Adeno-associated virus (AAV)–mediated gene transfer of factor IX (F.IX) to the liver results in long-term expression of transgene in experimental animals, but only short-term expression in humans. Loss of F.IX expression is likely due to a cytotoxic immune response to the AAV capsid, which results in clearance of transduced hepatocytes. We used a nonhuman primate model to assess the safety of AAV gene transfer coupled with an anti–T-cell regimen designed to block this immune response. Administration of a 3-drug regimen consisting of mycophenolate mofetil (MMF), sirolimus, and the anti–IL-2 receptor antibody daclizumab consistently resulted in formation of inhibitory antibodies to human F.IX following hepatic artery administration of an AAV-hF.IX vector, whereas a 2-drug regimen consisting only of MMF and sirolimus did not. Administration of daclizumab was accompanied by a dramatic drop in the population of CD4+CD25+FoxP3+ regulatory T cells (Tregs). We conclude that choice of immunosuppression (IS) regimen can modulate immune responses to the transgene product upon hepatic gene transfer in subjects not fully tolerant; and that induction of transgene tolerance may depend on a population of antigen-specific Tregs.
Introduction
Adeno-associated virus (AAV)–mediated, liver-directed gene transfer has been successfully used to treat a wide variety of genetic disorders in animal models of human disease.1,,,,,,,–9 However, despite long-term efficacy data in experimental animals, in the only human study of AAV-mediated, liver-directed gene transfer, expression of the donated F.IX gene was short-term in hemophilic men,10 persisting for approximately 4 weeks, then gradually declining over the ensuing 6 weeks. Loss of F.IX expression was accompanied by a transient asymptomatic transaminase elevation, also beginning 4 weeks after vector infusion. Studies in a subsequent AAV-infused subject showed that vector infusion was followed by expansion, then contraction, of a population of AAV capsid-specific CD8+ T cells.10,11 This led to the hypothesis that humans, the only natural hosts for AAV-2, harbor a population of capsid-specific memory CD8+ T cells, formed during childhood infection with the wild-type virus, and that these become reactivated when the subject is re-exposed to AAV capsid via vector infusion. The memory capsid-specific CD8+ T cells expand and eventually lyse the transduced hepatocytes,11 leading to a transient increase in liver enzymes and the loss of F.IX expression.10
While several possible solutions to this dilemma have been proposed, including engineering of the capsid proteins to escape immune recognition, or use of alternate AAV serotypes in pursuit of the same goal,12 the highly polymorphic nature of the human MHC loci, and the considerable degree of sequence conservation among capsids of different serotypes, may substantially hinder these approaches. Transient immunosuppression (IS) given at the time of gene transfer and maintained until capsid antigen has been cleared from the target cells could be a feasible approach to blocking immune responses and achieving long-term efficacy after AAV-mediated gene transfer to liver. An important requirement for an IS regimen in an adult hemophilia population is documented safety in hepatitis C virus–positive (HCV+) individuals. In this regard, regimens based on mycophenolate mofetil (MMF) and sirolimus have been widely used in renal transplantation,13,14 where substantial numbers of transplant recipients are HCV+, due to transfusion dependence that predated effective screening of the blood supply for HCV. These regimens offer an excellent long-term safety profile. More recently, the addition of anti–IL-2 receptor antibodies (eg, daclizumab) to these regimens has further improved efficacy in the setting of organ transplantation.15
However, the use of IS poses additional concerns, because several laboratories have shown that hepatic gene transfer results in immunologic tolerance to the transgene product, mediated by the induction of antigen-specific CD4+CD25+FoxP3+ regulatory T cells.16,–18 Because tolerance is essential to avoiding a humoral response to the transgene product, and little is known about the timing of its induction, IS regimens used in liver-directed gene transfer must be designed to circumvent any interference with induction of regulatory T cells. This is particularly relevant in hemophilia, where the risk of developing neutralizing antibodies to the transgene product (clinically termed inhibitors) is a serious concern.19 In this regard, regimens containing sirolimus are of great interest, because the drug has been shown to promote the induction of regulatory T cells.20,–22
In this study, we used a nonhuman primate (NHP) model to assess the safety of IS regimens in AAV-mediated, liver-directed gene transfer. Specifically, we sought to determine whether coadministration of IS agents altered transduction efficiency or biodistribution of AAV, or the immune response to the transgene product. The results demonstrate that the choice of IS regimen can dramatically influence the outcome of liver-directed gene transfer. These findings provide critical preclinical safety data for a proposed trial of AAV-F.IX coupled with transient immunomodulation. In addition, they extend to an outbred large-animal model previous observations in mouse models16,–18 on the possible role of CD4+CD25+ regulatory T cells in the induction of transgene tolerance following AAV-mediated gene delivery to liver.
Materials and methods
Animal procedures
Male rhesus macaques were purchased from a breeding colony in China and housed at Charles River Laboratories, Sierra Biomedical Division (Sparks, NV). Study protocol was approved by the Institutional Animal Care and Use Committee of Charles River Laboratories and conducted in accordance with the U.S. Department of Agriculture guidelines for laboratory animals, and with Good Laboratory Practices.
Prior to inclusion in the study, animals were screened for pre-existing neutralizing antibodies to the AAV-2 capsid. Only animals with a titer of 1:3 or less were included in the study. Animals were randomly assigned to 3 groups (n = 3 per group, Table 1) receiving no immunosuppression (group 1); a combination of MMF, sirolimus, and daclizumab (group 2); or a combination of MMF and sirolimus (group 3). Vector was administered through a 30-gauge needle inserted into the hepatic artery and attached to a syringe infusion pump as previously described.23 Prior to infusion, the vector was thawed and diluted in PBS 5% sorbitol; infusion of the vector occurred at a constant flow rate of 0.2 mL/kg per minute. Clinical chemistry, hematology, and coagulation parameters, as well as weight and food consumption, were monitored weekly throughout the study.
AAV vectors
Recombinant AAV-2 vectors expressing human F.IX under the control of a liver-specific promoter were prepared by standard triple-transfection followed by double cesium gradient centrifugation methods.10 Empty capsids used for the anti–AAV-2 antibody enzyme-linked immunosorbent assay (ELISA) were isolated by cesium gradient centrifugation as a contaminant of vector preparations and titered by silver staining and OD reading.
Immunosuppressive drug regimens and monitoring
MMF (Roche, Nutley, NJ) was administered orally twice daily (25 mg/kg per dose) and sirolimus (Wyeth, Philadelphia, PA) orally once daily (2 or 4 mg/kg) via nasogastric tube. Daclizumab, a humanized IgG1 monoclonal antibody that binds the alpha subunit of the human IL-2 receptor, CD25 (Roche), was given twice intravenously (2 mg/kg at day − 1 and 1 mg/kg at day 15). Immunosuppression with MMF was initiated one week prior to vector administration and discontinued 10 weeks after gene transfer. Drug combinations, dose, and administration schedule for each group of animals are summarized in Table 1.
IS drugs doses were calculated to maintain target trough levels of 10 to 15 μg/L and 2 to 3.5 mg/L for sirolimus and mycophenolic acid (MPA), the active metabolite of MMF, respectively. Serum levels of MPA were determined by high-performance liquid chromatography (HPLC) on EDTA-anticoagulated plasma, and sirolimus levels by liquid chromatography mass spectroscopy on EDTA-anticoagulated whole blood at the Hospital of University of Pennsylvania, Pathology and Laboratory Medicine (Philadelphia, PA).
ELISA for hFIX and anti–hFIX IgG
Citrated plasma samples were collected and assayed weekly for hF.IX transgene expression levels and anti-hF.IX antibodies. F.IX levels were determined by ELISA as previously described23 using a coating antibody (Haematologic Technologies, Essex Junction, VT) able to recognize human F.IX in rhesus plasma. Antibody formation was measured with a capture assay; microtiter plates were coated overnight at 4°C with recombinant human F.IX protein (Wyeth) at a concentration of 1 μg/mL in 0.1 M carbonate coating buffer (pH 9.6). The day after, plates were blocked with 2% BSA, 0.05% Tween 20 in PBS for 2 hours at room temperature. Serial dilutions of samples in blocking buffer were loaded and incubated overnight at 4°C. An HRP-conjugated anti–rhesus IgG antibody (Research Diagnostics, Concord, MA) was used as detecting antibody; IgG concentration was determined against a standard curve made with serial dilutions of rhesus monkey purified total IgG (Research Diagnostics).
Bethesda assay
Bethesda assay was performed as previously described.24 Citrated plasma samples were collected and heat inactivated at 56°C for one hour to eliminate the endogenous rhesus F.IX. Serial dilutions of heat-inactivated rhesus plasma were then incubated with human plasma for 2 hours at 37°C. Residual clotting activity was measured by activated partial thromboplastin time (aPTT) assay and compared with a standard curve made with serial dilutions of human plasma in heat-inactivated naive rhesus plasma. Results are expressed in Bethesda units (BU); one BU is defined as the reciprocal of the dilution of test plasma at which 50% of human F.IX activity is inhibited.
Circulating anti–AAV-2 IgG and AAV-2 neutralizing antibody assay
Anti–AAV-2 capsid total IgG formation was measured with a capture assay; ELISA plates were coated with 5 × 1010 capsid particles/mL AAV-2 empty capsids. Plates were blocked with 2% BSA, 0.05% Tween 20 in PBS for 2 hours at room temperature; serial dilutions of samples in blocking buffer were loaded and incubated overnight at 4°C. An HRP-conjugated anti–rhesus IgG antibody (Research Diagnostics) was used as detecting antibody; IgG concentration was determined against a standard curve made with serial dilution of rhesus monkey purified total IgG (Research Diagnostics). Anti–AAV-2 neutralizing antibody titer was determined as previously described.10
Real-time quantitative PCR
Real-time quantitative polymerase chain reaction (Q-PCR) was used to determine the vector genome copy number in liver biopsies collected at week 8 after gene transfer and on various tissues isolated at the time of necropsy. Tissues were snap frozen in liquid nitrogen and DNA was extracted with a commercially available kit. The primers and probe set used for this assay have been previously described25 and span exons 5 and 6 of human F.IX cDNA, preventing amplification of rhesus genomic DNA and thus minimizing background of the assay. Linearized plasmid containing the transgene expression cassette flanked by the viral ITR sequences was used as standard for the reaction. Samples were acquired and analyzed on a 3500 Real-Time PCR System (Applied Biosystems, Foster City, CA).
Regulatory T-cell staining
Antibodies for CD4, CD25, FoxP3, isotype controls, and the kit for intracellular staining were all purchased form eBioscience (San Diego, CA). Staining was performed according to the company's protocol. Briefly, peripheral blood mononuclear cells (PBMCs) were thawed in PBS, 1% FBS and washed twice; surface staining for CD4 and CD25 was performed first, followed by fixation and permeabilization of the cells. After blocking with rat serum for 10 minutes at room temperature, the anti-FoxP3 antibody, or an isotype control, was added and incubated for 30 minutes at 4°C. Samples acquired on a Cyan flow cytometer (Dako Cytomation, Carpinteria, CA) and data were compensated and analyzed using FlowJo software (Treestar, Ashland, OR). For the analysis, lymphocytes were gated on forward and side scatter and single events selected on pulse width; CD4+CD25+ T cells were then gated and analyzed for the relative percentage of FoxP3+ cells.
IFN-γ enzyme-linked immunosorbent spot
PBMCs were collected at baseline and weeks 2, 3, 11, and 13, and stored frozen at − 70°C. At the time of assay, cells were thawed in a 37°C water bath, washed twice in AIM-V 3% human AB serum (HuAB; Valley Biomedical, Winchester, VA) containing 10 U/mL benzonase (Novagen, San Diego, CA), and counted.
IFN-γ enzyme-linked immunosorbent spot (ELISpot) was performed as previously described.10 Briefly, filter plates (Millipore, Billerica, MA) were coated overnight at 4°C with an anti–human IFN-γ antibody (Mabtech, Cincinnati, OH) cross-reacting with rhesus IFN-γ. On the day of the assay, plates were blocked with PBS 3% HuAB for 2 hours at room temperature. Cells were plated at 3 × 105 cells per well; as antigens, serial 2-fold dilutions from 20 μg/mL to 2.5 μg/mL human F.IX (Wyeth) or empty AAV-2 capsids was used. Cells were incubated at 37°C, 5% CO2 overnight, and IFN-γ secretion was detected with a streptavidin-conjugated anti–IFN-γ antibody (Mabtech). Spots were counted on a ELISpot reader (Cellular Technologies, Cleveland, OH) and analyzed with the Immunospot Software version 3 (Cellular Technologies). Wells were considered positive if the number of spots was more than 3 times the medium control and at least 10 spots. Phorbol myristate (PMA) (50 ng/mL) and ionomycin (1 μg/mL) (Sigma, St Louis, MO) were used as positive controls for cell viability.
Statistical analysis
Comparison of data obtained from distinct experimental groups was performed by unpaired Student t test and repeated measures ANOVA using GraphPad InStat version 3.0a (GraphPad Software, San Diego, CA). P values less than .05 were considered significant.
Results
A regimen consisting of MMF, sirolimus, and an anti–IL-2 receptor monoclonal antibody results in inhibitory antibodies to F.IX
To test the effect of transient IS on AAV-2–mediated liver transduction, 6 male rhesus macaques were selected for low anti–AAV-2 neutralizing antibodies and injected with a dose of 8 × 1012 vector genomes (vg)/kg of an AAV-2 vector expressing hF.IX under the control of a liver-specific promoter.10 Vector administration was performed by direct injection into the hepatic artery and was uneventful. Animals were randomly assigned to 2 groups: group 1 (n = 3) receiving the vector alone and group 2 (n = 3) receiving 10 weeks of an anti–T-cell regimen commonly used in human organ transplantation,13,14 consisting of MMF, sirolimus, and daclizumab. MMF and sirolimus were administered orally; the anti–IL-2 receptor antibody (daclizumab) was administered intravenously on days −1 and 15 for the induction phase of the regimen15 (see Table 1 for dosing and schedule of immunosuppressant drugs).
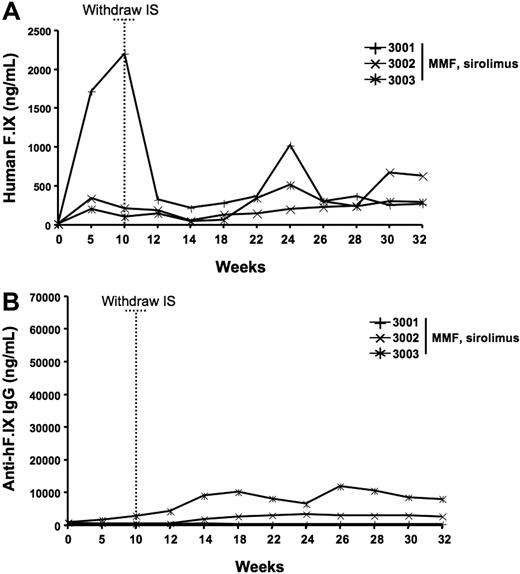
At the time of IS withdrawal (week 10 after gene transfer), 2 of the 3 animals from group 2 (3-drug IS regimen) had hF.IX-circulating levels of 100 and 150 ng/mL (Figure 1A); a third animal from the same group (no. 2002, Figure 1A) had declining levels of hF.IX starting from week 4 and returning to baseline (undetectable levels) by week 8. This animal did not have any rise in liver enzymes (data not shown) and by week 10 developed an inhibitor to F.IX with a titer of 4.8 BU (suggesting that the earlier drop in hF.IX levels reflected a low titer inhibitor not detected in our assay). Animals were followed for several weeks after gene transfer, and by week 27 all the animals on the 3-drug IS regimen (n = 3) developed high-titer inhibitory antibodies to the human F.IX transgene product (Figure 1B; Table 2). All the animals that received the AAV-2 vector alone (group 1, n = 3) had plateau levels of hF.IX expression between 200 and 479 ng/mL (4% to ∼ 10% of normal, within the therapeutic range, Table 2) without developing an inhibitor to hF.IX. None of the animals had altered liver function tests. PBMCs collected prior to and throughout the study (2, 3, 8, 11, and 13 weeks) were tested by IFN-γ and IL-10 ELISpot for response to AAV-2 capsid or to human F.IX. No T-cell responses were detected in either antigen (data not shown) in the nonimmunosuppressed animals, or in the animals that developed a neutralizing antibody to the F.IX transgene (3-drug IS regimen). Liver biopsy at week 8 and at necropsy (40 weeks) showed no evidence for lymphocytic infiltrates (data not shown). These findings confirmed that the loss of circulating human F.IX in the 3-drug IS group was due to a transgene-specific antibody response and not to a cytotoxic T-cell response against the AAV-2 capsid or hF.IX transgene. Furthermore, the absence of abnormalities in coagulation parameters (aPTT, PT, data not shown) confirmed that the inhibitory antibodies observed were specific to the human F.IX transgene product and did not cross-react with the endogenous rhesus F.IX.
hF.IX expression levels and anti-hF.IX antibody formation after AAV2-hF.IX hepatic gene transfer in rhesus macaques receiving MMF, sirolimus, and daclizumab and in nonimmunosuppressed controls. Time 0 represents baseline samples; dotted vertical line shows the time IS was withdrawn. Each line is representative of one individual animal. All animals received 8 × 1012 vg/kg of an AAV-2-hF.IX vector infused through the hepatic artery. Solid symbols indicate animals receiving a course of IS consisting of MMF, sirolimus, and daclizumab; open symbols, non-IS control animals. (A) Plasma human F.IX levels. (B) Plasma antihuman F.IX total IgG levels.
hF.IX expression levels and anti-hF.IX antibody formation after AAV2-hF.IX hepatic gene transfer in rhesus macaques receiving MMF, sirolimus, and daclizumab and in nonimmunosuppressed controls. Time 0 represents baseline samples; dotted vertical line shows the time IS was withdrawn. Each line is representative of one individual animal. All animals received 8 × 1012 vg/kg of an AAV-2-hF.IX vector infused through the hepatic artery. Solid symbols indicate animals receiving a course of IS consisting of MMF, sirolimus, and daclizumab; open symbols, non-IS control animals. (A) Plasma human F.IX levels. (B) Plasma antihuman F.IX total IgG levels.
In a separate experiment, a different IS regimen was tested consisting of MMF and sirolimus without daclizumab (2-drug IS regimen, group 3, n = 3). An initial loading dose of sirolimus (4 mg/kg daily, starting at day 1 for one week) was given as induction regimen (Table 1). At a dose of 8 × 1012 vg/kg AAV-2-hF.IX, all the animals receiving this IS regimen had plateau expression levels of circulating hF.IX at week 27 within the therapeutic range (240–365 ng/mL or 4.8%-7.3% of normal, Table 2) comparable with those of the non-IS group 1 (P = .952, Figures 1A, 2A). Two of the 3 animals from the 2-drug IS regimen (group 3) developed low-titer nonneutralizing antibodies (Figure 2B) as previously described in rhesus macaques receiving intravenous infusion of human F.IX protein26 and as observed in the non-IS group 1 in this study (Table 2).
hF.IX expression levels and anti-hF.IX antibody formation after AAV2-hF.IX hepatic gene transfer in rhesus macaques receiving MMF and sirolimus. Time 0 represents baseline samples; dotted vertical line shows the time IS was withdrawn. Each line is representative of one individual animal. All animals received 8 × 1012 vg/kg of an AAV-2-hF.IX vector infused through the hepatic artery. (A) Plasma human F.IX levels. (B) Plasma anti–human F.IX total IgG levels.
hF.IX expression levels and anti-hF.IX antibody formation after AAV2-hF.IX hepatic gene transfer in rhesus macaques receiving MMF and sirolimus. Time 0 represents baseline samples; dotted vertical line shows the time IS was withdrawn. Each line is representative of one individual animal. All animals received 8 × 1012 vg/kg of an AAV-2-hF.IX vector infused through the hepatic artery. (A) Plasma human F.IX levels. (B) Plasma anti–human F.IX total IgG levels.
Statistical analysis of results in animals receiving either no IS or a non–daclizumab-containing regimen (inhibitors present in 0/6 animals) versus those receiving a regimen containing daclizumab (inhibitors present in 3/3 animals) does not show a difference (P = .182). However, if these data are combined with our earlier published studies on absence of inhibitors in 6 NHPs receiving either no IS or a 2-drug non–daclizumab-containing regimen,23 the results are statistically significant (inhibitors present in 0/12 receiving no IS or 2-drug regimen, inhibitors present in 3/3 animals receiving 3-drug, daclizumab-containing regimen, P = .025).
Transient IS does not substantially alter transduction efficiency or vector biodistribution following AAV-2–mediated liver-directed gene therapy
In a previous study,23 Jiang et al showed that IS with MMF and tacrolimus did not alter hepatocyte transduction efficiency of an AAV-8 vector expressing human F.IX in rhesus macaques. To test whether the IS regimens proposed here for AAV-2 influence liver-directed gene transfer, needle biopsies were collected from the livers of all animals 8 weeks after vector administration to assess transduction efficiency. Gene copy number was determined by Q-PCR on DNA extracted from biopsies (Table 3). Overall, the use of IS, whether 2 or 3 drugs, at the time of AAV-2–mediated gene transfer did not lower transduction efficiency, as the vector gene copy number per diploid genome was not different (Table 3). Although intragroup variations were noted, this is most likely due to the sample collection procedure and the nonuniformity of liver transduction.
Forty weeks after gene transfer, one animal from each experimental group was killed, tissues were collected, and DNA was extracted for biodistribution studies. Quantitative PCR specific for the human F.IX transgene expression cassette showed a widespread positive signal in several tissues (Table 4), however the number of vector genomes per diploid genome was consistently low for the majority of tissues and in particular 1 to 2 logs lower than liver tissue collected from different liver lobes (Table 4). Spleen tissue had the highest number of vector genomes per diploid genome after liver tissue. Gene copy numbers document no difference in extent of liver transduction between animals that received IS (2 or 3 drugs) and the control animal.
The 3-drug regimen increases B-cell responses to AAV capsid
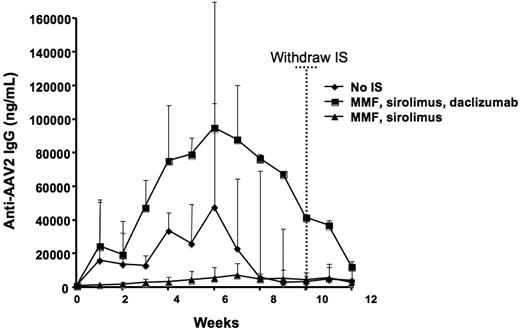
Serum samples were collected weekly for the duration of the study and anti–AAV-2 IgG production was assayed. All animals produced antibodies to the AAV-2 capsid upon vector infusion, with plasma levels of anti–AAV-2 IgG peaking around week 7 (Figure 3). Notably, animals receiving a course of IS consisting of MMF and sirolimus (group 3, 2-drug regimen) exhibited the lowest anti–AAV-2 antibody titers of all experimental groups (Figure 3), indicating that the 2-drug combination partially blocks B-cell responses to capsid. A marked difference in the antibody titer was measured among the groups studied, with animals receiving the 3-drug IS regimen based on MMF, sirolimus, and daclizumab (group 2) developing an antibody titer higher than animals receiving no IS (group 1) or a 2-drug regimen (group 3) (P < .05 for group 2 vs group 1 and group 2 vs group 3; P > .05 for group 1 vs group 3). While it is not surprising that anti–T-cell regimens do not completely block B-cell responses to a readily available antigen (ie, the AAV-2 capsid), the boosting effect of daclizumab is somewhat unexpected, as a combination of IS drugs would be expected at least to diminish the magnitude of humoral responses.
Anti–AAV-2 antibody formation following vector delivery. Time 0 represents the time of vector administration; dotted vertical line shows the time IS was withdrawn. All animals received 8 × 1012 vg/kg of an AAV-2-hF.IX vector infused through the hepatic artery. Each line represents the average value (+ SD) of a group of 3 animals.
Anti–AAV-2 antibody formation following vector delivery. Time 0 represents the time of vector administration; dotted vertical line shows the time IS was withdrawn. All animals received 8 × 1012 vg/kg of an AAV-2-hF.IX vector infused through the hepatic artery. Each line represents the average value (+ SD) of a group of 3 animals.
In all 3 groups, the anti–AAV-2 antibody titer declined to similar levels by week 13 and, more importantly, no second peak in the titer was observed following discontinuation of IS regimen, suggesting that a duration of 10 weeks of IS may be adequate to prevent an immune response to capsid in this model.
Addition of daclizumab is associated with marked reduction of CD4+CD25+FoxP3+ cells
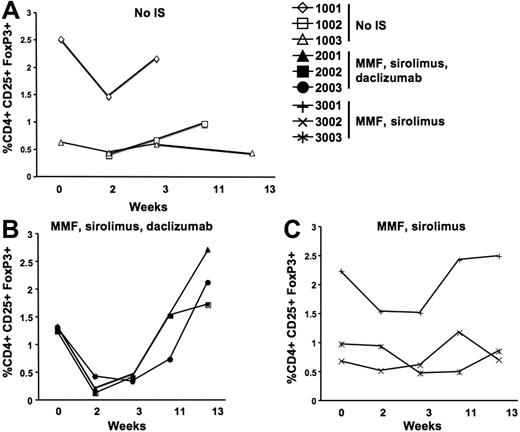
Analysis of antibody responses to both the human F.IX transgene product and the AAV-2 capsid suggests that the use of daclizumab enhances B-cell responses to antigen. Previous studies indicate that regulatory T cells (Tregs) that are CD4+CD25+FoxP3+ are indeed involved in induction of tolerance to the transgene product after hepatic gene transfer.16,17 However, these cells are also a target of daclizumab, an antibody directed against the IL-2 receptor CD25. To test the influence of the IS regimens used in our study on Tregs, PBMCs were collected at baseline and at weeks 2, 3, 11, and 13; cells were surface-stained for CD4 and CD25 and intracellularly stained for FoxP3. Administration of daclizumab at day −1 and day 15 (group 2, Table 1) caused a dramatic decrease in the number of CD4+CD25+FoxP3+ cells (Figure 4B), to almost undetectable levels. The Treg population returned to levels similar to baseline by week 11 as previously observed in humans receiving a similar IS regimen.27 The number of CD4+CD25+FoxP3+ T cells remained unchanged throughout the study in animals that received no IS (group 1) or MMF and sirolimus (group 3) (Figure 4A,C).
Staining for regulatory T cells on PBMCs isolated before and after AAV-2-hF.IX hepatic gene transfer. Each line represents one individual animal. y-axis shows the percent of CD4+CD25+FoxP3+ T cells. Cells were gated on CD4+CD25hi and analyzed for FoxP3. (A) Animals receiving only the AAV vector with no IS. (B) Animals receiving both the vector and an IS regimen consisting of MMF, sirolimus, and daclizumab. (C) Animals receiving the vector and MMF and sirolimus.
Staining for regulatory T cells on PBMCs isolated before and after AAV-2-hF.IX hepatic gene transfer. Each line represents one individual animal. y-axis shows the percent of CD4+CD25+FoxP3+ T cells. Cells were gated on CD4+CD25hi and analyzed for FoxP3. (A) Animals receiving only the AAV vector with no IS. (B) Animals receiving both the vector and an IS regimen consisting of MMF, sirolimus, and daclizumab. (C) Animals receiving the vector and MMF and sirolimus.
Discussion
Our previous study in men with severe hemophilia B10 established that infusion of AAV-F.IX vector at a dose of 2 × 1012 vg/kg results in therapeutic levels of F.IX expression (10%-12%). However, expression lasted for only a few weeks, and was terminated by a CD8+ T-cell response to the AAV capsid.11 The purpose of the experiments described here was to determine whether a short course of IS, administered to block the CD8+ T-cell response to capsid, would alter any of the characteristics of AAV vector transduction or immune response to F.IX in a large-animal model.
We chose to use NHP for these studies for the following reasons: (1) there is considerable experience with IS drugs28,–30 and with AAV vector infusion31,–33 in NHP, so the baseline toxicities for both types of agents are well-described in these animals; (2) IS drugs such as daclizumab (the humanized anti–IL-2 receptor antibody) developed for human use are active in NHP, due to a high degree of conservation of protein domain sequences between primates, but not in other mammals; and (3) use of a human F.IX transgene in rhesus macaques models the immune challenge in hemophilia gene transfer in humans, because neither the nonhuman primate nor the hemophilic human is necessarily tolerant to the human F.IX transgene expressed by the vector. The rhesus F.IX sequence differs at 11 of 461 residues from the human sequence26 ; for some hemophilia subjects bearing a single point mutation in the F.IX gene, gene transfer of wild-type human F.IX represents a less severe immunologic challenge than in the rhesus model presented here; for those with an early stop codon, gene transfer will represent a more severe challenge, due to the greater loss of genetic material.34
A reasonable starting point for the design of a regimen to block the CD8+ T-cell response to AAV capsid is to use regimens used in organ transplantation. The goal in organ transplantation is to block T-cell responses to thousands of eukaryotic antigens on the donated organ, while in AAV-mediated gene transfer, the goal is to block the T-cell response to the vector capsid, a single antigen that is only transiently present and is not expressed. An added layer of complexity in the setting of hemophilia is that the regimen identified must block the T-cell response to capsid without breaking tolerance to F.IX. Earlier work by us and by others has established that AAV-mediated hepatic expression of a transgene promotes tolerance to the transgene product. In mice, this effect is mediated by a subset of CD4+CD25+ T cells.17
Because the safety of a renal transplantation regimen including MMF, sirolimus, and the interleukin-2 receptor alpha (IL-2Rα) antagonist daclizumab is well established in HCV+ patients,13,14 it is a reasonable choice for the patient population of interest to us (ie, adults with severe hemophilia) many of whom are HCV+.35 Our goal was to determine whether the regimen would be safe in the setting of AAV-mediated hepatic gene transfer, but we recognized that the inclusion of an anti-CD25 antibody (daclizumab) might alter the induction of regulatory T cells. However, because tolerance to the transgene product is thought to result from more than one mechanism, including Fas-FasL–mediated cell death16,17 and T-cell anergy,17 and because the role of the timing of regulatory T-cell induction in development of tolerance is unclear (especially in large-animal models), we hypothesized that the regimen would not completely abrogate the induction of tolerance to human F.IX that is routinely observed on AAV-mediated gene transfer of human F.IX to rhesus liver.31,–33 This study in NHP demonstrated that, although the regimen did not result in any significant change in transduction efficiency or biodistribution of the AAV-2 vector, the immune response to the transgene product was altered in an unacceptable way. Specifically, as we showed here, the marked reduction in the CD4+CD25+FoxP3+ cell population with administration of daclizumab was associated with the formation of inhibitory antibodies to F.IX. Moreover, even after a prolonged period off IS, these antibodies did not disappear. This establishes two important conclusions: first, anti-CD25 antibodies cannot be used as part of an IS regimen for hepatic gene transfer in subjects who are not fully tolerant to the transgene product; and second, induction of antigen-specific regulatory T cells, if they are indeed involved, must occur at or around the time of vector administration to promote tolerance to the transgene product. As we subsequently showed, omission of daclizumab from the regimen allows this cell population to persist, and appears to spare the induction of regulatory T cells as evidenced by the absence of anti-F.IX inhibitory antibodies.
Recent findings27 in humans undergoing kidney transplantation show that 8 weeks after administration of a single dose of daclizumab, a population of CD4+CD25+ Tregs is present and does not appear to differ from the pre-IS Tregs in its capacity to inhibit lymphocyte proliferation to alloantigens in vitro. The kinetics of Treg depletion and recovery after daclizumab administration in nonhuman primates as shown here closely follows that seen in humans. However, here we have shown that the restoration of a population of CD4+CD25+FoxP3+ T cells in the nonhuman primate model of gene transfer is not associated with restoration of tolerance to the transgene product, as inhibitory antibodies to the human F.IX transgene product persist over time. These results suggest that this daclizumab-sensitive T-cell population is required early during the onset of transgene expression to establish tolerance to the newly synthesized protein.
The critical question that cannot be answered from these studies is whether a regimen composed solely of MMF and sirolimus will be adequate to suppress the human memory CD8+ T-cell response to AAV capsid, because it has so far been impossible to generate an animal model of the memory CD8+ T-cell response to capsid seen in humans.11,36 If the regimen is not adequate to suppress the memory CD8+ T-cell response to capsid, then one would expect to see transaminase elevation and loss of F.IX expression occurring several weeks after vector infusion, as previously described, even if these IS drugs are coadministered with AAV-F.IX in men with severe hemophilia B.10
Earlier studies by Jiang et al23 demonstrated that a regimen composed of MMF and tacrolimus (FK-506) results in no change in transduction efficiency or biodistribution of AAV-8-F.IX in NHPs. However, because of reports of the association of the AAV ITR with FK-506–binding protein37 and resulting concerns that the effects of this interaction might be difficult to predict, we wished to avoid tacrolimus.
Because the capsid antigen is present only transiently, one would predict that only a short course of IS would be required. In this regard, it is of interest that withdrawal of IS at 10 weeks after vector infusion was not accompanied by any rise in titer of anti-AAV antibodies. This complements findings reported by Jiang et al that showed elevation in antibody titer when IS was stopped at 6 weeks.23
We showed that blockade of the IL-2 receptor with daclizumab results in a boost of the anti–AAV-2 antibody titer and in formation of neutralizing antibody to the human F.IX transgene. Similarly, findings in mice show that in vivo depletion of CD25+ cells enhances humoral responses against foreign protein antigens administered in complete Freund adjuvant,38 and that interference with the CTLA-4 receptor, highly expressed in CD4+CD25+FoxP3+ T cells, results in a boost of effector T-cell responses in a T-cell–mediated model of colitis.39
One other point of interest concerns the factor IX levels expressed from the AAV-2-F.IX vector. The average plateau hF.IX levels of 289 ng/mL in the nonimmunosuppressed animals (group 1, dosed at 8 × 1012 vg/kg) are comparable with the plateau hF.IX levels of 269 ng/mL in NHPs we previously treated with an AAV-8-hF.IX vector (dosed at 5 × 1012 vg/kg).23 This would suggest that the marked dose advantage seen with AAV-8 in mice may not be observed in higher species.
In summary, in response to findings in a clinical study of AAV-mediated gene transfer to liver in men with severe hemophilia B, in which expression of the donated gene was terminated by a CD8+ T-cell response to AAV capsid, resulting in destruction of the transduced cells, we have designed and tested an adjunctive regimen designed to block the CD8+ T-cell response to capsid. In a nonhuman primate model, we have shown that a commonly used organ transplantation regimen consisting of MMF and sirolimus does not alter AAV transduction efficiency, nor does it promote formation of antibodies to the transgene product F.IX. Addition of the anti–IL-2 receptor daclizumab to this regimen, however, consistently resulted in formation of anti-F.IX antibodies in the NHP, likely due to interference with induction of CD4+CD25+FoxP3+ regulatory T cells at an early time after vector infusion. We conclude that anti–IL-2 receptor antibodies cannot be used as part of an IS regimen for hepatic gene transfer in subjects who are not fully tolerant of the transgene product, and that timing of induction of regulatory T cells is critical to establishment of transgene tolerance in AAV-mediated, liver-directed gene transfer.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants R01 HL084220 (V.R.A.) and P01 HL078810 (K.A.H.) and the Howard Hughes Medical Institute. N.C.H. was supported by training grant NIH T32 HL007150 and D.J.H., by training grant NIH T32 HL07439.
We gratefully thank Jitin Bajaj, Bernd Hauck, Sonali Joyce, Alex Tai, Olga Zelenaia, and Yi Zhao for assistance with AAV vector preparation.
National Institutes of Health
Authorship
Contribution: F.M. organized and supervised the nonhuman primate studies, coordinated experimental activities, performed gene expression and immunology studies, and drafted the paper; N.C.H. performed the biodistribution studies, the lymphocyte isolation, and the ELISpot assays; E.B.-T. performed the staining for regulatory T cells; S.A.E. performed the ELISA for transgene expression levels, the antibody assays, the Bethesda assay, and the PBMC isolation; D.J.H. assisted with tissue processing and lymphocyte isolation; D.E.S. provided essential input to experimental design; S.Z. and J.F.W. prepared the AAV vectors used in the studies; H.J. and G.F.P. provided input to experimental design and collaborated with paper preparation; V.R.A. collaborated on study design, data interpretation, and paper preparation; K.A.H. directed experimental design, conducted data analysis and interpretation, and drafted the paper.
Conflict-of-interest disclosure: J.F.W. and K.A.H. hold patents related to AAV gene therapy that are licensed to Genzyme. Two of the authors (G.F.P. and H.J.) were employed by a company (Avigen) whose potential product is discussed in this work. All other authors declare no competing financial interests.
Correspondence: Katherine A. High, The Children's Hospital of Philadelphia, Abramson Research Center Rm 302, 3615 Civic Center Blvd, Philadelphia, PA 19104; e-mail:high@email.chop.edu.