Linker for activation of T cells (LAT) is an adaptor protein required for organization of the signaling machinery downstream of the platelet collagen receptor, the glycoprotein VI (GPVI). Here, we investigated the effect of LAT mutations on specific signaling pathways and on platelet functions in response to GPVI triggering by convulxin (Cvx). Using mice containing tyrosine to phenylalanine mutations of the adaptor, we show the crucial role played by the tyrosine residues at positions 175, 195, and 235 in the phosphorylation of LAT and in the whole pattern of protein tyrosine phosphorylation in response to Cvx. These 3 C-terminal tyrosine residues are important to recruit the tyrosine kinase Fyn, which may be involved in LAT phosphorylation. Efficient phosphoinositide 3-kinase (PI3K) activation requires the 3 C-terminal tyrosine residues of LAT but not its tyrosine 136. Interestingly, single mutation of the tyrosine 136 results in the loss of phospholipase C γ2 (PLCγ2) activation without affecting its PI3K-dependent membrane association, and is sufficient to impair platelet responses to Cvx. Thus, activation of PLCγ2 via GPVI is dependent on 2 complementary events: its interaction with the tyrosine 136 of LAT and its membrane location, which itself requires events mediated by the 3 C-terminal tyrosines of LAT.
Introduction
Subendothelial collagen fibers play an important role in platelet adhesion and activation at sites of vessel damage.1 Besides α2β1, a major receptor supporting platelet adhesion to collagen, the glycoprotein VI (GPVI) is considered as the receptor mediating collagen-induced platelet activation.2 GPVI is a member of the immunoglobulin superfamily of type I transmembrane proteins and is noncovalently associated with the Fc receptor (FcR) γ-chain. It stimulates platelets through a tyrosine kinase-based signaling pathway.2 The initial step of tyrosine phosphorylation of the immunoreceptor tyrosine-based activation motif (ITAM) in the FcR γ-chain by Src family kinases leads to the recruitment and the activation of the tyrosine kinase Syk and in turn to the phosphorylation of a number of signaling proteins. Several adaptor molecules, including linker for activation of T cells (LAT) and Src homology 2 domain-containing leukocyte phosphoprotein of 76 kDa (SLP-76) coordinate the assembly of a multiprotein signaling complex essential for GPVI-induced activation of key enzymes such as phospholipase Cγ2 (PLCγ2) and phosphoinositide 3-kinase (PI3K).3,,,–7
LAT is a membrane-associated adaptor molecule containing a short extracellular domain, a single transmembrane-spanning region, and a long cytosolic tail with 9 tyrosines conserved in mouse and human sequences. It is thought that the palmitoylation of cysteine residues near the plasma membrane anchors LAT to lipid rafts. This hematopoietic-specific adaptor protein is expressed in early B cells, T cells, mast cells, natural killer cells, megakaryocytes, and platelets. It plays a crucial role in T-cell activation and thymocyte development.8 LAT-deficient mice (Lat−/−) have revealed a role of this adaptor protein during T-cell maturation.9 LAT is also involved in the FcϵRI activating pathway in mast cells10,11 and might facilitate early B-cell differentiation.12,13 Studies in T lymphocytes have shown the importance of the 4 C-terminal tyrosine residues (Y132, Y171, Y191, and Y226 in humans, and their homologs in mice: Y136, Y175, Y195, and Y235) for most of the signaling activity of LAT.14 The 3 membrane-distal tyrosine residues Y171, Y191, and Y226 bind Gads and Grb2, while Y132 is required for PLCγ1 interaction.15 Mice expressing LAT with mutations in the 4 distal tyrosines exhibit defective thymocyte development.16 LAT knock-in harboring point mutations in the Y175, Y195, and Y235 results in the selective development and expansion of γδ T cells.17 Mice bearing a single Y136F mutation have an aberrant αβ T-cell proliferation characterized by an exaggerated polyclonal differentiation into CD4 cells that secreted abnormally high levels of T helper 2 (Th2) cytokines.18,19 It is thus becoming clear that subtle mutations in LAT, leading to a defective LAT signalosome, have important effects on the differentiation and function of multiple immune cell types.20,21
Platelets from Lat−/− mice exhibit a defective response to GPVI agonists such as the collagen-related peptide (CRP)3,4 and the C-type lectin present in the venom of the rattlesnake Crotalus durissus terrificus convulxin (Cvx).22 Lat−/− platelets are still able to aggregate in response to high concentrations of GPVI agonists,23 suggesting that this adaptor may be dispensable for some platelet functions in certain conditions. However, LAT appears essential for the platelet procoagulant response24 and the full surface expression of P-selectin.23 As GPVI-null mice,25 Lat−/− mice show no signs of hemorrhage, suggesting that other agonists or adhesive proteins using different signaling pathways can compensate for these deficiencies. Recent evidence suggests that, in vivo, the type of initiating event appears to determine the relative importance of GPVI-mediated platelet activation in thrombus formation.26 It is also important to note that GPVI is involved in thrombotic disorders.1,27 Besides its important role in signal transduction upon GPVI triggering, LAT is also involved downstream of FcγRIIa28 and GpIb.29 It plays an essential role in platelet stimulation by the C-terminal peptide of thrombospondin-1.30 Most tyrosine kinase-dependent platelet activation pathways actually use LAT to efficiently orchestrate the spatio-temporal assembly of signaling proteins to obtain a complete and rapid platelet response.
There are similarities of LAT functions in immune cells and in platelets, but several important differences in the signaling mechanisms involving SLP-76 and LAT have also been observed between both systems.31 Here, we investigated the effects of LAT mutations on the disruption of specific signaling pathways downstream of GPVI and analyzed their functional consequences on platelet responses. We demonstrate the crucial role of the 3 C-terminal tyrosines (Y175, Y195, and Y235) in the phosphorylation of the adaptor itself, in the whole pattern of protein tyrosine phosphorylation, and in platelet responses to GPVI triggering. PI3K activation is strongly inhibited upon mutation of the 3 distal tyrosine residues of LAT but not of the Y136 residue. Conversely, a single mutation of the Y136 residue of LAT results in the loss of PLCγ2 activation but spares its PI3K-dependent association with the membrane.
Materials and methods
Materials
The anti-Fyn monoclonal antibody and the anti-PLCγ2 polyclonal antibody were from Santa Cruz Biotechnology (Santa Cruz, CA). The mouse antiphosphotyrosine 4G10 was from Upstate Biotechnology (Lake Placid, NY). 5-hydroxy[14C]tryptamine (2072 MBq/mmol [56.0 mCi/mmol]) and SuperSignal West Pico Chemiluminescent substrate were from Amersham International (Little Chalfont, United Kingdom). [γ-32P] ATP (11.1 × 1013 Bq/mmol [3000 Ci/mmol]) was from New England Nuclear (Boston, MA). GST-LAT cDNA was generated by inserting the full-length cytosolic domain of LAT obtained by reverse transcriptase-polymerase chain reaction (RT-PCR) from megakaryocytic DAMI cells into pGEX-KG vector (Pharmacia, Uppsala, Sweden). Polyclonal LAT antibody was produced by immunizing rabbit with this fusion protein. Cvx was purified from the venom of Crotalus durissus terrificus as previously described.32 Horm collagen from equine tendon was from Nycomed (Munich, Germany). Thin-layer chromatography (TLC) plates were from Merck (Darmstadt, Germany). Wortmannin, LY294002, and other reagents were from Sigma (St Louis, MO).
Mice
Mice deficient in recombination activation gene 1 (Rag1−/− mice) were originally obtained from E. Spanopoulou (Mount Sinai School of Medicine, New York, NY).33 Mice deficient in LAT (Lat−/−), and mice homozygous for a mutation that replaced with phenylalanine tyrosine 136 (LatY136F) or the last 3 carboxy-terminal tyrosines (Lat3YF) were previously described.17,18 To prevent the development of noxious T cells, mice deficient for the Rag1 gene and homozygous for the LatY136F or Lat3YF mutations were used. Mice were housed under specific pathogen-free conditions in accordance with institutional guidelines approved by French laws and were used between 6 and 10 weeks of age.
Platelet preparation and in vitro aggregation studies
Whole blood was collected by puncturing the inferior vena cava with syringes containing acid citrate dextrose (1/9 vol) from anesthetized mice. Pooled blood samples were diluted in 1 vol of modified HEPES Tyrode buffer (137 mM NaCl, 2 mM KCl, 12 mM NaHCO3, 0.3 mM NaH2PO4, 1 mM MgCl2, 5.5 mM glucose, and 5 mM HEPES [pH 6.7]) containing 0.35% human serum albumin, and platelet-rich plasma was obtained by centrifugation (4 minutes, 250g, 37°C). Thereafter, prostaglandin I2 at a final concentration of 500 nM was added, and platelets were pelleted once by centrifugation (4 minutes, 1000g, 37°C). The platelet pellet was finally resuspended in modified HEPES-Tyrode buffer (pH 7.38) containing 2 mM CaCl2, at a density of 5 × 108 platelets/mL in the presence of 0.02 U/mL of the ADP scavenger apyrase (adenosine-5′-triphosphate diphosphohydrolase), and incubated for 45 minutes at 37°C before stimulating with Cvx. Optical aggregation experiments were monitored by a turbidimetric method using a dual-channel Payton aggregometer (Payton Associates, Scarborough, ON) with continuous stirring (900 rev/min) at 37°C.
Dense granule secretion
Dense granule secretion was investigated by platelet 5-hydroxy[14C]tryptamine release in response to Cvx stimulation under stirring conditions, as described previously.34
In vitro flow-based adhesion studies
Glass microcapillaries were coated with 500 μg/mL Horm collagen from equine tendon for 1 hour at 37°C. The flow chamber, mounted on an epifluorescence microscope (Axiovert 200; Carl Zeiss, Iena, Germany), allowed direct visualization of the platelet adhesion and aggregation process, which was recorded with a CCD camera (Cool Snap HQ; Roper Scientific, Tucson, AZ). Mouse blood was drawn into lepirudin (200 IU/mL) and DiOC6 (2 μM; 30 minutes at 37°C) was used to label platelets in whole blood. Labeled blood was then perfused through collagen-coated glass microcapillaries for 2 minutes at a wall shear rate of 1500 s−1 (15 dyn/cm2), followed by washing for 2 minutes at the same shear rate with phosphate-buffered saline. Thrombus formation was visualized with a 40×/1.3 NA long working distance objective in real time (acquisition rate: 1 frame every 5 seconds) for both fluorescent and transmitted light microscopy. Image sequences of the time-lapse recording and analysis of surface coverage were performed offline on a single frame using the Metamorph software (Universal Imaging, Auburn Hills, MI). After deconvolution, a lower-intensity threshold was applied to distinguish platelets from the background, and a similar threshold was then used for analyzing all Z-stacks collected for a given experiment. Thrombus volume was calculated as the summation of partial volumes measured from the area occupied by platelets in each plane of Z-stacks as described previously.35
Lipid extraction and analysis
Platelets were labeled with 22.2 MBq/mL (0.6 mCi/mL) [32P]orthophosphate during 45 minutes in a phosphate-free HEPES-Tyrode buffer (pH 6.5) at 37°C. [32P]-labeled platelets were then washed once in the same buffer and finally suspended at a final concentration of 1 × 109 platelets/mL in modified HEPES-Tyrode buffer (pH 7.38). After stimulation, reactions were stopped by addition of chloroform/methanol (1/1 vol/vol) containing 0.4 N HCl, and lipids were immediately extracted as described previously.34,36 For phosphatidylinositol 3,4-bisphosphate (PtdIns(3,4)P2), phosphatidylinositol 3,4,5-trisphosphate (PtdIns(3,4,5)P3), and phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2) quantification, lipids were first resolved by TLC using chloroform/acetone/methanol/acetic acid/water (80/30/26/24/14 vol/vol). The spots corresponding to PtdIns(3,4)P2, PtdIns(3,4,5)P3, and PtdIns(4,5)P2 were then scraped off, immediately deacylated, and analyzed by high-performance liquid chromatography (HPLC) on a Whatman Partisphere 5 SAX column (Whatman International, Maidstone, United Kingdom) as described.36 For phosphatidic acid (PtdOH) quantification, lipids were resolved by TLC using chloroform/methanol/10 N HCl (87/13/0.5 vol/vol) as described previously.34 The spots corresponding to PtdOH were scraped off and directly quantified by liquid-scintillation counting.
Immunoprecipitations and immunoblotting
A total of 500 μL of 7.5 × 108/mL resting or stimulated platelets were lysed in RIPA buffer at final concentrations of 150 mM NaCl, 20 mM Tris-HCl (pH 7.7), 4 mM EDTA, 0.5% Triton X-100, 1 mM sodium orthovanadate, 1 mM phenyl-methylsulfonyl fluoride, 10 μg/mL leupeptin, and 10 μg/mL aprotinin. The lysates were submitted to standard immunoprecipitation and immunoblotting protocols. For reimmunoprecipitation, the immunoprecipitated complexes were dissociated by addition of 60 μL of a buffer containing 50 mM Tris-HCl (pH 7.5), 10 mM DTT, and 1% SDS and boiled for 5 minutes. The final volume was then brought to 1.5 mL by RIPA buffer containing 0.5% Triton X-100, and the second immunoprecipitation was performed with the appropriate antibody.
Biotinylated Cvx (2 μg/mL), prepared as described previously,37 was used to analyze GPVI expression and was probed with horseradish peroxidase (HRP)–streptavidin.
In vitro kinase assay
Samples were immunoprecipitated as described and washed twice with RIPA buffer and once with kinase buffer (50 mM Tris-HCl, 2.5 mM MnCl2, and 5 mM MgCl2 [pH 7.4]). The beads were resuspended in 40 μL of kinase buffer containing 0.37 MBq (10 μCi) of [γ-32P] ATP with 10 μM cold ATP and incubated at 30°C for 30 minutes under shaking.28 Reactions were terminated by addition of 10 μL of 5 × Laemli buffer and submitted to a 10% SDS-PAGE. To perform reimmunoprecipitation, the immune complexes from the in vitro kinase (IVK) assay were submitted to the protocol described using LAT antibody.
Cytosol depletion
After stimulation, platelets were centrifuged (3000g for 30 seconds) and suspended in 20 mM PIPES buffer (pH 6.8) containing 150 mM KCl, 2 mM EDTA, and 30 μg/mL saponin. After 5 minutes at room temperature under shaking, supernatant and pellet fractions were separated by centrifugation (12 000g for 40 seconds). The pellet, containing membranes and cytoskeleton, was suspended in Laemli sample buffer, and PLCγ2 was probed by immunoblotting using anti-PLCγ2 antibody.
Results
The 3 C-terminal tyrosine residues of LAT and tyrosine 136 are critical for GPVI-induced platelet activation
To address the role of the C-terminal tyrosine residues of LAT, platelets from wild-type (WT) or knock-in mice expressing LAT with combinations of tyrosine mutations were stimulated with the potent GPVI agonist Cvx. The platelet counts of the mice with LAT mutated either on the last 3 C-terminal tyrosine residues 175, 195, and 235 (Lat3YF) or on the tyrosine residue 136 only (LatY136F) were normal (492 000 ± 36 000/mm3 for WT versus 470 000 ± 47 000/mm3 for LatY136F and 447 000 ± 97 000/mm3 for WT(Rag−/−) versus 541 000 ± 37 000/mm3 for Lat3YF; n = 5) and the platelet expression level of GPVI, LAT, and PLCγ2 were fairly comparable (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
As expected, stimulation of WT platelets with increasing concentrations of Cvx resulted in an irreversible platelet aggregation in a dose-dependent manner (Figure 1A,B; left panels). Conversely, platelets from Lat3YF (Figure 1A) or from LatY136F (Figure 1B) mice did not aggregate in response to low doses of Cvx (< 5 nM) and slightly aggregated at the highest concentrations of Cvx tested (up to 20 nM). Lat−/− platelets did not aggregate on 5 nM Cvx but, as reported previously,23 a significant aggregation response was observed upon high concentrations of Cvx (> 20 nM; not shown). To preserve the selectivity of the Cvx/GPVI activation pathway, we used a dose of 5 nM Cvx in our biochemical studies, since higher concentrations may induce GPVI-independent events. It is noteworthy that platelets from LatY136F and Lat3YF mice aggregated as WT platelets in response to 0.2 IU/mL thrombin (Figure S2C,D). Platelet-dense granule secretion was nearly maximal at 10 nM Cvx in WT platelets but was hardly detectable in Lat3YF (Figure 1C) and LatY136F (Figure 1D) platelets upon stimulation with Cvx concentrations up to 10 nM.
Mutations of the 3 C-terminal tyrosines or tyrosine 136 of LAT inhibit platelet aggregation and secretion in response to Cvx. (A,B) Platelets from the different mouse strains were stimulated with increasing concentrations of Cvx, and aggregation was assessed using a Chrono-log (Payton Associates) dual channel aggregometer under stirring at 900 rev/minute. The profiles shown are representative of 4 independent experiments. (C,D) To measure dense granule secretion, 5-hydroxy[14C]tryptamine[e]labeled platelets were stimulated by different concentration of Cvx. Results are expressed as a percentage of 5-hydroxy[14C]tryptamine (serotonine) secretion and are means (± SEM) of 5 independent determinations.
Mutations of the 3 C-terminal tyrosines or tyrosine 136 of LAT inhibit platelet aggregation and secretion in response to Cvx. (A,B) Platelets from the different mouse strains were stimulated with increasing concentrations of Cvx, and aggregation was assessed using a Chrono-log (Payton Associates) dual channel aggregometer under stirring at 900 rev/minute. The profiles shown are representative of 4 independent experiments. (C,D) To measure dense granule secretion, 5-hydroxy[14C]tryptamine[e]labeled platelets were stimulated by different concentration of Cvx. Results are expressed as a percentage of 5-hydroxy[14C]tryptamine (serotonine) secretion and are means (± SEM) of 5 independent determinations.
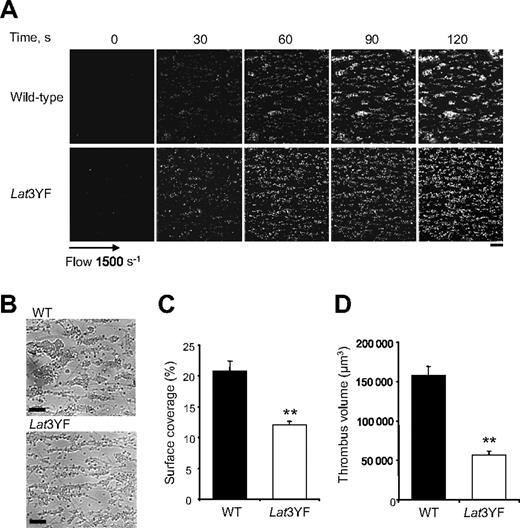
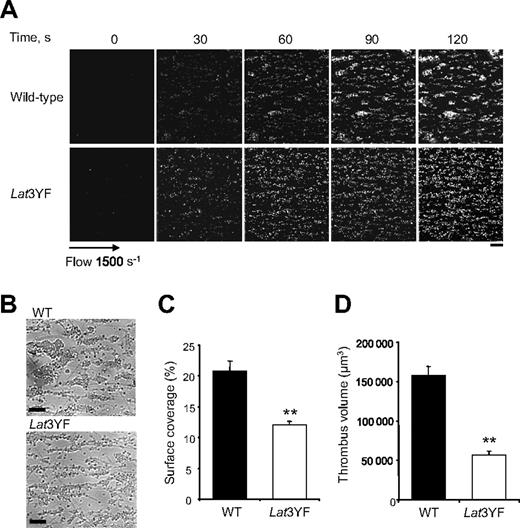
To further investigate the functional role of the C-terminal tyrosine residues of LAT on platelets adhesion and aggregation, we have examined platelets under physiologic flow conditions using an in vitro flow-based platelet aggregation assay. Fluorescently labeled platelets in whole blood were perfused over a matrix of collagen at a shear rate of 1500 s−1 (15 dyn/cm2). Blood from control mice exhibited robust formations of densely packed platelet thrombi on collagen. In marked contrast, Lat3YF platelets attached along the length of the collagen fibers, but the formation of platelet thrombi was strongly deficient (Figure 2A,B). These results are consistent with the notion that these platelets formed a single layer on the collagen fibers in contrast to the multilayer platelet thrombi observed in control blood as shown with the differential interference contrast pictures and the total fluorescence intensity. As shown in Figure 2C,D, the surface covered by platelet thrombi (12% ± 0.6% for Lat3YF versus 20.9% ± 1.6% for WT platelets) and the thrombus volume (0.6 ± 0.01 × 105 μm3 for Lat 3YF versus 1.6 ± 0.1 × 105 μm3 for WT platelets) were significantly reduced. Similar results were obtained with LatY136F platelets (not shown).
Mutation of the 3 C-terminal tyrosines of LAT affect platelet thrombi formation on collagen under flow. DiOC6-labeled platelets in whole blood were perfused through a collagen-coated microcapillary at a shear rate of 1500 s−1 for 2 minutes. (A) Thrombus formation was visualized with a 40×/1.3 NA; long working distance objective in real time and then imaged using transmitted light microscopy. (B) After a washing step with phosphate-buffered saline for 2 minutes at the same shear rate to remove nonadherent cells, slides were visualized using differential interference contrast microscopy. Representative images of platelet adhesion from WT and Lat3YF mice are shown. A representative time course for both WT (top panel) and Lat3YF (bottom panel) platelet accumulation on collagen is shown (see “In vitro flow-based adhesion studies” for complete image acquisition information). (C) Area covered by platelet thrombi and (D) thrombus volume were measured at 2 surface locations in each of 3 different experiments (mean ± SEM). **P < .005 versus WT, according to Student t test. Scale bar equals 20 μm.
Mutation of the 3 C-terminal tyrosines of LAT affect platelet thrombi formation on collagen under flow. DiOC6-labeled platelets in whole blood were perfused through a collagen-coated microcapillary at a shear rate of 1500 s−1 for 2 minutes. (A) Thrombus formation was visualized with a 40×/1.3 NA; long working distance objective in real time and then imaged using transmitted light microscopy. (B) After a washing step with phosphate-buffered saline for 2 minutes at the same shear rate to remove nonadherent cells, slides were visualized using differential interference contrast microscopy. Representative images of platelet adhesion from WT and Lat3YF mice are shown. A representative time course for both WT (top panel) and Lat3YF (bottom panel) platelet accumulation on collagen is shown (see “In vitro flow-based adhesion studies” for complete image acquisition information). (C) Area covered by platelet thrombi and (D) thrombus volume were measured at 2 surface locations in each of 3 different experiments (mean ± SEM). **P < .005 versus WT, according to Student t test. Scale bar equals 20 μm.
All together, these results strongly suggest that the docking properties of the 4 distal tyrosine residues of LAT link GPVI to intracellular signaling pathways essential for platelet functions.
The 3 C-terminal tyrosine residues of LAT are critical for most GPVI-induced tyrosine phosphorylations
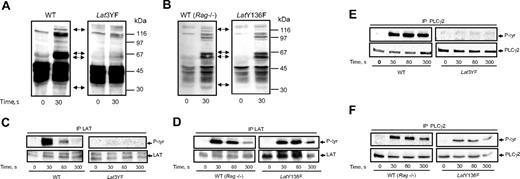
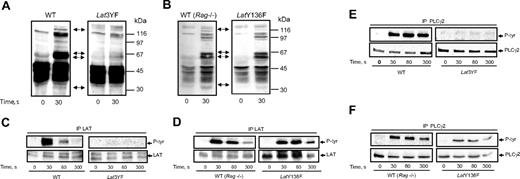
To analyze the signaling pathways linked to the 3 distal tyrosine residues of LAT, we first investigated the tyrosine phosphorylation events occurring in platelets from the different mice strains stimulated by Cvx. Stimulation with 5 nM Cvx led to a rapid and dramatic increase in the level of tyrosine phosphorylation of several proteins in WT platelets (Figure 3A). Particularly, a marked increase in the phosphorylation of 70-kDa proteins (matching Syk and SLP-76) was observed. An increase in the tyrosine phosphorylation of proteins of about 120 kDa and 145 kDa (matching PLCγ2) was also clearly detected. Interestingly, a combination of Y175F, Y195F, and Y235F mutations of LAT dramatically decreased the phosphorylation of the 70 kDa and the 145 kDa proteins in response to Cvx (Figure 3A). Conversely, the tyrosine phosphorylation pattern of platelets from knock-in mice presenting a single mutation (Y136F) was comparable to that of WT platelets in response to Cvx (Figure 3B) except a reduction in the degree of phosphorylation of the 145-kDa protein (matching PLCγ2). As suggested in Figure 3A, the heavy tyrosine phosphorylation of LAT observed in response to Cvx in WT mice platelets was abolished by mutation of its 3 C-terminal tyrosine residues (Figure 3C). Thus, although LAT has 9 tyrosine residues, mutations of its last 3 C-terminal tyrosines are sufficient to abolish its own phosphorylation in response to GPVI triggering. The single mutation Y136F did not significantly affect the degree of tyrosine phosphorylation of LAT in response to Cvx (Figure 3D). This Y136 residue of LAT is the major binding site for PLCγ1 in T lymphocytes.15 Two forms of PLCγ have been identified, and PLCγ2 is the form predominantly expressed in platelets. GPVI-induced PLCγ2 tyrosine phosphorylation was fully inhibited in Lat3YF platelets (Figure 3E). Interestingly, although strongly affected, a tyrosine phosphorylation of PLCγ2 was still detectable in LatY136F platelets (Figure 3F). These results suggest that besides an important role of the Y136 residue, the 3 distal tyrosine residues of LAT (Y175, Y195 and Y235) are required for PLCγ2 phosphorylation.
Effects of mutations of the C-terminal tyrosines of LAT on the tyrosine phosphorylations of PLCγ2 in response to Cvx. (A,B) Platelets from WT, Lat3YF, and LatY136F mice were stimulated by 5 nM Cvx for the time indicated. Reactions were stopped by addition of RIPA buffer, protein separated by a 12.5% SDS-PAGE, and transferred onto nitrocellulose; the tyrosine-phosphorylated proteins were detected by immunoblotting with the antiphosphotyrosine antibody 4G10. The membrane was stripped and reprobed for LAT, Syk, SLP-76, and PLCγ2; their positions are indicated by the black arrow (from the bottom of the nitrocellulose). Platelets from WT (C,D) (left panel), Lat3YF (C) (right panel), and LatY136F (D) (right panel) mice were stimulated by 5 nM Cvx for the time indicated. Reactions were stopped by addition of RIPA buffer, and LAT was immunoprecipitated using a specific antibody. Tyrosine phosphorylation of LAT was assessed by immunoblotting using the antiphosphotyrosine antibody 4G10 (top panels). The membrane was stripped and reprobed for LAT with the anti-LAT antibody (bottom panels). PLCγ2 was immunoprecipitated from the lysate of platelets from WT (E,F) (left panel), Lat3YF (E) (right panel), and LatY136F (F) (right panel) mice stimulated by 5 nM Cvx for the indicated times. Tyrosine phosphorylation of PLCγ2 was assessed by immunoblotting using the antiphosphotyrosine antibody 4G10 (top panels). The membrane was stripped and reprobed for PLCγ2 with the anti-PLCγ2 antibody (bottom panels). Results are representative of 3 independent experiments.
Effects of mutations of the C-terminal tyrosines of LAT on the tyrosine phosphorylations of PLCγ2 in response to Cvx. (A,B) Platelets from WT, Lat3YF, and LatY136F mice were stimulated by 5 nM Cvx for the time indicated. Reactions were stopped by addition of RIPA buffer, protein separated by a 12.5% SDS-PAGE, and transferred onto nitrocellulose; the tyrosine-phosphorylated proteins were detected by immunoblotting with the antiphosphotyrosine antibody 4G10. The membrane was stripped and reprobed for LAT, Syk, SLP-76, and PLCγ2; their positions are indicated by the black arrow (from the bottom of the nitrocellulose). Platelets from WT (C,D) (left panel), Lat3YF (C) (right panel), and LatY136F (D) (right panel) mice were stimulated by 5 nM Cvx for the time indicated. Reactions were stopped by addition of RIPA buffer, and LAT was immunoprecipitated using a specific antibody. Tyrosine phosphorylation of LAT was assessed by immunoblotting using the antiphosphotyrosine antibody 4G10 (top panels). The membrane was stripped and reprobed for LAT with the anti-LAT antibody (bottom panels). PLCγ2 was immunoprecipitated from the lysate of platelets from WT (E,F) (left panel), Lat3YF (E) (right panel), and LatY136F (F) (right panel) mice stimulated by 5 nM Cvx for the indicated times. Tyrosine phosphorylation of PLCγ2 was assessed by immunoblotting using the antiphosphotyrosine antibody 4G10 (top panels). The membrane was stripped and reprobed for PLCγ2 with the anti-PLCγ2 antibody (bottom panels). Results are representative of 3 independent experiments.
Fyn interacts with LAT in response to GPVI triggering: a role for the 3 C-terminal tyrosines of LAT
To check whether a kinase that would phosphorylate LAT interacts with this adaptor through its 3 distal tyrosine residues, we performed an in vitro kinase assay in LAT immunoprecipitates. As shown in Figure 4A, a strong in vitro LAT phosphorylation was observed after 30 seconds of Cvx stimulation of WT platelets. This phosphorylation was only very weak in Lat3YF platelets. These results suggest that the last 3 tyrosine residues of LAT are important for the binding of a protein kinase involved in its phosphorylation. The Src family kinase member Fyn is known to form part of the GPVI signaling cascade.38,39 Interestingly, Fyn interacted with LAT upon Cvx activation in WT mice platelets (Figure 4B). This interaction was no longer observed when LAT was mutated on its last 3 C-terminal tyrosine residues. These results were confirmed by reverse experiment. Indeed, phosphorylated LAT was detected in Fyn immunoprecipitates obtained from WT mice platelets, while only a weak signal was detected when the immunoprecipitate was performed from Lat3YF mice platelets (Figure 4C).
The interaction of Fyn with LAT is abolished by mutations in the 3 distal C-terminal tyrosines of LAT. (A) Platelets from WT (left panel) and Lat3YF (right panel) mice were stimulated by 5 nM Cvx for the time indicated, and LAT was immunoprecipitated and submitted to an in vitro kinase (IVK) assay in the presence of [γ-32P]ATP. After separation by 10% SDS-PAGE, the radioactivity incorporated in LAT was assessed by PhosphorImager (PerkinElmer, Shelton, CT) analysis. (B) Alternatively, following immunoprecipitation with the anti-LAT antibody, immunoprecipitated complexes were dissociated and reimmunoprecipitated with anti-Src family antibody. After separation by 10% SDS-PAGE and transfer onto nitrocellulose, the reimmunoprecipitate was immunoblotted with a specific anti-Fyn antibody. (C) Fyn was immunoprecipitated from lysate of WT (left panel) or Lat3YF (right panel) platelets stimulated by 5 nM Cvx for the indicated times. Immunoprecipitated complexes were submitted to an in vitro kinase assay in the presence of [γ-32P]ATP and reimmunoprecipitated with the anti-LAT antibody as described. The radioactivity incorporated in LAT was assessed by PhosphorImager analysis. Results are representative of 2 independent experiments.
The interaction of Fyn with LAT is abolished by mutations in the 3 distal C-terminal tyrosines of LAT. (A) Platelets from WT (left panel) and Lat3YF (right panel) mice were stimulated by 5 nM Cvx for the time indicated, and LAT was immunoprecipitated and submitted to an in vitro kinase (IVK) assay in the presence of [γ-32P]ATP. After separation by 10% SDS-PAGE, the radioactivity incorporated in LAT was assessed by PhosphorImager (PerkinElmer, Shelton, CT) analysis. (B) Alternatively, following immunoprecipitation with the anti-LAT antibody, immunoprecipitated complexes were dissociated and reimmunoprecipitated with anti-Src family antibody. After separation by 10% SDS-PAGE and transfer onto nitrocellulose, the reimmunoprecipitate was immunoblotted with a specific anti-Fyn antibody. (C) Fyn was immunoprecipitated from lysate of WT (left panel) or Lat3YF (right panel) platelets stimulated by 5 nM Cvx for the indicated times. Immunoprecipitated complexes were submitted to an in vitro kinase assay in the presence of [γ-32P]ATP and reimmunoprecipitated with the anti-LAT antibody as described. The radioactivity incorporated in LAT was assessed by PhosphorImager analysis. Results are representative of 2 independent experiments.
Both PLCγ2 and PI3K activations are abolished by mutations of the 3 last C-terminal tyrosines of LAT, while the Y136F mutation selectively affects PLC and spares PI3K activation
Activation of PLC and PI3K are recognized as critical processes in the stimulation of platelets by GPVI. To monitor PLC activity in [32P]-labeled platelets, it is typical to follow the production of [32P]-PtdOH, a metabolite of 1,2-diacylglycerol. PI3K activation is usually measured by following the levels of the different [32P]-labeled phosphoinositides by an HPLC technique.
In contrast to WT platelets, which rapidly form PtdIns(3,4,5)P3 in response to Cvx, platelets from Lat3YF mice produced only very small amounts of this PI3K product (Figure 5A). Moreover, PtdIns(3,4)P2, known to accumulate in an integrin-engagement and a platelet aggregation-dependent manner, was no longer produced in platelets from Lat3YF mice (Figure 5A). As shown by the lack of PtdOH production, the activation of PLC was fully inhibited in platelets from Lat3YF mice (Figure 5A). In agreement, the weak decrease in PtdIns(4,5)P2, the substrate of PLC, observed in WT platelets stimulated by Cvx was not observed in platelets from Lat3YF mice (Figure 5A). Similar results were obtained with Lat−/− platelets (not shown). In contrast, PtdIns(3,4,5)P3 was still produced in platelets from LatY136F mice stimulated by Cvx, with only a slight decrease in the maximal synthesis of this second messenger after 1 minute of activation (Figure 5B). The initial production of PtdIns(3,4)P2 observed at 30 seconds of Cvx stimulation was preserved by the Y136F mutation of LAT, but the accumulation of this lipid was abolished, likely because aggregation was impaired (Figure 5B). Interestingly, mutation of the PLCγ-binding site of LAT (Y136) fully inhibited the production of PtdOH and the decrease in PtdIns(4,5)P2 (Figure 5B). It is noteworthy that this mutation only partly affected the phosphorylation of PLCγ2 in response to Cvx (Figure 3F).
Impact of mutations in the C-terminal tyrosines of LAT on PI3K and PLC activation in response to GPVI triggering. [32P]-labeled platelets from WT (♦) and Lat3YF (■) (A) or from WT (Rag−/−) (♦) and LatY136F mice (●) (B) platelets were stimulated by 5 nM Cvx for the indicated times and the levels of [32P]-PtdIns(3,4,5)P3, [32P]-PtdIns(3,4)P2, [32P]-PtdIns(4,5)P2, and [32P]-PtdOH were analyzed as indicated in “Platelet preparation and in vitro aggregation studies.” Results are means (± SEM) of 4 experiments.
Impact of mutations in the C-terminal tyrosines of LAT on PI3K and PLC activation in response to GPVI triggering. [32P]-labeled platelets from WT (♦) and Lat3YF (■) (A) or from WT (Rag−/−) (♦) and LatY136F mice (●) (B) platelets were stimulated by 5 nM Cvx for the indicated times and the levels of [32P]-PtdIns(3,4,5)P3, [32P]-PtdIns(3,4)P2, [32P]-PtdIns(4,5)P2, and [32P]-PtdOH were analyzed as indicated in “Platelet preparation and in vitro aggregation studies.” Results are means (± SEM) of 4 experiments.
These results show that a full PI3K activation requires the 3 distal tyrosine residues of LAT (Y175, Y195, and Y235) but not its Y136 residue. Conversely, mutation of the Y136 residue of LAT results in the loss of PLCγ2 activation but spares PI3K activation in response to Cvx.
PLCγ2 association with the platelet membrane/cytoskeleton is not affected by the Y136F mutation of LAT but is abolished by mutations of the 3 distal tyrosine residues
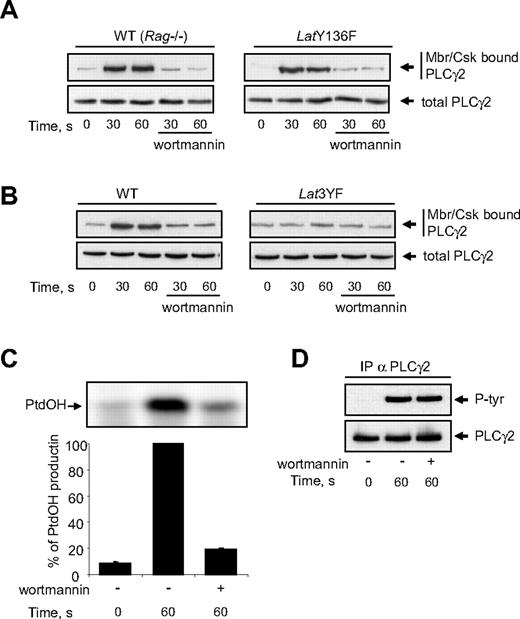
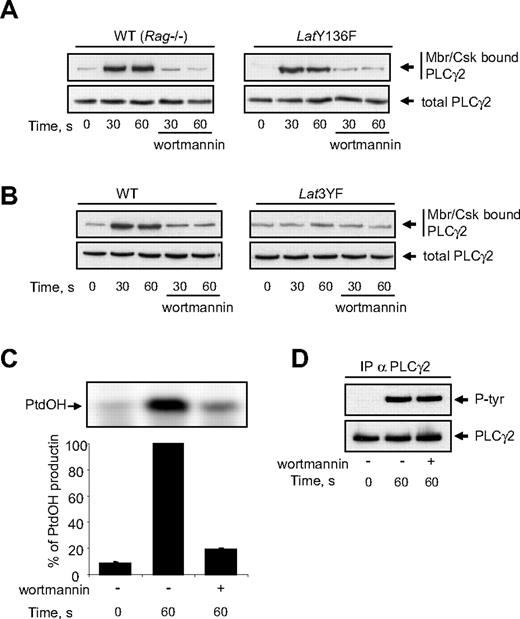
The stable recruitment of activated PLCγ2 to the membrane is critical for the enzyme to hydrolyse PtdIns(4,5)P2 and to produce inositol-1,4,5 trisphosphate (InsP3) and diacylglycerol. Since Y136F mutation of LAT abolishes PtdOH production, we checked whether this mutation also affected the association of PLCγ2 with the membrane/cytoskeleton. As shown in Figure 6A, Cvx stimulation induced a rapid interaction of PLCγ2 with the membrane fraction of platelets from both WT and LatY136F mice. In both cases, this interaction was fully inhibited by the PI3K inhibitor wortmannin (Figure 6A) and the other unrelated PI3K inhibitor LY294002 (not shown). The fact that Y136F mutation of LAT did not affect the association of PLCγ2 with the membrane/cytoskeleton clearly shows that this step is not sufficient to allow PLC activation. Interestingly, mutations of the 3 distal tyrosine residues of LAT which dramatically inhibited PI3K activation (Figure 5A) also strongly prevented the relocation of PLCγ2 (Figure 6B). The association of PLCγ2 with the membrane/cytoskeleton is essential since in platelets from WT mice, PI3K inhibitors abolished PtdOH production (Figure 6C) while sparing PLC γ2 phosphorylation (Figure 6D).
Mutation of tyrosine 136 of LAT does not affect GPVI-dependent membranes/cytoskeleton recruitment of PLCγ2. WT, LatY136F (A), and Lat3YF (B) platelets treated or not with the PI3K inhibitor wortmannin (50 nM) were stimulated by 5 nM Cvx for the indicated times. (A,B) Half of the cells were analyzed for the total amount of PLC-γ2 (bottom panels), and the other half were immediately permeabilized by saponin for cytosol depletion. After centrifugation (12 000g for 40 seconds), the pellet (corresponding to the membranes and cytoskeleton fraction; Mbr/Csk) was suspended in Laemli sample buffer, and the amount of PLC-γ2 was analyzed by immunoblotting (top panels). The effect of PI3K inhibition by wortmannin on PtdOH formation (C) and PLCγ2 tyrosine phosphorylation (D) of platelets from WT mice was then analyzed. Results are means plus or minus SEM of 3 experiments.
Mutation of tyrosine 136 of LAT does not affect GPVI-dependent membranes/cytoskeleton recruitment of PLCγ2. WT, LatY136F (A), and Lat3YF (B) platelets treated or not with the PI3K inhibitor wortmannin (50 nM) were stimulated by 5 nM Cvx for the indicated times. (A,B) Half of the cells were analyzed for the total amount of PLC-γ2 (bottom panels), and the other half were immediately permeabilized by saponin for cytosol depletion. After centrifugation (12 000g for 40 seconds), the pellet (corresponding to the membranes and cytoskeleton fraction; Mbr/Csk) was suspended in Laemli sample buffer, and the amount of PLC-γ2 was analyzed by immunoblotting (top panels). The effect of PI3K inhibition by wortmannin on PtdOH formation (C) and PLCγ2 tyrosine phosphorylation (D) of platelets from WT mice was then analyzed. Results are means plus or minus SEM of 3 experiments.
Discussion
In this study, we show that tyrosine-to-phenylalanine mutations in the C-terminal part of LAT has profound effects on signaling and on platelet responses upon GPVI triggering by Cvx. Concomitant mutation in the 3 membrane distal tyrosine residues at positions 175, 195, and 235 result in the inhibition of the phosphorylation of LAT itself and in a dramatic reduction of the whole pattern of protein tyrosine phosphorylation in response to Cvx. Accordingly, platelet secretion and aggregation are impaired under these conditions. In T cells, the last 4 tyrosine residues of LAT also have a critical role as the mutant mice, with the last 4 tyrosines mutated to phenylalanine having a similar phenotype as that of LAT-deficient mice with an early block in T-cell differentiation.16,40 ZAP-70, a kinase activated after T-cell–receptor stimulation, is involved in the direct phosphorylation of LAT in T cells.41,42 However, other kinases such as Itk and Lck have been suggested to participate in its phosphorylation.20 It has been reported that in human T cells, the phosphorylation of tyrosine residues at position 191 is required for the phosphorylation of the tyrosine residues at position 132, suggesting that LAT phosphorylation occurs in different waves involving different tyrosine kinases.43 In platelets, little is know about the kinase(s) responsible for the direct phosphorylation of LAT. This adaptor becomes phosphorylated downstream of Syk activation, but whether this kinase is responsible for the direct phosphorylation of LAT is unknown. Asazuma et al22 previously observed a coimmunoprecipitation of LAT and the tyrosine kinase Lyn. Here, we show that another member of the Src-family, Fyn, coprecipitates with LAT upon Cvx stimulation in mice platelets. Interestingly, the 3 C-terminal tyrosine residues of LAT are required for the rapid recruitment of Fyn. Moreover, in vitro kinase assays performed on LAT immunoprecipitates indicate that a kinase associated to these 3 C-terminal tyrosines, possibly Fyn, is able to phosphorylate LAT itself. Lyn and Fyn are known to play a crucial role in the very early phase of GPVI pathway since they phosphorylate the ITAM of the FcR γ-chain, enabling the binding of the tyrosine kinase Syk.39,44 Our results suggest that these kinases also participate in the direct phosphorylation of LAT, which is likely a sequential event upon GPVI triggering. Thus, in addition to Syk and Lyn, Fyn may directly participate in the maximal phosphorylation of LAT.
Our results clearly show the importance of the 3 C-terminal tyrosine residues of LAT in platelet activation via GPVI. They are essential for the tyrosine phosphorylation of most proteins in response to GPVI, efficient PI3K activation, and PLC activation. These mutations strongly affect platelet secretion and aggregation in washed platelet activation assays and thrombus growth in whole blood under flow conditions. The tyrosine residue at position 136, known to interact with PLCγ1 in T lymphocytes, is also critical. Mutation of this particular tyrosine residue of LAT results in the loss of PLCγ2 activation in response to Cvx and in platelet responses. Interestingly, this point mutation does not affect significantly PI3K activation and spares the tyrosine phosphorylation of most proteins in response to GPVI triggering. The tyrosine phosphorylation of PLCγ2, which is impaired by mutations of the last 3 tyrosine residues of LAT, is strongly reduced but still detectable by mutation of tyrosine 136. These results suggest that other proteins, depending on the phosphorylation of the last 3 tyrosine residues of LAT, regulate some phosphorylation sites of PLCγ2 in the absence of its docking site on the adaptor. However, this phosphorylation appears insufficient to activate the enzyme, or more likely concerns tyrosine residues not involved in its activation. Recently, it was shown in stimulated Jurkat T cells that in addition to LAT, several other phosphotyrosyl-proteins, including SLP-76, c-Cbl, and Vav1, coprecipitate with PLCγ1, the PLCγ isoform playing a major role in these cells.45 Particularly, the interaction of PLCγ1 with SLP-76 coincides with the activating tyrosine phosphorylation of the enzyme (ie, tyrosine 783) possibly via a Tec family kinase. Our result would fit with this model, as SLP-76 is essential in GPVI-induced PLCγ2 phosphorylation and activation in platelets.46
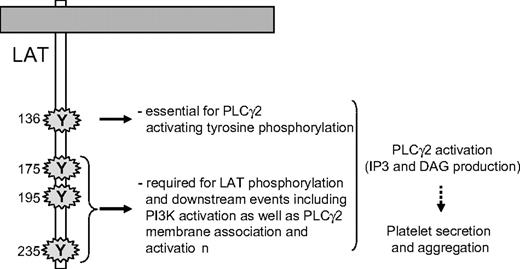
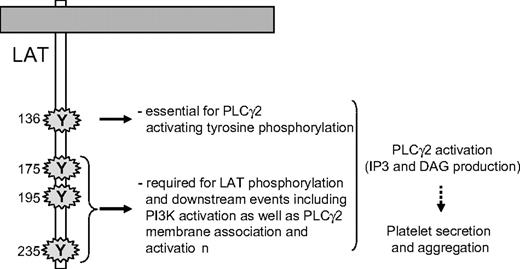
Although the recruitment of PLCγ2 and its phosphorylation are critical steps, they do not necessarily result in production of diacylglycerol and InsP3. Indeed, a stable and adequate interaction of the enzyme with its substrate in the membrane is mandatory for an efficient production of second messengers. In platelets, the PI3K product PtdIns(3,4,5)P3 is required for PLCγ2 activation but not for its tyrosine phosphorylation.34,47,48 Bobe et al have shown that in megakaryocytes, the translocation of PLCγ2 to the plasma membrane in response to GPVI activation is dependent on PI3K.49 Here, we show that the translocation of PLCγ2 to platelet membranes in response to GPVI-triggering is fully dependent on PI3K and is not affected by mutation of the tyrosine 136 residue of LAT. This result indicates that interaction of PLCγ2 with LAT, through tyrosine 136, is not required for the location of the phospholipase to the membrane/cytoskeleton. However, this PI3K-dependent stabilization is not sufficient to activate the production of the second messengers diacylglycerol and InsP3. Thus, both tyrosine 136 residue of LAT and PI3K activation through the last 3 C-terminal tyrosine residues of LAT are required for PLCγ2 activation in response to GPVI triggering (Figure 7). Thus, highly coordinated mechanisms, involving specific tyrosine residues of LAT, SLP-76, and PI3K, control PLCγ2 activation, and in turn platelet functions downstream of GPVI.
A schematic model highlighting the essential role of the tyrosine residues of LAT involved in PLCγ2 activation upon GPVI triggering in platelets.
A schematic model highlighting the essential role of the tyrosine residues of LAT involved in PLCγ2 activation upon GPVI triggering in platelets.
Interestingly, consistent with these observations, the mutation of tyrosine residue 136 or mutations of tyrosine residues 175, 195, and 235 of LAT strongly affect platelet aggregation in response to low concentrations of collagen (< 5 μg/mL; Figure S2). However, at concentrations higher than 6 μg/mL, this agonist can induce platelet aggregation in the presence of the mutation of tyrosine residue 136 of LAT. Even higher concentrations are required to promote aggregation in the presence of the mutation of tyrosine residues 175, 195, and 235. These results and previous reports in which Lat−/− platelets were analyzed23,50 indicate that, at high concentrations, collagen can induce platelet aggregation through signaling pathways independent of LAT and strengthen the idea of multiple receptors and/or alternative signaling mechanisms to allow platelet activation by this multivalent agonist.
In conclusion, using LAT mutants, we demonstrated the critical role of the last 3 tyrosine residues of LAT for most functions of the adaptor downstream of GPVI activation in platelets. These tyrosine residues are essential for the recruitment of Fyn, which may in turn contribute to LAT phosphorylation. Mutation of tyrosine 136 of LAT has a more subtle effect, as it does not affect the PI3K-dependent association of PLCγ2 with the membrane, partly inhibits the phosphorylation of the phospholipase, but impairs its activation and platelet responses upon GPVI triggering. These results indicate that, as in immune cells, subtle mutation of LAT affecting specific signaling pathway may alter platelet reactivity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We wish to thank M. Plantavid and C. Racaud-Sultan for stimulating discussions, G. Chicanne and C. Viala for technical assistance, and S. Allart from the Imaging Core Facility of IFR30 for her expert contribution in the flow-based studies. The Lat mutant mice were developed on the Plate-forme RIO-MNG (Marseille-Luminy) and housed in the service of Zoothechnie of IFR30 (Toulouse).
This work was supported by grants from the “Fondation de France” (cardiovascular; contract no. 2003005647) and the Région Midi-Pyrénées. S.S. was supported by grants from “Groupe d'Etude sur l'Hémostase et la Thrombose” and “Nouvelle Société Française d'Athérosclérose.”
Authorship
Contribution: A.R., S.S., and M-P.G. designed and performed most experiments and analyzed data; E.A., B.M., and M.M. produced transgenic mice; M.J.-P. purified Cvx; and J.R. and B.P. supervised the work, analyzed data, and wrote the paper. A.R. and S.S. contributed equally to the work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bernard Payrastre, INSERM U563, BP 3028, 31024 Toulouse Cedex 03, France; e-mail:payrastr@toulouse.inserm.fr.

![Figure 1. Mutations of the 3 C-terminal tyrosines or tyrosine 136 of LAT inhibit platelet aggregation and secretion in response to Cvx. (A,B) Platelets from the different mouse strains were stimulated with increasing concentrations of Cvx, and aggregation was assessed using a Chrono-log (Payton Associates) dual channel aggregometer under stirring at 900 rev/minute. The profiles shown are representative of 4 independent experiments. (C,D) To measure dense granule secretion, 5-hydroxy[14C]tryptamine[e]labeled platelets were stimulated by different concentration of Cvx. Results are expressed as a percentage of 5-hydroxy[14C]tryptamine (serotonine) secretion and are means (± SEM) of 5 independent determinations.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/7/10.1182_blood-2007-02-075432/2/m_zh80190707710001.jpeg?Expires=1769123972&Signature=VHkah5X7Zv~uPjMGiI8f-LsDVbTx9p4W6xbqrfhCQPi3FH~8a2ZP0kTrxaUj~vhDeI3ztf75h2AdB1PRrZPYkiIriAnLDSg8KDA3FK27byDrvnQZfEu1jO4ey8t5oWhKWXBAH5Ofk4ny7TZ7b0YC0Zl5sWrU6LLD2kW7ZE8QNl1wTjgZv4ND4rouUOLVGZTYaRWR8nRVrNpZYXFNFeDTHTasdFko-jJioOhQO~nHNLYGyVRcWERK2V4wZM2qgKAHpMmrkWmEeB1rP8A~LuJfQEksHxvYMDTs23beygeloLrHvuuE~vsgox7H91sRsrFy5nN~dxiyWGsTN47ZUricWA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. The interaction of Fyn with LAT is abolished by mutations in the 3 distal C-terminal tyrosines of LAT. (A) Platelets from WT (left panel) and Lat3YF (right panel) mice were stimulated by 5 nM Cvx for the time indicated, and LAT was immunoprecipitated and submitted to an in vitro kinase (IVK) assay in the presence of [γ-32P]ATP. After separation by 10% SDS-PAGE, the radioactivity incorporated in LAT was assessed by PhosphorImager (PerkinElmer, Shelton, CT) analysis. (B) Alternatively, following immunoprecipitation with the anti-LAT antibody, immunoprecipitated complexes were dissociated and reimmunoprecipitated with anti-Src family antibody. After separation by 10% SDS-PAGE and transfer onto nitrocellulose, the reimmunoprecipitate was immunoblotted with a specific anti-Fyn antibody. (C) Fyn was immunoprecipitated from lysate of WT (left panel) or Lat3YF (right panel) platelets stimulated by 5 nM Cvx for the indicated times. Immunoprecipitated complexes were submitted to an in vitro kinase assay in the presence of [γ-32P]ATP and reimmunoprecipitated with the anti-LAT antibody as described. The radioactivity incorporated in LAT was assessed by PhosphorImager analysis. Results are representative of 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/7/10.1182_blood-2007-02-075432/2/m_zh80190707710004.jpeg?Expires=1769123972&Signature=we2W-FqLqgz7wjw-8u6rxbpKodgCsOtDUE-7hpRPtQT5Lkm3KUJMQOLhR6d0ordwBeqYghtlqv0NdKAMh4QXADwuTICBbnHAXEmhVk21VdTOLpVmB3cunNsn7PQpUyfuSelGBqPrO2-e9ERRFv7xMZxTPPZ6fW9QGWwY1Llo2amrK0Q6pU7nxDSXTCW4yg0Okq~sU9nhdxOQ8wTeVE~-JyUYFYF2zL9HBxSngPRPuJA3V0qGXntytSjBTKy-4kGQ6tOFqkC-WVXiYX1uNbLKwop7j-dmn03RSc7RVNr2eocVwq86CCPW1DMCJh4DpeHs8XVjIHsoa6c1bPM2c~V6Ug__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Impact of mutations in the C-terminal tyrosines of LAT on PI3K and PLC activation in response to GPVI triggering. [32P]-labeled platelets from WT (♦) and Lat3YF (■) (A) or from WT (Rag−/−) (♦) and LatY136F mice (●) (B) platelets were stimulated by 5 nM Cvx for the indicated times and the levels of [32P]-PtdIns(3,4,5)P3, [32P]-PtdIns(3,4)P2, [32P]-PtdIns(4,5)P2, and [32P]-PtdOH were analyzed as indicated in “Platelet preparation and in vitro aggregation studies.” Results are means (± SEM) of 4 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/7/10.1182_blood-2007-02-075432/2/m_zh80190707710005.jpeg?Expires=1769123972&Signature=LsWIHmKF~JkbzHRXGdFelHW6KyalGFCGC~Yyc0A9Et280LcFuu~fjIdE~h3DJUiP2lmZR9ylr8NdeU07E7dsoeJ65-jJMxXm2h2v~pgSfbiQXSUBynR~KaRTHj-Qn3~A-R9BEgaiFJ0m5wKqBcxUhQMqIddkBwwqg44Aagc4Y8DRV~OApI7lJVyKcwgKrQDfNk6nxTW5yst2VUah66pVZUWCE32DplFBReXXz4uVygVvDNu~O-5xlqOu7GKTdwec9zddJrQsjtt8S58k-Z8JyGcaRKNHZm-oEmhxDd-dbJEwXlbz7Hb8vLuHAT4nLYW0lyhn6HCVkVfbmVEph7I5Cw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. Mutations of the 3 C-terminal tyrosines or tyrosine 136 of LAT inhibit platelet aggregation and secretion in response to Cvx. (A,B) Platelets from the different mouse strains were stimulated with increasing concentrations of Cvx, and aggregation was assessed using a Chrono-log (Payton Associates) dual channel aggregometer under stirring at 900 rev/minute. The profiles shown are representative of 4 independent experiments. (C,D) To measure dense granule secretion, 5-hydroxy[14C]tryptamine[e]labeled platelets were stimulated by different concentration of Cvx. Results are expressed as a percentage of 5-hydroxy[14C]tryptamine (serotonine) secretion and are means (± SEM) of 5 independent determinations.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/7/10.1182_blood-2007-02-075432/2/m_zh80190707710001.jpeg?Expires=1769123973&Signature=U~tmSMWlByTf7ipVvq9dhpRwO8CZ1FzZU61z0Q5XI5g1ZQ9mMKbQ1a5d376ttBh07z6B8KjseDvW7J~BX0SKxEBWLRbr9fDON9iOmRyAeLkDShyaO3iKG-5dCDhkcRcgTftRRaB-crp31y0vIFxZmG-NNNEWpvG4TTsx7vjv9NoYThbpsPYt9iZV1NsrmT5WfdpVSz4JsP3ZA6dN7WZNLN4d5pMLljraIKyYUw~~qAFmg3c-F8vdQdS3KkAa4oi7eqGiwOrRK5OJWb9~b74cwPuRphg3pS-12z8lHp9kaZvlCtg~wACyDdW0iXbxCFueld1uZmHhmqRgwl7rECr-qA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. The interaction of Fyn with LAT is abolished by mutations in the 3 distal C-terminal tyrosines of LAT. (A) Platelets from WT (left panel) and Lat3YF (right panel) mice were stimulated by 5 nM Cvx for the time indicated, and LAT was immunoprecipitated and submitted to an in vitro kinase (IVK) assay in the presence of [γ-32P]ATP. After separation by 10% SDS-PAGE, the radioactivity incorporated in LAT was assessed by PhosphorImager (PerkinElmer, Shelton, CT) analysis. (B) Alternatively, following immunoprecipitation with the anti-LAT antibody, immunoprecipitated complexes were dissociated and reimmunoprecipitated with anti-Src family antibody. After separation by 10% SDS-PAGE and transfer onto nitrocellulose, the reimmunoprecipitate was immunoblotted with a specific anti-Fyn antibody. (C) Fyn was immunoprecipitated from lysate of WT (left panel) or Lat3YF (right panel) platelets stimulated by 5 nM Cvx for the indicated times. Immunoprecipitated complexes were submitted to an in vitro kinase assay in the presence of [γ-32P]ATP and reimmunoprecipitated with the anti-LAT antibody as described. The radioactivity incorporated in LAT was assessed by PhosphorImager analysis. Results are representative of 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/7/10.1182_blood-2007-02-075432/2/m_zh80190707710004.jpeg?Expires=1769123973&Signature=1hQ30kTyGDevhnwx~oGysh0AEkPcRV6f75BVTSuTVmc2GBmuZcTUy1qwItsJV1uVAKFbQqkDeoVyzbOgqVp-1yASLolxy4dEGBQt9YL4FNmbaxF2ZLeSoPFq7tlDu4op5F8Li5WivGkA5T2~fSs8iwvENXP5mUJ2XpNp3MI8t82x6rChzLHFA7EI6D3De6wbRPr-xjXQE8xallO7haaLiSs0~dXkV2cbTo6OyXkIF5tNaF0mcJ8XJRmhgX5VBuh-JXv4S4D1z1Te0CEiwdfWAP9zLxMvnAD0P5josyYcZn~uYskf4NVpLb5uwk4gsNZ0EsSqOyVTjjWqwyRbtpyJLA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Impact of mutations in the C-terminal tyrosines of LAT on PI3K and PLC activation in response to GPVI triggering. [32P]-labeled platelets from WT (♦) and Lat3YF (■) (A) or from WT (Rag−/−) (♦) and LatY136F mice (●) (B) platelets were stimulated by 5 nM Cvx for the indicated times and the levels of [32P]-PtdIns(3,4,5)P3, [32P]-PtdIns(3,4)P2, [32P]-PtdIns(4,5)P2, and [32P]-PtdOH were analyzed as indicated in “Platelet preparation and in vitro aggregation studies.” Results are means (± SEM) of 4 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/7/10.1182_blood-2007-02-075432/2/m_zh80190707710005.jpeg?Expires=1769123973&Signature=D3j3ViTwzs9zAzRngwjtFCoThnCtTKOu9MBfqgVaMapn0VxFlZAneXaTkm4brwmH-gZCUV8wr5wlfWn6DGsiUxp9hQvzzUvDd~U5gOp11-z8gXwMKP31893bOUceDgmB47Rzrk~sj6f82nt1XJ3-LW8LoUlUjGDWffbnzshyM2M3P2hIAr1L2ZoQak3OeEbTILX0cjcITrX8UacE2RSNWHymekmLvBruKdewvNKu6gmjNOU210v52nlyV~d80YZ-ydQEbeonoAon-BwJDJSJPt5SWuLdTbud6cV9hvaUCK83veunA2Qt5FwghwVgg4tKVsfdZl4vAn~sCcQDCoTJMg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)