Abstract
Transfusion of crossmatch-incompatible red blood cells (RBCs) can result in antibody-mediated hemolysis. However, in some patients, crossmatch-incompatible RBCs lose the incompatible antigen from their surface, and then circulate normally (“antigen loss”). Although antigen loss has been reported in the settings of autoimmune hemolytic anemia and transfusion of crossmatch-incompatible RBCs, mechanistic understanding of this phenomenon is limited. Using an in vivo murine model of antigen loss, we report that, unlike polyclonal antisera, monoclonal antibodies did not induce antigen loss. However, the combination of 2 monoclonal antibodies that recognized separate epitopes on the same antigen induced antigen loss. This was not due to an increased number of Fc domains bound to the cell surface, because antigen loss still occurred when combining intact monoclonal IgG and F(ab′)2 fragments recognizing different epitopes. Together, these data lead to the hypothesis that antigen-antibody crosslinking is required for nonhemolytic antigen loss to occur.
Introduction
During transfusion of crossmatch-incompatible red blood cells (RBCs), recipient antibodies that bind to the transfused RBCs are well known to be able to induce hemolysis. In addition, autoantibodies to self-RBC antigens can form, leading to potentially fatal autoimmune hemolytic anemia. However, hemolysis is not the inevitable result of antibodies binding RBCs in either of these scenarios. In some patients, crossmatch-incompatible RBCs lose the incompatible antigen from their surfaces and then circulate normally (“antigen loss”).1–15 This phenomenon has been reported involving antibodies against several different blood group antigens, including single-pass transmembrane proteins (Kell and Kidd) and multipass transmembrane proteins (Duffy and Rh).1–14 Western blot analysis of human RBCs that have undergone antigen loss suggest that for at least one case of antigen loss in the Kell system, the entire protein that carries the blood group antigen is removed from the RBC, as opposed to just a destruction of the part of the molecule that constitutes the antigen.4
Although antigen loss has been described in multiple case reports, there is little mechanistic understanding of this phenomenon. We recently described an in vivo animal model of nonhemolytic antigen loss during transfusion of crossmatch-incompatible RBCs.15 mHEL mice, which are transgenic for a membrane-bound form of hen egg lysozyme (HEL),16 express the mHEL antigen on the RBC surface.15 Transfusion of mHEL RBCs into recipients that are actively or passively immunized against HEL (crossmatch-incompatible) leads to rapid antigen loss of mHEL but does not affect a separate RBC antigen on a different molecule; the resulting RBCs maintain a normal circulatory life-span.15
The reported human cases of antigen loss involve antibody-antigen pairs that are well known to cause hemolysis in other patients. There is currently no way to predict which patients will undergo hemolysis versus antigen loss. Accordingly, transfusions of crossmatch-incompatible RBCs are strictly avoided for antigen-antibody combinations with the potential to cause hemolysis. However, some chronically transfused patients mount alloantibodies against so many donor blood group antigens that identifying crossmatch-compatible blood is not possible. In these cases, patients must either forgo the potentially life-saving benefits of transfusion or undergo transfusion with incompatible RBCs and risk the dangers of hemolysis. Understanding factors that regulate hemolysis versus antigen loss may allow prediction of antigen loss in a given patient, which could lead to safe transfusion of crossmatch-incompatible RBCs.
In the current report, we analyzed the antibody responses that induce nonhemolytic antigen loss from transfused RBCs. We generated and characterized a panel of monoclonal anti-HEL antibodies to examine their ability to induce antigen loss. Together, the data presented herein suggest that the formation of a crosslinked antigen-antibody lattice on the RBC surface is required to produce nonhemolytic antigen loss.
Materials and methods
Mice
C57BL/6, BALB/c, and C3H mice were obtained from the Jackson Laboratory (Bar Harbor, ME). mHEL mice (the Jackson Laboratory) were bred by the Emory Division of Animal Resources Animal Husbandry Service (Atlanta, GA). Mice were maintained on standard rodent chow and water in a temperature- and light-controlled environment. Mice were used at 8 to 12 weeks of age, and all procedures were performed according to approved Institutional Animal Care and Use Committee (IACUC) protocols.
Generation of monoclonal anti-HEL antibodies and F(ab′)2 fragments
BALB/c mice were immunized with HEL in an emulsion of complete Freund adjuvant (HEL/CFA) followed by several boosts with HEL in incomplete Freund adjuvant (HEL/IFA). Anti-HEL titers were determined by HEL-specific enzyme-linked immunosorbent assay (ELISA), as described previously,15 and the ability of the polyclonal antisera to induce antigen loss was confirmed by transfusing with mHEL RBCs. Monoclonal antibodies were produced using standard hybridoma technology; each antibody was purified by cell culture services (BioExpress, West Lebanon, NH) using protein A chromatography. All antibodies were determined to be free of lipopolysaccharide before use. Monoclonal antibodies were directly conjugated using the Alexa Fluor 647 monoclonal antibody labeling kit according to the manufacturer's instructions (Molecular Probes division, Invitrogen, Carlsbad, CA). The 4B7 monoclonal antibody F(ab′)2 fragment was generated using the ImmunoPure IgG ficin kit (Pierce Biotechnology, Rockford, IL) according to the manufacturer's instructions. In brief, purified 4B7 protein was digested with ficin under conditions optimized to generate F(ab′)2 fragments. Intact IgG was then removed by protein A chromatography. The resulting F(ab′)2 fragments were tested for residual IgG by Western blot.
Antigen-loss assays
Recipient mice were injected with the indicated antibodies intravenously by tail vein injection. For each experiment, unless otherwise stated, 200 μg of antibody was used per animal. Antibodies included purified monoclonal anti-HEL antibodies, polyclonal murine anti-HEL antiserum (described previously),15 an anti-HEL IgG fraction purified by protein A chromatography using the MAPS buffer system (Bio-Rad Laboratories, Hercules, CA), or a purified monoclonal antibody against human glycophorin A (hGPA) (clone 10F7, IgG1).17,18 Blood was harvested, leukoreduced, and labeled with DiO [3,3′-dihexadecyloxacarbocyanine percholorate] and DiI [1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate] as described previously.15 All transfusions were carried out by tail-vein injection. Survival of transgenic RBCs was calculated as a ratio to the survival of wild-type RBCs and then the survival of transgenic RBCs in control mice (ie, crossmatch-compatible) was defined as 100%. Percentage survival of transgenic RBCs in incompatible mice was calculated by the formula [(transgenic RBC/wild-type RBC) in incompatible mice/(transgenic RBC/wild-type RBC) in compatible mice]. Staining with anti–mouse IgG (BD Pharmingen, San Jose, CA) and anti–mouse κ light chain (BD Pharmingen) were analyzed by flow cytometry.15
Results
Nonhemolytic antigen loss was not a general property of murine RBCs
We reported previously a flow cytometry-based method of tracking and characterizing transfused murine RBCs that are crossmatch-incompatible with the recipient mouse.15 This involves labeling transfused RBCs with a lipophilic dye that incorporates into the RBC plasma membrane. We used 2 dyes that fluoresce on distinct channels (DiO and DiI) to label crossmatch-compatible and -incompatible RBCs, respectively. By tracking survival of the incompatible population as a function of the compatible RBCs, we control for any volume loss as a result of phlebotomy, and precise survival curves are generated.
We used the mHEL mouse, which expresses RBC membrane-bound HEL, to generate a model of crossmatch incompatibility.15 Transfusing mHEL RBCs into wild-type HEL-immune mice expressing high anti-HEL titers, created a situation of crossmatch incompatibility. In this system, the transfused mHEL RBCs showed surprisingly low levels of RBC clearance, with most of the RBCs losing their mHEL antigen and then circulating normally.15 This effect was antigen-specific, in that no antigen loss occurred when mHEL RBCs were transfused into mice immunized against an irrelevant antigen (ie chicken ovalbumin). Moreover, only mHEL, but not a separate RBC surface molecule (ie TER 119), was lost after transfusion into HEL immunized mice.
Although nonhemolytic antigen loss has occasionally been documented in humans,1–14 hemolysis (and not antigen loss) is thought to be the usual result of crossmatch-incompatible RBC transfusion in humans. Therefore, it is possible that antigen loss in the mHEL system reflected a general biological difference between humans and mice, or was even an artifact of changes in the RBC membrane as a result of dye labeling. To test these hypotheses, we added a second model of crossmatch-incompatible transfusion using transgenic mice that express hGPA on their RBCs.19,20 Parallel experiments were performed comparing the mHEL and hGPA systems. Recipient mice were passively immunized with polyclonal anti-HEL antiserum, the IgG fraction from anti-HEL antiserum, or monoclonal anti-hGPA and then transfused with crossmatch-incompatible transgenic RBCs (either mHEL or hGPA) labeled with DiI mixed with compatible wild-type RBCs labeled with DiO.
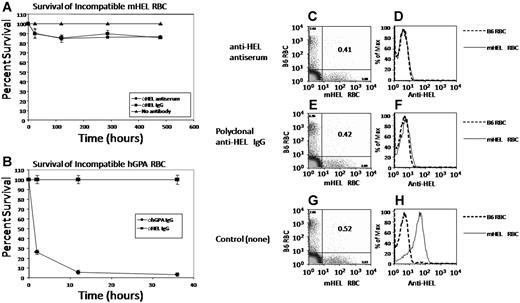
Similar to our previous findings,15 there was mild early clearance of mHEL RBCs from mice receiving either polyclonal anti-HEL antisera or the purified polyclonal anti-HEL IgG fraction (Figure 1A). However, after this early removal, most (ie, 85%-90%) of the transfused mHEL RBCs circulated with a normal lifespan. In contrast, transfusion of hGPA RBCs into mice with circulating anti-hGPA resulted in rapid clearance of incompatible RBCs, with greater than 90% clearance by 12 hours (Figure 1B). This was antigen-specific, in that no clearance of hGPA RBCs was seen in mice passively immunized with monoclonal anti-HEL (Figure 1B).
Non hemolytic antigen loss is not a general property of murine RBCs. (A) C57BL/6 mice were passively immunized with polyclonal anti-HEL antiserum or an anti-HEL IgG fraction from polyclonal antiserum. Control mice received no antibody. Mice were then transfused with a mixture of mHEL RBCs labeled with DiI and C57BL/6 RBCs labeled with DiO. Peripheral blood was obtained at the indicated time points and survival of the RBCs was determined by flow cytometry. (B) C57BL/6 mice were injected with the 10F7 anti-hGPA monoclonal antibody. Control mice received an IgG anti-HEL monoclonal antibody. Mice were then transfused with a mixture of hGPA RBCs labeled with DiI and C57BL/6 RBCs labeled with DiO and RBC survival was monitored. (C-H) Peripheral blood from the mice in panel A was stained with polyclonal anti-HEL antiserum followed by fluorescently labeled anti–mouse IgG and anti-HEL staining was measured by flow cytometry. Error bars in panels A and B represent standard deviation (SD). This experiment was reproduced 3 times with similar results. Representative flow plots and histograms are shown. In panels C, E, and G, the numbers in the upper left and lower right quadrants represent the percentage of circulating transfused B6 or mHEL RBCs, respectively. The numbers in the upper right quadrants represent the ratio of mHEL RBCs to B6 RBCs.
Non hemolytic antigen loss is not a general property of murine RBCs. (A) C57BL/6 mice were passively immunized with polyclonal anti-HEL antiserum or an anti-HEL IgG fraction from polyclonal antiserum. Control mice received no antibody. Mice were then transfused with a mixture of mHEL RBCs labeled with DiI and C57BL/6 RBCs labeled with DiO. Peripheral blood was obtained at the indicated time points and survival of the RBCs was determined by flow cytometry. (B) C57BL/6 mice were injected with the 10F7 anti-hGPA monoclonal antibody. Control mice received an IgG anti-HEL monoclonal antibody. Mice were then transfused with a mixture of hGPA RBCs labeled with DiI and C57BL/6 RBCs labeled with DiO and RBC survival was monitored. (C-H) Peripheral blood from the mice in panel A was stained with polyclonal anti-HEL antiserum followed by fluorescently labeled anti–mouse IgG and anti-HEL staining was measured by flow cytometry. Error bars in panels A and B represent standard deviation (SD). This experiment was reproduced 3 times with similar results. Representative flow plots and histograms are shown. In panels C, E, and G, the numbers in the upper left and lower right quadrants represent the percentage of circulating transfused B6 or mHEL RBCs, respectively. The numbers in the upper right quadrants represent the ratio of mHEL RBCs to B6 RBCs.
To assess antigen loss in the surviving mHEL RBCs, the transfused mHEL and wild-type RBCs were visualized by flow cytometry using DiI and DiO (Figure 1C,E,G). Antigen loss was measured by gating on these populations and then analyzing anti-HEL staining on a third channel. No mHEL antigen was detected on circulating RBCs transfused into mice receiving either polyclonal anti-HEL antiserum or the purified IgG fraction of anti-HEL antiserum (Figure 1D,F). In contrast, mHEL RBCs transfused into control mice reacted strongly with anti-HEL compared with wild-type RBCs (Figure 1H).
Taken together, these data demonstrate that murine RBCs expressing hGPA are rapidly cleared when transfused into mice immunized with monoclonal anti-hGPA. In contrast, mHEL RBCs continue to circulate and undergo loss of the mHEL surface antigen when transfused into mice passively immunized with polyclonal anti-HEL. Together, these data demonstrate that, like humans, transfusion of crossmatch-incompatible RBCs in mice can result in different outcomes, including either rapid clearance or nonhemolytic antigen loss.
Antigen loss occured in several different murine strains
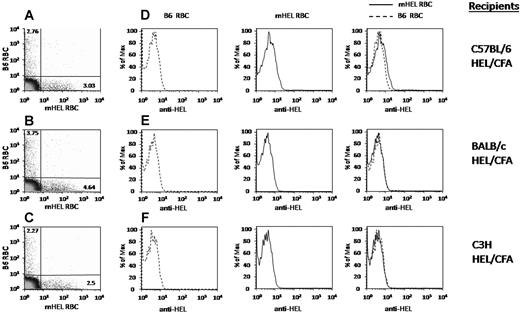
Our initial studies were performed with C57BL/6 recipients; however, hemolysis of antibody-coated RBCs can be strain-dependent.21 To test the strain dependence of antigen loss, C57BL/6, C3H, and BALB/c mice were each immunized with HEL/CFA. Two weeks later, serum was collected, and an anti-HEL response was confirmed by ELISA15 (data not shown). The immunized mice were transfused with a mixture of C57BL/6 RBCs labeled with DiO and mHEL RBCs labeled with DiI; RBC survival was determined by flow cytometry. In each case, most transfused mHEL RBCs continued to circulate, despite the presence of high titer anti-HEL antibodies (Figure 2A-C). In addition, in each case, the loss of detectable mHEL antigen from the transfused mHEL RBCs was observed by day 4 after transfusion (Figure 2D-F, where B6 and mHEL histograms are shown individually and then overlaid). In contrast, no antigen loss was observed in negative control mice of any nonimmunized strain (data not shown). Thus, antigen loss in the mHEL system is observed in multiple genetic backgrounds.
Antigen loss occurs in multiple different murine strains. C57BL/6, BALB/c or C3H mice were actively immunized with HEL/CFA. Mice were transfused with a mixture of mHEL RBCs labeled with DiI and C57BL/6 RBCs labeled with DiO. Four days after transfusion, peripheral blood was obtained and stained with polyclonal anti-HEL antiserum followed by fluorescently labeled anti–mouse IgG; anti-HEL staining was measured by flow cytometry. (A-C) Numbers in the upper left and lower right quadrants represent the percentage of circulating transfused B6 or mHEL RBCs, respectively. (D-F) B6 and mHEL histograms are shown individually and then overlaid. This experiment was reproduced 3 times with similar results. Representative flow plots and histograms are shown.
Antigen loss occurs in multiple different murine strains. C57BL/6, BALB/c or C3H mice were actively immunized with HEL/CFA. Mice were transfused with a mixture of mHEL RBCs labeled with DiI and C57BL/6 RBCs labeled with DiO. Four days after transfusion, peripheral blood was obtained and stained with polyclonal anti-HEL antiserum followed by fluorescently labeled anti–mouse IgG; anti-HEL staining was measured by flow cytometry. (A-C) Numbers in the upper left and lower right quadrants represent the percentage of circulating transfused B6 or mHEL RBCs, respectively. (D-F) B6 and mHEL histograms are shown individually and then overlaid. This experiment was reproduced 3 times with similar results. Representative flow plots and histograms are shown.
Individual monoclonal anti-HEL antibodies did not induce antigen loss
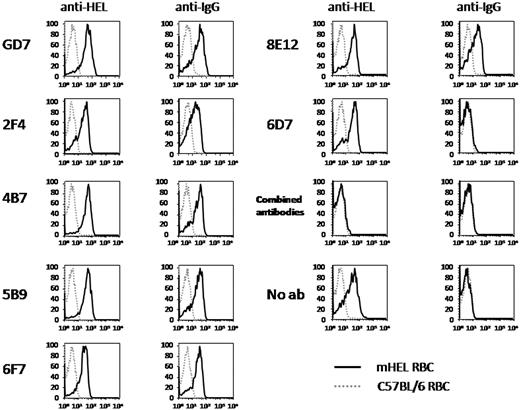
To characterize antibodies capable of inducing antigen loss, we generated a panel of monoclonal anti-HEL antibodies. Five new clones secreting IgG1 anti-HEL were isolated. In addition, 2 hybridoma clones, 2F4 and GD7, were acquired that secrete IgG1 and IgG2b anti-HEL, respectively (a generous gift from Dr Dennis Metzger, Albany Medical College, Albany, NY). Each monoclonal antibody specifically stained mHEL RBCs but not wild-type C57BL/6 RBCs, as measured by flow cytometry (data not shown). To test whether monoclonal anti-HEL induced antigen loss, C57BL/6 mice were infused with each antibody and then transfused with a mixture of DiO-labeled C57BL/6 RBCs and DiI-labeled mHEL RBCs. Three days later, peripheral blood was obtained and mHEL expression on the transfused RBCs was assessed by staining with anti-HEL. Specimens were also stained with anti-IgG alone to assess binding of the circulating monoclonal antibodies to mHEL RBCs in vivo (the equivalent of a direct antiglobulin test). As with polyclonal antisera, only minimal clearance of mHEL RBCs was observed, and most transfused mHEL RBCs continued to circulate despite the presence of anti-HEL IgG (data not shown). However, unlike the polyclonal anti-HEL antisera tested above, none of the anti-HEL monoclonal antibodies induced antigen loss (Figure 3). This was not a function of the monoclonal antibodies' not binding the RBCs in vivo, in that staining with anti-mouse IgG showed that the transfused mHEL RBCs were indeed coated with antibody. The one exception to this was monoclonal antibody 6D7, which, despite strong staining of mHEL RBCs in vitro, failed to bind mHEL RBCs in vivo. This is a consistent finding with 6D7; therefore, 6D7 was omitted from subsequent analysis.
Individual monoclonal anti-HEL antibodies do not induce antigen loss. C57BL/6 mice were passively immunized with the indicated anti-HEL monoclonal antibodies. Control mice received no antibody. Mice were transfused with a mixture of mHEL RBCs labeled with DiI and C56BL/6 RBC labeled with DiO. Three days after transfusion, peripheral blood was obtained, stained with polyclonal anti-HEL antiserum followed by fluorescently labeled anti-mouse IgG, and anti-HEL staining was measured by flow cytometry. This experiment was reproduced 3 times with similar results. Representative flow plots and histograms are shown.
Individual monoclonal anti-HEL antibodies do not induce antigen loss. C57BL/6 mice were passively immunized with the indicated anti-HEL monoclonal antibodies. Control mice received no antibody. Mice were transfused with a mixture of mHEL RBCs labeled with DiI and C56BL/6 RBC labeled with DiO. Three days after transfusion, peripheral blood was obtained, stained with polyclonal anti-HEL antiserum followed by fluorescently labeled anti-mouse IgG, and anti-HEL staining was measured by flow cytometry. This experiment was reproduced 3 times with similar results. Representative flow plots and histograms are shown.
To imitate the polyclonal nature of antiserum, additional mice were passively immunized with a polyclonal mixture containing each of the monoclonal antibodies; interestingly, antigen loss was observed (Figure 3). This was not due to increased overall anti-HEL binding activity, because decreased amounts of each monoclonal antibody were included in the mixture such that the total anti-HEL IgG in the mixture was the same as for the individual monoclonal infusions.
The failure of individual monoclonal antibodies to induce antigen loss was also not due to decreased kinetics, because extended experiments demonstrated that despite adequate monoclonal antibody binding to the transfused RBCs, they still circulated normally, and the HEL antigen persisted for at least 20 days (data not shown). In this way, the interaction of mHEL RBCs with monoclonal anti-HEL mimics “clinically insignificant” human blood group antigens, for which crossmatch-incompatible RBCs can be transfused without hemolysis (ie, JMH, Chido-Rodgers, Knops).22 Taken together, our data indicate that antigen loss depends on the cooperative interaction of more than one anti-HEL antibody.
Antigen loss required the simultaneous binding of at least 2 antibodies with different epitope specificities
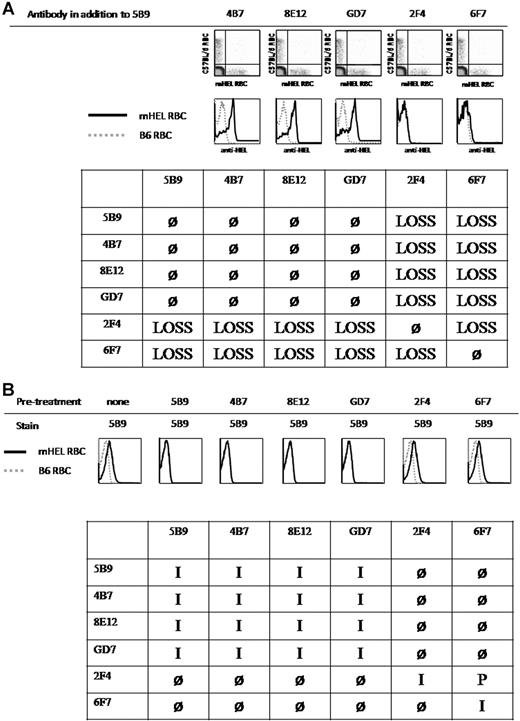
To characterize the nature of antibody cooperativity for inducing antigen loss, all possible combinations of 2 anti-HEL monoclonal antibodies were tested for their ability to induce antigen loss. Antibodies were mixed and infused into mice that subsequently received a transfusion of C57BL/6 and mHEL RBCs labeled with DiO and DiI, respectively. Six days later, the presence of mHEL antigen on the transfused RBCs was determined; an example with monoclonal antibody 5B9 is presented in Figure 4. Whereas no antigen loss was observed when 5B9 is combined with 4B7, 8E12, or GD7, antigen loss was observed when 5B9 is combined with 2F4 and 6F7 (Figure 4A).
Antigen-loss requires the simultaneous binding of antibodies with different epitope specificities. (A) C57BL/6 mice were injected with the indicated combinations of monoclonal antibodies. Mice were transfused with a mixture of mHEL RBCs labeled with DiI and C57BL/6 RBCs labeled with DiO. Six days after transfusion, peripheral blood was obtained and stained with polyclonal anti-HEL antiserum followed by fluorescently labeled anti–mouse IgG; anti-HEL staining was measured by flow cytometry. The combinations are arranged to maximize functional groupings of antibodies, and a complete table is presented that shows each condition twice but allows easier pattern analysis. Antigen loss is designated as “LOSS,” whereas no antigen loss is indicated by “ø.” The outcome of combining an antibody with itself, which is the same as injecting the antibody alone, was taken from the data with isolated antibodies in Figure 3. This experiment was reproduced in its entirety 2 times with identical results. Additional experiments tested smaller groups of antibody combinations with identical findings. (B) Each monoclonal antibody was directly conjugated to Alexa Fluor 647. mHEL RBCs were preincubated with the indicated unconjugated monoclonal antibodies followed by staining with the indicated conjugated antibodies. Blocking by unconjugated antibodies was measured by flow cytometry. Blocking in both directions is defined as epitope identity (I), failure to block as nonidentity (ø), and blocking in only one direction as partial identity (P). This experiment was reproduced 2 times with identical results.
Antigen-loss requires the simultaneous binding of antibodies with different epitope specificities. (A) C57BL/6 mice were injected with the indicated combinations of monoclonal antibodies. Mice were transfused with a mixture of mHEL RBCs labeled with DiI and C57BL/6 RBCs labeled with DiO. Six days after transfusion, peripheral blood was obtained and stained with polyclonal anti-HEL antiserum followed by fluorescently labeled anti–mouse IgG; anti-HEL staining was measured by flow cytometry. The combinations are arranged to maximize functional groupings of antibodies, and a complete table is presented that shows each condition twice but allows easier pattern analysis. Antigen loss is designated as “LOSS,” whereas no antigen loss is indicated by “ø.” The outcome of combining an antibody with itself, which is the same as injecting the antibody alone, was taken from the data with isolated antibodies in Figure 3. This experiment was reproduced in its entirety 2 times with identical results. Additional experiments tested smaller groups of antibody combinations with identical findings. (B) Each monoclonal antibody was directly conjugated to Alexa Fluor 647. mHEL RBCs were preincubated with the indicated unconjugated monoclonal antibodies followed by staining with the indicated conjugated antibodies. Blocking by unconjugated antibodies was measured by flow cytometry. Blocking in both directions is defined as epitope identity (I), failure to block as nonidentity (ø), and blocking in only one direction as partial identity (P). This experiment was reproduced 2 times with identical results.
This approach demonstrated that some monoclonal antibody combinations resulted in antigen loss, whereas others did not. In this context, it was possible to group different antibodies together based on their pattern of antigen loss. Pattern 1 was shown by 2F4 and 6F7, which each induced antigen loss when combined with any of the other antibodies. Pattern 2 was shown by monoclonal antibodies 5B9, 4B7, 8E12, and GD7, which did not induce antigen loss when combined with each other, but did cause antigen loss when combined with 2F4 or 6F7.
Blocking studies were performed to test the nature of the epitopes recognized by each of these monoclonal antibodies. After direct conjugation to the fluorescent molecule Alexa Fluor 647, each conjugated antibody-stained mHEL RBCs but not wild-type C57BL/6 RBCs, confirming proper maintenance of antigen binding (data not shown). Each antibody was then incubated with C57BL/6 RBCs, mHEL RBCs, or mHEL RBCs that had been preincubated with an unconjugated monoclonal antibody. A representative example is shown for antibody 5B9 (Figure 4B). Although baseline staining is seen by flow cytometry when mHEL RBCs are not pretreated, the shift is smaller than that observed in Figure 2, because in those experiments, staining was performed using unconjugated anti-HEL and labeled secondary antibodies, which provides signal amplification. Nevertheless, the observed shift is reproducible and significant, especially because the fluorescence is shown on a log scale.
As predicted, preincubation of mHEL RBCs with unconjugated 5B9 completely blocked binding with conjugated 5B9 (Figure 4B). Preincubation of mHEL RBCs with 4B7, 8E12, or GD7 also completely blocked binding of 5B9. In contrast, preincubation with 2F4 or 6F7 did not block binding of 5B9.
Thirty-six conditions were tested, evaluating each conjugated monoclonal antibody after preincubation with each of the other unconjugated monoclonal antibodies (Figure 4B). Blocking in both directions was defined as identity (I), blocking in only one direction as partial identity (P), and if no blocking occurred, as none (Ø) (Figure 4B). The complete table is presented in the same format as the functional data in Figure 4A. A clear pattern emerges by juxtaposing these tables. No antigen loss occurred when 2 antibodies blocked each other, and thus presumably recognized the same epitope (I). In contrast, antigen loss was observed if the antibodies did not block each other (Ø) or only partially blocked (P), suggesting that binding to different epitopes was required for antigen loss, even if there was partial identity. In each condition testing antigen loss, both monoclonal antibodies circulated before transfusion, such that mHEL RBCs were simultaneously exposed to both monoclonal antibodies, allowing both of the antibodies to bind mHEL RBC, even if they recognized the same epitope and must bind separate mHEL molecules. However, because antigen loss occurred only when the 2 antibodies bound distinct epitopes, even when they were related (ie, partial identity), this indicated that engagement of multiple epitopes was required for antigen loss.
The requirement for multiple antibodies binding different epitopes was not due to increased numbers of bound IgG molecules
These data demonstrate that antigen loss does not occur if only one epitope is recognized. Assuming that any given epitope occurs only once per molecule and that the molecule is monomeric, then the highest order molecular complex that can form in the presence of a single antibody is dimers of the antigen (Figure 5A,B). However, when 2 antibodies recognizing separate epitopes are present, then 2 new events occur (Figure 5C). First, crosslinking can occur, allowing a multimeric complex of molecules to form in the membrane of a given RBC. Second, the number of bound antibodies doubles. From this analysis, 2 hypotheses emerge: (1) crosslinking is required for antigen loss to occur and (2) a critical number of Fc domains is required to induce antigen loss, which is only reached when more antibodies are bound. Because we reported previously that antigen loss requires FcγRIII,15 and thus probably involves the Fc domains of RBC-bound antibodies, then increasing the number of antibodies on the mHEL RBC may cross the threshold for engaging a critical number of FcγRIII molecules required to induce antigen loss.
Model for molecular differences when 2 antibodies simultaneously bind to distinct epitopes on a single surface antigen. (A,B) A single IgG monoclonal antibody recognizing an epitope that occurs once on a surface molecule forms antigen dimers, but not multimolecular complexes. (C) The combination of 2 antibodies recognizing 2 distinct epitopes doubles the number of antibodies bound but also leads to multimolecular crosslinking. (D) The combination of intact IgG and F(ab′)2 fragments, each of which recognizes different epitopes, also induces multimolecular crosslinking but does not have an increased number of exposed Fc domains compared with a single IgG monoclonal antibody.
Model for molecular differences when 2 antibodies simultaneously bind to distinct epitopes on a single surface antigen. (A,B) A single IgG monoclonal antibody recognizing an epitope that occurs once on a surface molecule forms antigen dimers, but not multimolecular complexes. (C) The combination of 2 antibodies recognizing 2 distinct epitopes doubles the number of antibodies bound but also leads to multimolecular crosslinking. (D) The combination of intact IgG and F(ab′)2 fragments, each of which recognizes different epitopes, also induces multimolecular crosslinking but does not have an increased number of exposed Fc domains compared with a single IgG monoclonal antibody.
To test these hypotheses, it is necessary to separate the 2 events of multimeric crosslinking and increased numbers of Fc domains on RBCs. Therefore, we generated F(ab′)2 fragments from monoclonal anti-HEL antibodies. F(ab′)2 fragments maintain 2 antigen binding arms but have lost the Fc domain. By incubating mHEL RBCs with intact IgG anti-HEL against one epitope and with F(ab′)2 fragments that recognize a different epitope, crosslinking is maintained, but there is no increase in the absolute number of Fc domains present on the RBC surface (Figure 5D).
Conditions optimally generating monoclonal 4B7 antibody derived F(ab′)2 fragments by ficin digestion were determined by inspecting Coomassie Brilliant Blue–stained sodium dodecyl sulfate-polyacrylamide gels for the conversion of intact IgG (∼150 kDa) into F(ab′)2 fragments (∼100 kDa) (data not shown). Any residual intact IgG was removed by protein A chromatography. Because the Fc domain was removed, 4B7 F(ab′)2 binding activity was tested using a fluorescently conjugated, κ light chain-specific secondary antibody. 4B7 F(ab′)2 fragments maintained anti-HEL binding activity by anti-HEL ELISA and flow cytometry (data not shown). Western blots were performed using an anti-Fc specific antibody to determine the level of contaminating intact 4B7 IgG; at a detection limit of 1 ng/μL, no 4B7 IgG was detected in the 4B7 F(ab′)2 preparation (data not shown).
The ability of 4B7 F(ab′)2 to induce antigen loss was tested in combination with monoclonal antibody 2F4. Antibody and F(ab′)2 fragment combinations were incubated with DiI-labeled mHEL RBCs and DiO-labeled C57BL/6 RBCs; after transfusion into C57BL/6 mice, RBC survival and antigen loss were determined. Because the bioavailability and circulatory half-life of antibodies may change when converted into F(ab′)2 fragments, RBCs in these experiments were preincubated with the indicated immunoglobulin combinations before transfusion. In addition, because 4B7 F(ab′)2 fragments lack the Fc domain, a κ light chain-specific secondary antibody was used to detect bound 4B7 F(ab′)2.
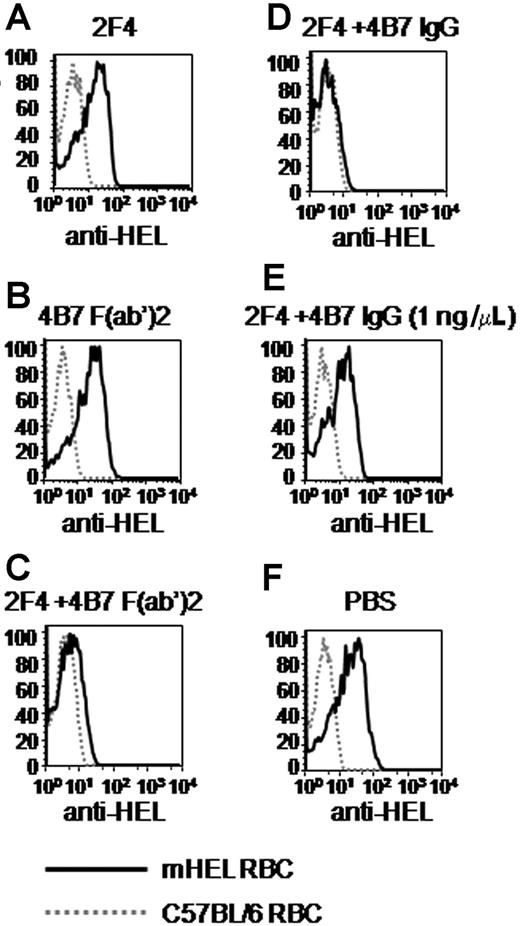
As reported above, no antigen loss was observed with 2F4 alone (Figure 6A). Likewise, no antigen loss was observed with 4B7 F(ab′)2 alone (Figure 6B). However, antigen loss was observed when intact 2F4 was combined with 4B7 F(ab′)2 (Figure 6C). As expected, combination of 2F4 and intact 4B7 IgG induced antigen loss (Figure 6D), and no antigen loss was observed in the absence of antibodies (Figure 6F). The legitimacy of the conclusion that 4B7 F(ab′)2 induces antigen loss when combined with 2F4 assumes that a low level of contaminating intact 4B7 IgG in the 4B7 F(ab′)2 preparation does not cause the effect. By Western blot analysis, there was a maximum of 1 ng/μL of 4B7 IgG in the 4B7 F(ab′)2 preparation; however, 2F4 combined with 1 ng/μL purified 4B7 IgG did not induce antigen loss (Figure 6E). Thus, contaminating 4B7 IgG is not responsible for antigen loss caused by the 4B7 F(ab′)2 fragment preparation. Taken together, these data exclude the hypothesis that the requirement for 2 separate antibodies recognizing different epitopes is due to the increased absolute amounts of Fc domains on the RBC surface.
The requirement for multiple antibodies binding different epitopes is not due to increased numbers of Fc domains on the RBC surface. A mixture of mHEL RBCs labeled with DiI and C57BL/6 RBCs labeled with DiO was incubated in vitro with the indicated monoclonal antibody and/or F(ab′)2 fragment combinations or PBS. After 30 minutes, the mixtures were transfused into C57BL/6 mice. Peripheral blood was obtained 3 days later, stained with polyclonal anti-HEL antisera followed by fluorescently labeled anti–mouse κ light chain, and anti-HEL staining was measured by flow cytometry. This experiment was reproduced 3 times with identical results. Representative histograms are shown.
The requirement for multiple antibodies binding different epitopes is not due to increased numbers of Fc domains on the RBC surface. A mixture of mHEL RBCs labeled with DiI and C57BL/6 RBCs labeled with DiO was incubated in vitro with the indicated monoclonal antibody and/or F(ab′)2 fragment combinations or PBS. After 30 minutes, the mixtures were transfused into C57BL/6 mice. Peripheral blood was obtained 3 days later, stained with polyclonal anti-HEL antisera followed by fluorescently labeled anti–mouse κ light chain, and anti-HEL staining was measured by flow cytometry. This experiment was reproduced 3 times with identical results. Representative histograms are shown.
Discussion
We used a murine model of nonhemolytic antigen loss to investigate the molecular characteristics of the antibodies that induce this phenomenon. We demonstrated that separate antibodies must simultaneously bind to at least 2 distinct epitopes on the target antigen for antigen loss to occur. These data are equally consistent with 2 hypotheses: (1) a requirement for increased numbers of Fc domains, in that approximately twice as many antibodies will be bound if they recognize 2 separate epitopes or (2) the requirement for antigen crosslinking into a multimeric molecular complex. Because antigen loss still occurs when an F(ab′)2 fragment of one monoclonal antibody is combined with a second intact monoclonal antibody, the first hypothesis is excluded. Although this does not provide direct experimental evidence that crosslinking is required, and other mechanisms may explain the requirement for simultaneous binding of 2 antibodies against different epitopes, our findings suggest that formation of multimeric complexes may be essential for the antigen loss process.
The data in this report relating to mechanisms of antigen loss are limited to the analysis of a single model blood group antigen on murine RBCs. Thus, it is not yet clear to what extent these findings bear on similar processes observed in humans. Nevertheless, such observations may provide insight into human antigen loss and lead to testable hypotheses. Why antigen loss occurs in some human cases of crossmatch-incompatible transfusion1,3,4 whereas brisk hemolysis occurs in others remains a mystery. If crosslinking is required for antigen loss in humans, it seems likely that the nature of the antisera may play a role. Because many blood group antigens consist of a single amino acid change (eg, the Duffy [Fya/b] and Kidd [Jka/b] polymorphisms), the number of novel epitopes may be limited. Therefore, it is possible that some patients make polyclonal monospecific responses that recognize one epitope, whereas other patients' antisera recognize multiple overlapping epitopes; this difference may determine whether incompatible RBCs undergo antigen loss or hemolysis. However, there are currently no data to support or refute this speculative concept. It is also worth noting that, in some cases, single amino acid polymorphisms can lead to conformational changes in tertiary structure that result in multiple epitopes.
An important consideration in the context of this model is why antigen loss is not consistently seen with anti-RhD (Rh blood group, D antigen), given that the whole RhD molecule is foreign to RhD-negative recipients, giving rise to multiple B-cell epitopes. However, the kinetics of hemolysis and antigen loss may vary widely with any given antisera. Thus, any given antisera may induce both antigen loss and hemolysis at different respective rates and with both processes occurring simultaneously. A race between the 2 processes may then ensue, with some RBCs undergoing sufficient antigen loss before being cleared, such that they escape hemolysis. Indeed, even under conditions that favor antigen loss in the mHEL system, 15%-20% of the RBCs are cleared rapidly. It is interesting to note that with isolated monoclonal anti-HEL IgG, where antigen loss does not occur, the RBCs nevertheless continued circulating normally while bound with anti-HEL IgG. Several human blood group antigens are not “clinically significant” and do not result in hemolysis when transfused into crossmatch-incompatible patients (ie, JMH, Chido-Rodgers, Knops).22 It may be that mHEL more closely resembles such antigens. This may provide mHEL RBCs additional time for antigen loss to occur, resulting in a model in which antigen loss is more easily observed.
The mHEL and hGPA model systems take a similar approach but yield different outcomes. In both cases, the antigen is expressed as a transgene on murine RBCs, and the antigens are each inserted into the lipid bilayer as single-pass transmembrane proteins. Moreover, this is not an effect of different antibody effector functions, in that both the anti-hGPA and anti-HEL were IgG1. However, some important differences may provide insight into the mechanisms of RBC clearance versus antigen loss. First, the antigens are expressed at different densities, with significantly more hGPA than mHEL molecules per RBC.20,23 Thus, the density of antibody bound to hGPA RBCs or mHEL RBCs is likely to differ after crossmatch-incompatible transfusion. Second, hGPA may be more resistant to antigen loss because at least some hGPA molecules are bound to band 3, thereby linked to the membrane cytoskeleton.20 Third, the affinity of anti-HEL and anti-hGPA monoclonal antibodies may differ. Future experiments comparing and contrasting the characteristics of the mHEL and hGPA systems may provide additional insights into factors that regulate clearance versus antigen loss.
The phenomenon of antigen loss is not always desirable. Indeed, with monoclonal antibody-based cancer therapies, antigen loss allows malignant cells to escape destruction. For example, anti-CD20 (rituximab) is a monoclonal antibody that induces regression of chronic lymphocytic leukemia (CLL).24 In some cases of incomplete tumor toxicity, resulting in subsequent recrudescence of CLL, neoplastic cells escape by selectively shedding CD20 molecules.25,26 It is unclear to what extent CD20 “shaving” in CLL is similar to loss of mHEL from incompatible RBCs, but the processes have similar characteristics and may be mechanistically related. Therefore, neoplastic cells may co-opt a normally self-protective mechanism to evade monoclonal antibody based therapies.
It is not obvious why rituximab would crosslink CD20, because rituximab is a single-IgG monoclonal antibody, and monoclonal antibodies do not induce antigen loss in the mHEL system. However, CD20 itself forms multimolecular complexes.27 Therefore, CD20 molecules have multiple epitopes displayed on a complex, thereby allowing crosslinking induced by one monoclonal antibody. On the other hand, it is worth noting that in the mHEL system, the 6F7 monoclonal antibody occasionally induced antigen loss on its own (data not shown). When these samples of 6F7 were subjected to size-exclusion chromatography, homodimer aggregates of antibody were readily observed. Purified monoclonal antibodies are known to form complexes during storage at 4°C, and the ability of 6F7 to induce antigen loss by itself seemed to increase with prolonged storage times. Because homodimerized 6F7 would be able to crosslink antigens, this observation may directly support a crosslinking mechanism.
In summary, the current results in a murine model provide insight into the mechanism by which antibodies to RBC antigens induce antigen loss. To the extent that these findings model antigen loss in humans, the clinical importance of this understanding lies in the fact that predicting (or even promoting) antigen loss would be beneficial in transfusion medicine. Conversely, because antigen loss is detrimental when it allows neoplasia to escape monoclonal antibody therapy, the development of approaches that inhibit crosslinking may increase the efficacy of these therapeutic reagents.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Seema Deshpande for additional technical assistance.
Authorship
Contribution: J.C.Z., C.M.C., T.E.C., S.L.S., D.A.S., C.A.P., and C.D.H. designed the research and analyzed and interpreted the data. J.C.Z., C.M.C., T.E.C., S.L.S., D.A.S., and T.W. performed the research. J.C.Z., C.M.C., S.L.S., D.A.S., and C.D.H. drafted and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James C. Zimring, MD, PhD, Center for Transfusion and Cellular Therapies, Department of Pathology and Laboratory Medicine, Emory University School of Medicine, Woodruff Memorial Building Suite 7301, 101 Woodruff Circle, Atlanta, GA 30322; e-mail: jzimrin@emory.edu.