroblasts per macrophage than did the Rb status of the cells. Differences in numbers of erythroblasts per macrophage as a function of oxygen tension (21% vs 1% O2) were significant in the absence of Cre expression (P < .001) and in the presence of Cre expression (P < .001). The numbers of islands formed at 21% compared with 1% O2 were also significant (P < .04 for Cre− and P < .02 for Cre+). Error bars represent standard deviation from the mean value for numbers of erythroblasts per island (A) and numbers of islands (B). (C-H) The effect of hypoxia on erythroblast island integrity was visualized by staining of adherent (C-F) and floating (G,H) cells from purified island cultures exposed to 21% oxygen for 36 hours (C,D) or to 21% oxygen for 18 hours and subsequently to 1.0% oxygen for 18 hours (E-H). Significant differences were observed for the number of erythroblasts per macrophage in E13.5 wild-type and Rb-null fetal liver after 4 hours (P = .001) or 16 hours (P = .001) in culture at 21% oxygen and between Rb-positive and Rb-deficient cultures derived from Rbflox mice when cultured at 1% oxygen versus 21% oxygen for 12 hours (P = .001 for Cre+; P = .001 for Cre−) but not between wild type and Rb null earlier in gestation or possessing a wild-type placenta (Rbflox;Cre+ vs Cre−). See ″Fluorescence microscopy and island counts″ for image acquisition information. (B) Island numbers were reduced in E13.5 Rb-null fetal liver compared with wild type (P = .01) and in conditionally targeted fetal livers following 16 hours at 1% oxygen when compared with normal tissue culture (P = .02 and P = .04, respectively) Notably, placental rescue increased Rb-deficient island number (P = .03; Student t test).
Discussion
The role of macrophages in providing a specialized microenvironment in which erythroblasts expand and mature has been of long-standing interest to hematologists.1,3 Insight into the role of macrophages in erythroblast islands and in red cell maturation has been aided by the use of novel mouse strains with phenotypes that affect the ability of macrophages and erythroblasts to interact, or inhibit the ability of macrophages to engulf extruded red cell nuclei. For example, gene targeting of DNase II in mice resulted in a deficit of macrophages in the developing fetal liver and mice died from anemia, leading the authors to propose a critical role for macrophages in promoting red cell enucleation.26 However, subsequent work has shown that defects in phagocytic macrophages resulted in interferon-β production and an inflammatory response to undigested DNA that actively killed developing erythroid cells.27 This report combined with recent elegant studies showing how externalization of phosphotidyl serine on the membrane of extruded nuclei was required for engulfment by macrophages have defined a critical role for macrophages in engulfing already extruded nuclei, as opposed to necessarily promoting the enucleation process itself.7 The notion that erythroid enucleation is primarily a cell autonomous process has been further backed up by analysis of erythroblasts deficient for the erythroblast macrophage protein (Emp) protein that are defective for erythroid enucleation despite attaching to wild-type macrophages, by other in vitro studies that demonstrate proficient terminal maturation of red cells in the absence of macrophages,28 and by studies in PU1-deficient mice that produce mature enucleated red cells despite developmental defects leading to the absence of mature macrophages.29,30
However, work examining the role of macrophages in the erythroid defect in Rb-null mice seemed to revise the appreciation of enucleation as a cell-intrinsic property of red cells, by proposing that defective macrophage function in the fetal liver of Rb-null mice underlies a non—cell-autonomous defect in red cell maturation.14 However, this concept was difficult to reconcile with the amelioration of Rb-null erythroid defects in mice provided with a wild-type placenta but harboring Rb-null macrophages.9 Our current work provides an explanation for these seemingly contradictory results by showing that Rb-null macrophages harvested from healthy fetal liver or bone marrow are not defective for erythroblast island formation in the absence of exogenous stress, that hypoxia can disrupt erythroblast islands in vitro, that macrophage coculture does not affect the level of red cell enucleation, and that the Rb-null fetal liver is hypoxic at times when erythroid islands are increasing in size and number in wild-type fetal liver.4 Thus, erythroblastic island defects in Rb-null mice and in ex vivo islands derived from these mice likely stem from the deleterious effects on macrophage-erythroblast interactions by hypoxia and the rapidly deteriorating microenvironment.
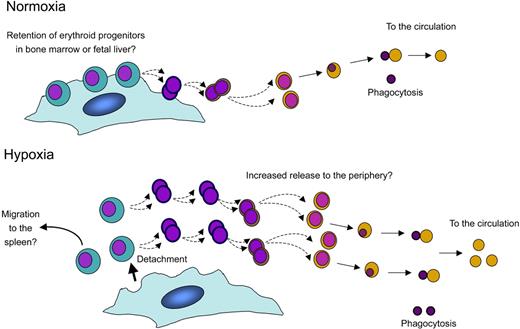
The idea that macrophage-erythroblast contacts are sensitive to oxygen concentration is intriguing. Under conditions of hypoxia, the animal requires increased numbers of mature red blood cells in the periphery to maximize oxygen transport. Does the role we propose for hypoxia in promoting dissociation of erythroblasts from the macrophage make sense in terms of the ability of the animal to respond to hypoxic stress? By modulating adhesion and associated signaling between erythroblasts and macrophages, hypoxia could have the practical effect of increasing the numbers of reticulocytes (a common sign of stress) and erythrocytes entering the bloodstream (Figure 6). Hypoxia could also increase the number of immature erythroblast progenitors released from the bone marrow that are capable of seeding the spleen, a major site of stress erythropoiesis,31 thereby contributing indirectly to erythroblast expansion under hypoxic stress. Nevertheless, it should be emphasized that cultures performed at atmospheric oxygen concentration (21%), as described in this paper, resulted in release of erythroblasts from macrophages earlier in terminal differentiation than reported in vivo.2,32 Further studies will be required to examine the effect of hypoxia on erythroblast island integrity in vivo. However, our data predict that the expression and/or interaction of key adhesion molecules on the surfaces of the red cells and macrophages may be sensitive to oxygen tension. It will therefore be interesting to determine whether adhesion molecules known to mediate macrophage-erythroblast interactions, such as ICAM-4 and α5-integrin33 or α4-integrin and VCAM-1,34 are altered in their expression or activity by changes in oxygen tension.
Model: a putative role for macrophages in the homeostatic response to hypoxia, based on in vitro analyses. In response to hypoxia, we propose that erythroblasts lose their tight adherence to central macrophages of erythroblastic islands, possibly promoting unrestrained maturation of erythroid progenitors, and increasing the number of red cells getting into the circulation. This may also promote the migration of immature erythroid progenitors to organs such as the spleen where their expansion is enhanced under stress conditions.31 In the Rb-null embryo, severe hypoxia and fetal liver necrosis disrupt erythropoietic islands concomitant with, but independent of, red cell enucleation defects and embryonic lethality.
Model: a putative role for macrophages in the homeostatic response to hypoxia, based on in vitro analyses. In response to hypoxia, we propose that erythroblasts lose their tight adherence to central macrophages of erythroblastic islands, possibly promoting unrestrained maturation of erythroid progenitors, and increasing the number of red cells getting into the circulation. This may also promote the migration of immature erythroid progenitors to organs such as the spleen where their expansion is enhanced under stress conditions.31 In the Rb-null embryo, severe hypoxia and fetal liver necrosis disrupt erythropoietic islands concomitant with, but independent of, red cell enucleation defects and embryonic lethality.
In summary, although macrophages may require Rb in other contexts, our work demonstrates that the capacity of Rb-null macrophages to mature and adhere to red cells is sufficient for them to form erythroblastic islands in a manner comparable with that of wild-type cells. Furthermore, our work suggests that both wild-type and Rb-null islands may be exquisitely sensitive to environmental cues including oxygen tension and that the hypoxia-induced cell death and fetal liver microenvironment deterioration that occur in the Rb-null mouse approaching the limits of its viability are the primary cause of erythroblast island defects in Rb-null fetal liver.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Work on this project was funded by RO1 HL080262 to K.F.M. from the National Heart Lung & Blood Institute of the National Institutes of Health.
The authors thank Yimei Chen for excellent technical assistance with electron microscopy and Jamie Zhou and Xinmin Li for technical work obtaining real-time PCR data.
National Institutes of Health
Authorship
Contribution: B.T.S., B.C.D., and K.F.M. participated in designing the research; B.T.S. and B.C.D. carried out the experimental work; K.F.M. wrote the paper; B.T.S., B.C.D., and K.F.M. edited and reviewed the final version of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kay F. Macleod, The Ben May Department for Cancer Research, GCIS-W338, The University of Chicago, 929 E 57th St, Chicago IL 60637; e-mail: kmacleod@huggins.bsd.uchicago.edu.