OX40 is a recently identified T-cell costimulatory molecule that belongs to the TNF/TNFR superfamily. OX40 can be expressed by both activated T effector cells and Foxp3+ Tregs. It is well known that OX40 delivers a potent costimulatory signal to T effector cells, but very little is known about the role of OX40 in regulating the suppressor properties of Foxp3+ Tregs and the de novo generation of new inducible Foxp3+ Tregs from T effector cells. In the present study, we found, by using a newly created foxp3gfp knockin model, that OX40 was dispensable for the genesis and suppressor functions of naturally arising CD4+Foxp3+ Tregs, but stimulating OX40 on the Foxp3+ Tregs abrogated their ability to suppress T effector cell proliferation, IFN-γ production, and T effector cell-mediated allograft rejection. OX40 costimulation did not significantly affect proliferation and survival of the naturally arising Foxp3+ Tregs, but profoundly inhibited Foxp3 gene expression. Importantly, OX40 costimulation to T effector cells prevented the induction of new inducible Foxp3+ Tregs from T effector cells. Our study identified OX40 as a key negative regulator of Foxp3+ Tregs and may have important clinical implications in models of transplantation and autoimmunity.
Introduction
T cells with regulatory properties are critical to the induction of self-tolerance and acquired tolerance.1,2 Among the cell types that exhibit potent suppressor functions, the CD4+Foxp3+ regulatory T cells (Tregs) are particularly important, as deficiency or functional impairment of this cell type often leads to the development of autoimmunity and the failure to establish acquired tolerance,3,4 albeit other regulatory cell types also contribute to tolerance via different mechanisms.5
The CD4+Foxp3+ Tregs are not a uniform cell type. Depending on the origin of these cells, the CD4+Foxp3+ T cells can be divided into those that are developed in the thymus (natural Tregs) and those that are induced in the periphery (induced Tregs).6 Natural Foxp3+ Tregs are selected and matured in the thymus, and then exported to the periphery where they suppress potentially cytopathic T cells.7 It is well known that lineage commitment of the natural Foxp3+ Tregs requires Foxp3,8,9 and their survival and expansion demand the presence of IL-2 and expression of IL-2 receptors.10 However, some activated T effector cells can be converted to Foxp3+ Tregs in the periphery and such induced Foxp3+ Tregs also act as potent suppressor cells.11,12 From a therapeutic point of view, therapies that can preserve or expand the Foxp3+ Tregs and at the same time inhibit cytopathic T effector cells would be highly desirable in the induction of transplant tolerance or in the treatment of autoimmune diseases.
Phenotypically, Foxp3+ Tregs and activated T effector cells often express similar cell surface molecules. For example, both cell types express CD25, CD28, CD154, GITR, CTLA-4, and others, although the functions of such molecules are not always the same in both cell types.4 Recently, it has been shown that the CD4+CD25+ Tregs constitutively express OX40 (also called CD134),13 a new costimulatory molecule that belongs to the TNF-R superfamily.14 Also, T effector cells, though they do not express OX40 at resting state, can readily express OX40 upon activation,13 and OX40 engagement delivers a potent costimulatory signal to T effector cells.15 The recent finding that deliberately stimulating OX40 in vivo can break tolerance to peptide antigens16 and that blocking OX40 costimulation can enable allograft survival in stringent transplant models17 suggests that the impact of OX40 signaling on a regulatory type of immune response is likely to be profound. However, very little is known about the role of OX40 in regulating the Foxp3+ Tregs. There are 2 reports in the literature suggesting that OX40 may be capable of modifying the suppressor functions of Tregs, but the findings appear to be contradictory.18,19 As OX40, like CD25, can be expressed by both Foxp3+ Tregs and activated T effector cells, partition of such functionally distinct T-cell subsets in the initial studies based solely on the CD25 marker has obvious limitations. Moreover, activated T effector cells, which express both CD25 and OX40, can be converted to Foxp3+ Tregs, and such converted Tregs also function as potent suppressor cells.11,12 These new findings suggest that the impact of OX40 on the Foxp3+ Tregs is likely to be far more complex than initially anticipated. As manipulation of Tregs is an important and clinically relevant issue, a clear understanding of the role of OX40 in regulating Foxp3+ Tregs is of quintessential importance.
In the present study, we used a newly developed foxp3gfp knockin model that allows a clear separation of GFP-tagged Foxp3+ Tregs and Foxp3− T effector cells to critically examine the role of OX40 in the genesis and regulatory properties of natural Foxp3+ Tregs. We also examined the role of OX40 costimulation in the de novo generation of new Foxp3+ Tregs from activated T effector cells. We found that OX40 negatively regulates Foxp3 expression, and OX40 costimulation abrogates the suppressor functions of natural Foxp3+ Tregs and prevents the induction of new Foxp3+ Tregs from T effector cells. Our data identified OX40 as a negative regulator of Foxp3+ Tregs and our new findings may have important clinical implications.
Materials and methods
Animals
C57BL/6 (H-2b), DBA/2 (H-2d), Rag-1−/− were purchased from The Jackson Laboratory (Bar Harbor, ME). Generation of OX40−/−, OX40L−/−, and OX40Ltg mice, all of which are on the C57BL/6 background, has already been described.18,20,21 Foxp3gfp knock-in mice (foxp3gfpKI) on the C57BL/6 background were generated by introducing the bicistronic EGFP reporter gene into the Foxp3 locus and used as previously reported.22 Foxp3gfpKI mice that are deficient in OX40 were generated by crossing the foxp3gfpKI mice with the OX40−/− mice and selected by polymerase chain reaction (PCR)-assisted genotyping. Animal care and use conformed to the guidelines established by the Animal Care Committee at Harvard Medical School in Boston, Massachusetts.
Monoclonal antibodies
The following antimouse mAbs used for cell surface staining were obtained from BD PharMingen (San Diego, CA): cychrome-anti-CD4, PE-Cy5-anti-CD4 (clone GK1.5), PE-anti-CD25 (clone PC61), PE-anti-OX40 (clone OX86), FITC-anti-CD90.1, PE-annexin V, and isotype control Abs. Antimouse IFN-γ capturing antibody (clone R4-6A2), biotin-anti-IFN-γ detection antibody (clone XMG1.2), and horseradish peroxidase (HRP)–streptavidin for enzyme-linked immunosorbent spot (ELISPOT) assay were also obtained from BD PharMingen. A PE-antimouse Foxp3 mAb (clone FJK-16s) was purchased from eBiosciences (San Diego, CA).
An agonist antimouse OX40 mAb (clone OX86) and the antimouse CD154 (clone MR1) were produced from hybridoma cell lines by BioExpress (West Lebanon, NH) and were used in some in vitro and in vivo studies.
Cell staining and flow cytometry
Spleen and lymph node cells were harvested and single cell suspension was prepared as previously reported.17 Cells were resuspended in PBS/0.5% BSA and stained with fluorochrome-conjugated Abs on ice for 20 minutes. The cells were washed twice in PBS/BSA and fixed in 1% paraformaldehyde prior to fluorescence-activated cell sorting (FACS) analysis. All samples were acquired using the FACScan (BD Biosciences, Mountain View, CA). Data analysis was performed using the FlowJo software (Treestar, Ashland, OR).
Sorting of CD4+Foxp3+ Tregs and CD4+Foxp3− T effector cells
Male foxp3gfpKI mice, 6 to 10 weeks of age, were killed; spleen and lymph nodes were harvested; and single-cell suspension was prepared. Cells were stained with PE-antimouse CD4. The CD4+GFP(Foxp3)− T effector cells and the CD4+GFP(Foxp3)+ Tregs were identified, electronically gated, and then sorted using the MoFlo high speed cell sorter (Dako-Cytomation, Ft Collins, CO). The purity of cells sorted using this method was consistently more than 96%.23
Preparation of APCs as stimulator cells
Spleen cells were prepared from donor mice, and CD3+ T cells were depleted by positive selection using magnetic-activated cell sorter (MACS)-assisted cell sorting (Miltenyi Biotec, Auburn, CA). The T-cell–depleted fraction was collected and treated with mitomycin C at 50 μg/mL (Sigma-Aldrich, St Louis, MO) at 37°C for 20 minutes. Cells were then washed and used as antigen-presenting cells (APCs) for all the in vitro cultures.
Conversion of T effector cells to Foxp3+ Tregs in vitro
CD4+GFP(Foxp3)− T effector cells were FACS sorted from foxp3gfpKI mice; the sorted T effector cells were stimulated in vitro (1 × 105 cells/well) with plate-bound anti-CD3 (3 μg/mL, clone 2C11; BD PharMingen) and soluble anti-CD28 (5 μg/mL) in the presence or absence of recombinant human TGF-β1 (2 ng/mL; Biosource, Camarillo, CA) for 3 to 5 days. Cells were then harvested and stained with PE-Cy5-anti-CD4 mAb. Induction of GFP(Foxp3)+ cells in the CD4+ fraction was analyzed by FACS.
In some experiments, CD4+GFP(Foxp3)− T effector cells from foxp3gfpKI mice were stimulated with anti-CD3 (clone 2C11, 3 μg/mL) plus an equal number of syngeneic APCs with or without TGF-β1 (2 ng/mL) for 3 to 5 days, and induction of GFP(Foxp3)+ cells was determined by FACS.
T-cell suppression assays in vitro
CD4+GFP(Foxp3)− T effector cells sorted from wt foxp3gfpKI mice or OX40−/−foxp3gfpKI mice were mixed with FACS-sorted CD4+GFP(Foxp3)+ Tregs at different effector to Treg ratios, then plated into 96-well tissue culture plates. The cells were stimulated with anti-CD3 plus syngeneic APCs at 37°C for 4 days. For the last 16 hours of culture, cells were pulsed with 1 μCi (0.037 MBq) 3H-TdR/well (Amersham, Boston, MA), and incorporation of 3H-TdR was determined using a Beta Plate scintillation counter (Perkin-Elmer, Wellesley, MA). Data were collected as mean CPM of triplicate assays. Inhibition of effector cell proliferation was calculated based on proliferation of T effector cells in the absence of Tregs.
ELISPOT assay
ELISPOT assay for IFN-γ was performed as previously reported.24 Briefly, sorted CD4+GFP− T effector cells (2 × 105 cells/well) from foxp3gfpKI-OX40−/− mice were cocultured with wt CD4+GFP(Foxp3)+ Tregs (6 × 105 cells/well) in immunospot plates. The cell mixture was stimulated with anti-CD3 plus syngeneic APCs (4 × 105 cells/well) at 37°C for 2 days, and IFN-γ spots were developed using a specific detecting Ab. The IFN-γ spots were quantitated using a computer-assisted ELISPOT image analyzer (T Spot Image Analyzer; Cellular Technology, Cleveland, OH) and presented as mean plus or minus standard deviation of triplicate assays.
Real-time PCR
Total cellular RNA was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA) and reverse transcribed into cDNA with ABI Prism TaqMan reverse-transcription method. Expression of genes of interest and of GAPDH control was assessed in simplex reverse-transcription (RT)–PCR with FAM and VIC probes (Applied BioSystems, Foster City, CA). All the TaqMan primers and probe sets (Foxp3, CTLA-4, GITR) were purchased from Applied BioSystems. Transcript levels of target genes were calculated according to the 2−ddCt method as supplied by the manufacturer (ABI PRISM 7700 user bulletin; Applied BioSystems) and expressed as arbitrary unit (AU).
Annexin V staining
Sorted CD4+GFP(Foxp3)+ Tregs from foxp3gfpKI mice were stimulated in vitro with anti-CD3 (2C11, 2 μg/mL) plus equal number of syngeneic APCs, OX40Ltg APCs, or OX40L−/− APCs. At different time points after the culture, cells were collected and stained with PE-conjugated annexin V in a calcium-rich annexin-binding buffer (BD PharMingen) on ice for 20 minutes. Cell survival was analyzed by FACS and annexin V-positive cells were gated and regarded as apoptotic cells.23
Induction of new Foxp3+ Tregs in vivo
CD4+CD25− T effector cells were FACS sorted from congenic CD90.1 C57BL/6 mice and adoptively transferred into wt C57BL/6 mice and OX40Ltg mice (CD90.2) via the tail vein (12 × 106/mice). The host mice were then treated with a tolerizing protocol consisting of donor-specific transfusion (DST) and anti-CD154 mAb.25 Briefly, the host mice were injected with DBA/2 splenic cells (10 × 106 /mouse) and then treated with anti-CD154 mAb (clone MR1, 0.5 mg/day, intraperitoneally) on days 0, +1, +3, and +5 starting at the time of adoptive cell transfer. The host mice were then killed on days +6 and +10, and single cell suspension was prepared from the spleen and lymph node. Cells were stained with cychrome-antimouse CD4 and FITC-anti-CD90.1, followed by intracellular staining for the expression of Foxp3. All samples were analyzed by FACS and expression of Foxp3 in the CD4+CD90.1+ fraction was determined and compared among different groups.
Skin transplantation
Full-thickness tail skin grafts from donor mice were transplanted onto the thoracic wall of recipients as previously reported.17 Graft survival was assessed by daily visual inspection and rejection was defined as a complete loss of viable tissue grafts. In some experiments, recipient mice were treated with an agonist anti-OX40 mAb (0.5 mg, intraperitoneally on days 0, 2, 4, and 8) following skin grafting and day 0 was the time of skin transplantation.
Statistics
Analysis of levels of Foxp3+ Tregs, cell proliferation, and ELISPOT data was performed using the Student t test. Allograft survival was compared using the log-rank test. A P value of less than .05 was considered as significant.
Results
OX40 is expressed by functionally different T-cell subsets
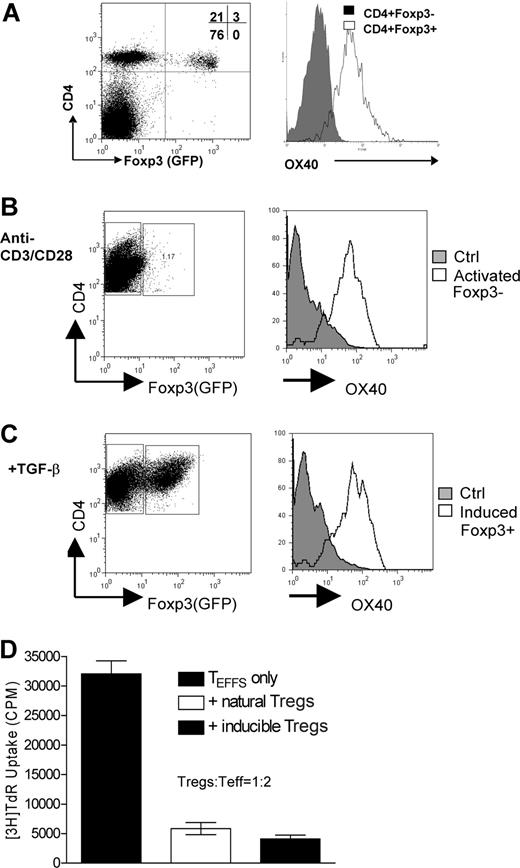
To examine the cell types that express OX40, and therefore, are likely regulated by OX40 costimulation, we used a newly developed foxp3gfpKI model in which the endogenous Foxp3 locus is genetically linked to the reporter protein EGFP.22 In naive foxp3gfpKI mice, CD4+GFP(Foxp3)+ Tregs in either the spleen or the lymph nodes constitutively expressed OX40 on the cell surface, whereas the CD4+ GFP(Foxp3)− T effector cells did not (Figure 1A). However, when CD4+GFP(Foxp3)− T effector cells were FACS sorted and activated in vitro with anti-CD3 and anti-CD28, OX40 expression was readily induced on the cell surface (Figure 1B open histogram). Furthermore, stimulation of CD4+GFP(Foxp3)− T effector cells with anti-CD3/anti-CD28 in the presence of TGF-β consistently converted a subset of GFP(Foxp3)− T effector cells to GFP(Foxp3)+ T cells, and as high as 30% of CD4+GFP(Foxp3)− T effector cells became GFP(Foxp3)+ T cells 3 days later. Interestingly, such converted Foxp3+ T cells remained OX40+ (Figure 1C). Similar to the natural GFP(Foxp3)+ Tregs, the converted Foxp3+ T cells could effectively suppress the proliferation of CD4+GFP− T effector cells in a coculture assay (Figure 1D), confirming that the converted CD4+GFP(Foxp3)+ T cells are bona fide suppressor cells. This is consistent with several other reports showing that CD4+Foxp3+ Tregs can be induced from activated T effector cells.11,12 Clearly, OX40 is not confined to the natural Foxp3+ Tregs or activated T effector cells; the converted Foxp3+ Tregs can also express OX40 on the cell surface.
OX40 expression by CD4+Foxp3+ Tregs and CD4+Foxp3— T effector cells. (A) Spleen cells from naive foxp3gfpKI mice were stained with cychrome-anti-CD4 and PE-anti-OX40. CD4+GFP(Foxp3)+ cells and CD4+GFP(Foxp3)− cells were selectively gated and expression of OX40 on the Foxp3+ and Foxp3− subsets was analyzed. A representative plot of 5 experiments is shown. (B) FACS sorted CD4+ GFP(Foxp3)− T effector cells were stimulated in vitro with anti-CD3 and anti-CD28, and expression OX40 on the activated T cells was analyzed and shown. Naive CD4+ T effector cells were included as a control (the solid histogram). (C) The sorted CD4+ GFP(Foxp3)− T effector cells were stimulated with anti-CD3/anti-CD28 plus TGF-β for 4 days, and the induction of GFP(Foxp3)+ cells was determined. Expression of OX40 on the GFP(Foxp3)− T effector cells and the converted GFP(Foxp3)+ Tregs was shown. (D) CD4+GFP(Foxp3)− T effector cells were cocultured with the natural CD4+GFP(Foxp3)+ Tregs or the converted CD4+GFP(Foxp3)+ Tregs. The cell mixture was stimulated with anti-CD3 plus APCs, and suppression of T effector cell proliferation was shown as mean (CPM ± SD) of triplicate assays. Representative data of 3 individual experiments are shown.
OX40 expression by CD4+Foxp3+ Tregs and CD4+Foxp3— T effector cells. (A) Spleen cells from naive foxp3gfpKI mice were stained with cychrome-anti-CD4 and PE-anti-OX40. CD4+GFP(Foxp3)+ cells and CD4+GFP(Foxp3)− cells were selectively gated and expression of OX40 on the Foxp3+ and Foxp3− subsets was analyzed. A representative plot of 5 experiments is shown. (B) FACS sorted CD4+ GFP(Foxp3)− T effector cells were stimulated in vitro with anti-CD3 and anti-CD28, and expression OX40 on the activated T cells was analyzed and shown. Naive CD4+ T effector cells were included as a control (the solid histogram). (C) The sorted CD4+ GFP(Foxp3)− T effector cells were stimulated with anti-CD3/anti-CD28 plus TGF-β for 4 days, and the induction of GFP(Foxp3)+ cells was determined. Expression of OX40 on the GFP(Foxp3)− T effector cells and the converted GFP(Foxp3)+ Tregs was shown. (D) CD4+GFP(Foxp3)− T effector cells were cocultured with the natural CD4+GFP(Foxp3)+ Tregs or the converted CD4+GFP(Foxp3)+ Tregs. The cell mixture was stimulated with anti-CD3 plus APCs, and suppression of T effector cell proliferation was shown as mean (CPM ± SD) of triplicate assays. Representative data of 3 individual experiments are shown.
OX40 is dispensable for genesis and suppressor functions of CD4+Foxp3+ Tregs
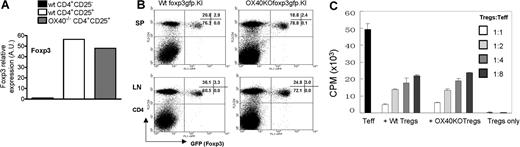
To probe whether OX40 is required for the generation of natural CD4+Foxp3+ Tregs, we first compared Foxp3 gene transcripts in CD4+CD25+ T cells sorted from wt C57BL/6 mice and age-matched OX40−/− mice by real-time PCR. As shown in Figure 2A, the Foxp3 transcripts were expressed at comparable levels in CD4+CD25+ T cells sorted from wt C57BL/6 and OX40−/− mice. To further address this issue, we crossed the foxp3gfpKI mice with the OX40−/− mice and generated foxp3gfpKI mice that are deficient for OX40; and then compared the CD4+GFP(Foxp3)+ T cells in OX40−/− foxp3gfpKI mice with that in wt foxp3gfpKI mice. As shown in Figure 2B, GFP(Foxp3)+ T cells could be readily identified in either the spleen (SP) or the lymph nodes (LNs) of OX40−/− foxp3gfpKI mice. In fact, the OX40−/− foxp3gfpKI mice and the wt foxp3gfpKI mice expressed similar levels of CD4+GFP(Foxp3)+ Tregs, suggesting that OX40 is not absolutely required for the genesis of natural CD4+Foxp3+ Tregs.
Role of OX40 in the genesis and suppressor functions of CD4+Foxp3+ Tregs. (A). Real-time RT-PCR analysis of Foxp3 gene transcripts in CD4+CD25+ Tregs sorted from wt C57BL/6 and OX40KO mice. Data shown are representative of 3 individual experiments. (B) Spleen (SP) and lymph node (LN) cells from wt foxp3gfpKI mice and OX40KO foxp3gfpKI mice were stained with cychrome–anti-CD4 and then compared for the presence of CD4+GFP(Foxp3)+ T cells by FACS. The data shown are representative data of 4 individual experiments. Numbers in quadrants are the relative percentage of cells in each region given by the flow cytometer. (C) CD4+GFP− T effector cells sorted from foxp3gfpKI mice were mixed with CD4+GFP(Foxp3)+ Tregs from wt and OX40KO foxp3gfpKI mice at different ratios, and suppression of T effector cell proliferation was shown. The data shown are representative of 4 individual experiments. Error bars represent SD of triplicate assays.
Role of OX40 in the genesis and suppressor functions of CD4+Foxp3+ Tregs. (A). Real-time RT-PCR analysis of Foxp3 gene transcripts in CD4+CD25+ Tregs sorted from wt C57BL/6 and OX40KO mice. Data shown are representative of 3 individual experiments. (B) Spleen (SP) and lymph node (LN) cells from wt foxp3gfpKI mice and OX40KO foxp3gfpKI mice were stained with cychrome–anti-CD4 and then compared for the presence of CD4+GFP(Foxp3)+ T cells by FACS. The data shown are representative data of 4 individual experiments. Numbers in quadrants are the relative percentage of cells in each region given by the flow cytometer. (C) CD4+GFP− T effector cells sorted from foxp3gfpKI mice were mixed with CD4+GFP(Foxp3)+ Tregs from wt and OX40KO foxp3gfpKI mice at different ratios, and suppression of T effector cell proliferation was shown. The data shown are representative of 4 individual experiments. Error bars represent SD of triplicate assays.
To determine whether OX40 is needed for the natural CD4+Foxp3+ Tregs to execute their suppressor functions, CD4+GFP(Foxp3)+ Tregs were FACS sorted from OX40−/− foxp3gfpKI mice and mixed with wt CD4+GFP− T effector cells at different ratios; the cell mixture was stimulated with anti-CD3 plus syngeneic APCs in vitro; and the ability of OX40-deficient GFP(Foxp3)+ Tregs to suppress T effector cell proliferation was determined 3 days later and compared with that from wt foxp3gfpKI mice. As shown in Figure 2C, both wt GFP(Foxp3)+ Tregs and OX40−/− Foxp3+ Tregs strongly suppressed the proliferation of CD4+GFP(Foxp3)− T effector cells, and at different effector to Tregs ratios examined, the suppressor activities between the wt GFP(Foxp3)+ Tregs and the OX40−/−GFP(Foxp3)+ Tregs were comparable, suggesting that OX40 is unlikely to be critical to the suppressor functions of natural Foxp3+ Tregs.
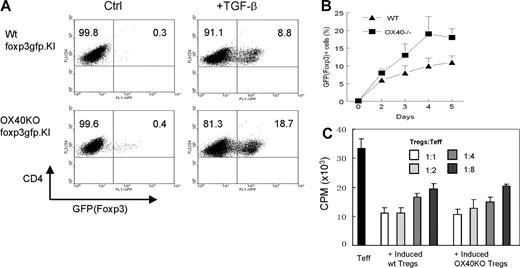
CD4+Foxp3− T effector cells, once activated, also express OX40, and certain Foxp3− T effector cells can be converted into Foxp3+ Tregs (Figure 1). It is likely that such conversion may require OX40 costimulation. To test this possibility, CD4+GFP(Foxp3)− T effector cells were FACS sorted from wt foxp3gfpKI mice and OX40−/− foxp3gfpKI mice and stimulated in vitro with anti-CD3 plus syngeneic APCs in the presence of TGF-β. Conversion of T effector cells to GFP(Foxp3)+ Tregs at different time points was determined by flow cytometry. As shown in Figure 3A, OX40-deficient CD4+GFP(Foxp3)− T effector cells could be readily converted to GFP(Foxp3)+ Tregs. As compared with the wt controls, there was a small but consistent increase in the conversion of CD4+GFP(Foxp3)− T effector cells to GFP(Foxp3)+ Tregs in the absence of OX40 at any of the time points examined (Figure 3B). The converted GFP(Foxp3)+ Tregs were FACS sorted and further examined in a secondary culture for their suppressor functions in vitro. As shown in Figure 3C, the GFP(Foxp3)+ Tregs converted either from wt T effector cells or from OX40-deficient T effector cells strongly suppressed the proliferation of freshly prepared CD4+GFP(Foxp3)− T effector cells, and their suppression activities were also comparable. Thus, conversion of T effector cells to Foxp3+ Tregs and the suppressor functions of such induced Foxp3+ Tregs are also independent of OX40 costimulation.
Effect of OX40 costimulation on the induction of new CD4+Foxp3+ Tregs from T effector cells. (A) CD4+GFP(Foxp3)− T effector cells were sorted from wt foxp3gfpKI mice and OX40KO foxp3gfpKI mice, The T effector cells were stimulated with anti-CD3 plus APCs in the presence or absence of TGF-β for 2 to 5 days. Induction of new GFP(Foxp3)+ T cells in the CD4+ fraction was determined by FACS. The dot plot shown is one of 3 individual experiments 4 days after the culture. Numbers in quadrants are the relative percentage of cells in each region given by the flow cytometer. (B) Induction of new GFP(Foxp3)+ Tregs calculated from 3 individual experiments. The conversion shown is the mean ± SD of 3 independent experiments at different time points. (C) Suppression of T effector cell proliferation by CD4+GFP(Foxp3)+ Tregs converted from either the wt or the OX40KO T effector cells. Data shown are representative of 3 independent experiments. Error bars represent SD of triplicate assays.
Effect of OX40 costimulation on the induction of new CD4+Foxp3+ Tregs from T effector cells. (A) CD4+GFP(Foxp3)− T effector cells were sorted from wt foxp3gfpKI mice and OX40KO foxp3gfpKI mice, The T effector cells were stimulated with anti-CD3 plus APCs in the presence or absence of TGF-β for 2 to 5 days. Induction of new GFP(Foxp3)+ T cells in the CD4+ fraction was determined by FACS. The dot plot shown is one of 3 individual experiments 4 days after the culture. Numbers in quadrants are the relative percentage of cells in each region given by the flow cytometer. (B) Induction of new GFP(Foxp3)+ Tregs calculated from 3 individual experiments. The conversion shown is the mean ± SD of 3 independent experiments at different time points. (C) Suppression of T effector cell proliferation by CD4+GFP(Foxp3)+ Tregs converted from either the wt or the OX40KO T effector cells. Data shown are representative of 3 independent experiments. Error bars represent SD of triplicate assays.
OX40 stimulation on CD4+Foxp3+ Tregs abrogates their suppressor functions
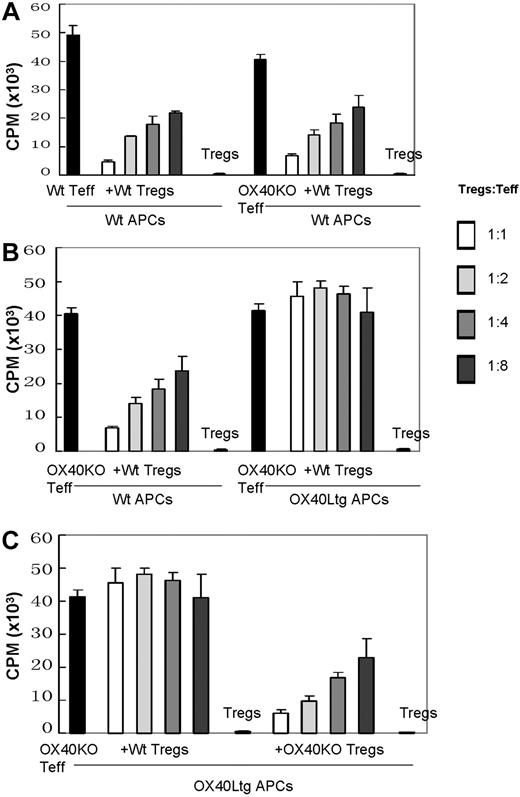
Clearly, OX40 does not significantly affect the genesis and suppressor functions of natural Foxp3+ Tregs, despite the fact that Foxp3+ Tregs express OX40 on the cell surface. However, it remains possible that OX40 on natural Foxp3+ Tregs may be capable of modifying their suppressor activities. To probe this possibility, we performed a series of in vitro suppression assays to determine whether selective stimulation of OX40 on the natural GFP(Foxp3)+ Tregs would alter their suppressor functions. As shown in Figure 4A, either wt or OX40 KO CD4+GFP(Foxp3)− T effector cells proliferated vigorously when stimulated with anti-CD3 plus syngeneic APCs, and such proliferation was strongly suppressed by the addition of wt CD4+GFP(Foxp3)+ Tregs. In these experiments, the wt and OX40KO T effector cells were equally susceptible to Foxp3+ Treg-mediated suppression; and the inhibition of T effector cell proliferation by the GFP(Foxp3)+ Tregs was as high as 90%. To our surprise, in cultures in which OX40Ltg APCs were used to selectively stimulate OX40 on the GFP(Foxp3)+ Tregs, the ability of GFP(Foxp3)+ Tregs to suppress OX40KO T effector cell proliferation was completely abolished (Figure 4B). In these cultures, the CD4+GFP(Foxp3)+ Tregs alone did not proliferate at all when stimulated with anti-CD3 plus OX40Ltg APCs (Figure 4B). In a similar set of experiments in which OX40KO T effector cells were stimulated with anti-CD3 plus OX40Ltg APCs, OX40-deficient CD4+GFP(Foxp3)+ Tregs were added into the cultures. As shown in Figure 4C, addition of OX40KO Foxp3+ Tregs into the cultures resulted in the suppression of T effector cell proliferation. Collectively, these data suggest that OX40 costimulation to the Foxp3+ Tregs results in the loss of their suppressor functions.
Stimulation of OX40 on CD4+GFP(Foxp3)+ Tregs abrogates their suppressor functions. (A) CD4+GFP(Foxp3)− T effector cells sorted from wt and OX40KO foxp3gfpKI mice were stimulated with anti-CD3 plus wt APCs. T effector cell proliferation in the presence or absence of wt CD4+GFP(Foxp3)+ Tregs at different Tregs to T effector ratios was shown. Data shown are mean (CPM ± SD) of triplicate assays. (B) OX40 deficient CD4+GFP(Foxp3)− T effector cells were stimulated with anti-CD3 plus wt APCs or OX40Ltg APCs in the presence or absence of wt CD4+GFP(Foxp3)+ Tregs. Cell proliferation was determined 3 days later by 3H-TdR uptake. Data shown are mean (CPM ± SD) of triplicate assays. (C) OX40 deficient CD4+GFP(Foxp3)− T effector cells were stimulated with anti-CD3 plus OX40Ltg APCs. In these cultures, graded numbers of wt or OX40KO CD4+GFP(Foxp3)+ Tregs were added, and cell proliferation was determined 3 days later by 3H-TdR uptake. Data shown are mean (CPM ± SD) of triplicate assays. In all the suppression assays, representative data of 3 independent experiments are shown.
Stimulation of OX40 on CD4+GFP(Foxp3)+ Tregs abrogates their suppressor functions. (A) CD4+GFP(Foxp3)− T effector cells sorted from wt and OX40KO foxp3gfpKI mice were stimulated with anti-CD3 plus wt APCs. T effector cell proliferation in the presence or absence of wt CD4+GFP(Foxp3)+ Tregs at different Tregs to T effector ratios was shown. Data shown are mean (CPM ± SD) of triplicate assays. (B) OX40 deficient CD4+GFP(Foxp3)− T effector cells were stimulated with anti-CD3 plus wt APCs or OX40Ltg APCs in the presence or absence of wt CD4+GFP(Foxp3)+ Tregs. Cell proliferation was determined 3 days later by 3H-TdR uptake. Data shown are mean (CPM ± SD) of triplicate assays. (C) OX40 deficient CD4+GFP(Foxp3)− T effector cells were stimulated with anti-CD3 plus OX40Ltg APCs. In these cultures, graded numbers of wt or OX40KO CD4+GFP(Foxp3)+ Tregs were added, and cell proliferation was determined 3 days later by 3H-TdR uptake. Data shown are mean (CPM ± SD) of triplicate assays. In all the suppression assays, representative data of 3 independent experiments are shown.
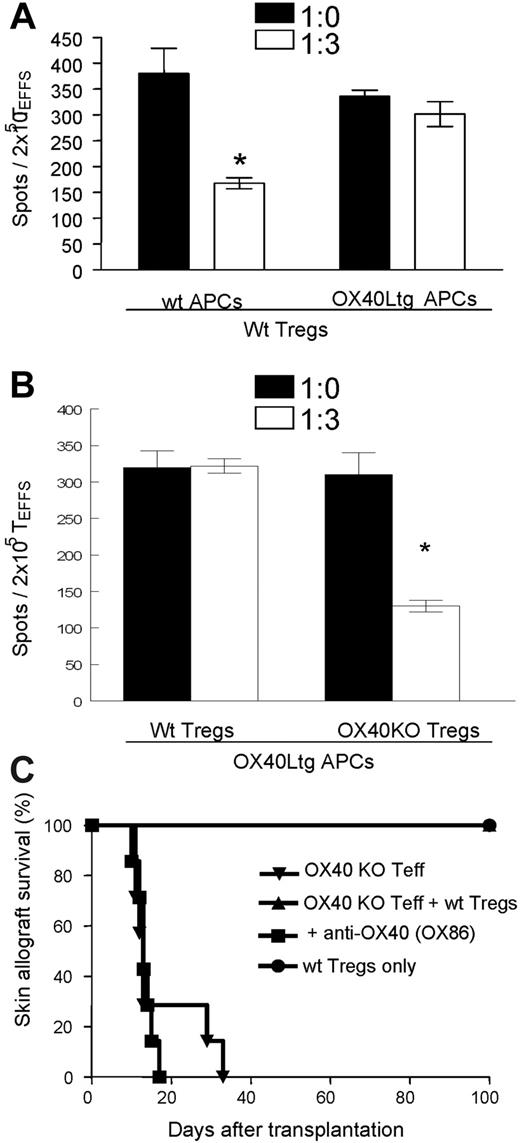
In a different set of experiments, we also examined the production of IFN-γ by OX40-deficient T effector cells when stimulated with anti-CD3 plus wt APCs or OX40Ltg APCs in the presence or absence of wt CD4+GFP(Foxp3)+ Tregs. As shown in Figure 5A, OX40KO T effector cells produced copious amounts of IFN-γ when stimulated with anti-CD3 plus APCs as analyzed by the ELISPOT assay (380 ± 49 spots/2 × 105 T effector cells [TEFFS]), and such IFN-γ production was markedly inhibited by the addition of CD4+GFP(Foxp3)+ Tregs (168 ± 11 spots/2 × 105 TEFFS). Once again, suppression of IFN-γ production by the Foxp3+ Tregs was abrogated in cultures in which OX40Ltg APCs were used to selectively stimulate OX40 on the Foxp3+ Tregs. However, OX40-deficient CD4+GFP(Foxp3)+ Tregs markedly inhibited the production of IFN-γ by OX40KO T effector cells when stimulated by anti-CD3 and OX40Ltg APCs (Figure 5B).
Suppression of IFN-γ production by CD4+GFP(Foxp3)+ Tregs with or without OX40 stimulation. (A) CD4+GFP(Foxp3)− T effector cells sorted from OX40KO foxp3gfpKI mice were stimulated with anti-CD3 plus wt APCs or OX40Ltg APCs, IFN-γ production by the T effector cells in the presence or absence of wt CD4+GFP(Foxp3)+ Tregs was analyzed by ELISPOT assay. Data shown are mean (± SD) of 3 experiments. (B) OX40 deficient CD4+GFP(Foxp3)− T effector cells were stimulated with anti-CD3 plus OX40Ltg APCs. In these cultures, wt or OX40 deficient CD4+GFP(Foxp3)+ Tregs were added as indicated, and suppression of IFN-γ production was shown. Data shown are mean (± SD) of 3 independent experiments. (C) CD4+GFP(Foxp3)− T effector cells from OX40KO foxp3gfpKI mice were transferred into syngeneic Rag−/− hosts (5 × 105 cells/mouse), groups of host mice were also transferred with equal number of wt CD4+GFP(Foxp3)+ Tregs. The host mice were then grafted with DBA/2 skin grafts and treated with an agonist anti-OX40 mAb, and skin allograft survival was shown. (*) P < .05
Suppression of IFN-γ production by CD4+GFP(Foxp3)+ Tregs with or without OX40 stimulation. (A) CD4+GFP(Foxp3)− T effector cells sorted from OX40KO foxp3gfpKI mice were stimulated with anti-CD3 plus wt APCs or OX40Ltg APCs, IFN-γ production by the T effector cells in the presence or absence of wt CD4+GFP(Foxp3)+ Tregs was analyzed by ELISPOT assay. Data shown are mean (± SD) of 3 experiments. (B) OX40 deficient CD4+GFP(Foxp3)− T effector cells were stimulated with anti-CD3 plus OX40Ltg APCs. In these cultures, wt or OX40 deficient CD4+GFP(Foxp3)+ Tregs were added as indicated, and suppression of IFN-γ production was shown. Data shown are mean (± SD) of 3 independent experiments. (C) CD4+GFP(Foxp3)− T effector cells from OX40KO foxp3gfpKI mice were transferred into syngeneic Rag−/− hosts (5 × 105 cells/mouse), groups of host mice were also transferred with equal number of wt CD4+GFP(Foxp3)+ Tregs. The host mice were then grafted with DBA/2 skin grafts and treated with an agonist anti-OX40 mAb, and skin allograft survival was shown. (*) P < .05
We then used a skin transplant model to further examine whether OX40 stimulation would interfere with the Foxp3+ Treg-mediated suppression of skin allograft rejection in vivo. For this purpose, the OX40KO CD4+GFP(Foxp3)− T effector cells were FACS sorted and adoptively transferred into syngeneic Rag−/− mice with or without transferring equal numbers of wt CD4+GFP(Foxp3)+ Tregs, the host mice were then grafted with the fully MHC-mismatched DBA/2 skin allografts, and the skin allograft survival was determined. As shown in Figure 5C, adoptive transfer of OX40KO CD4+Foxp3− T effector cells induced prompt rejection of DBA/2 skin allografts (MST = 17 days, n = 7). Cotransfer of an equal number of wt CD4+GFP(Foxp3)+ Tregs prevented the rejection response, and all the skin allografts survived for more than 100 days (n = 5). In host mice transferred with OX40KO T effector cells and wt GFP(Foxp3)+ Tregs, treatment with an agonist anti-OX40 mAb (OX86) precipitated rapid skin allograft rejection, and all the skin allografts were promptly rejected within 20 days after transplantation (n = 6), suggesting that suppression of T effector cell-mediated rejection by Foxp3+ Tregs was abrogated by selectively stimulating OX40 on the Foxp3+ Tregs. Taken together, these findings strongly suggest that OX40 costimulation to the Foxp3+ Tregs antagonizes their suppressor properties both in vitro and in vivo.
OX40 costimulation negatively regulates Foxp3 expression
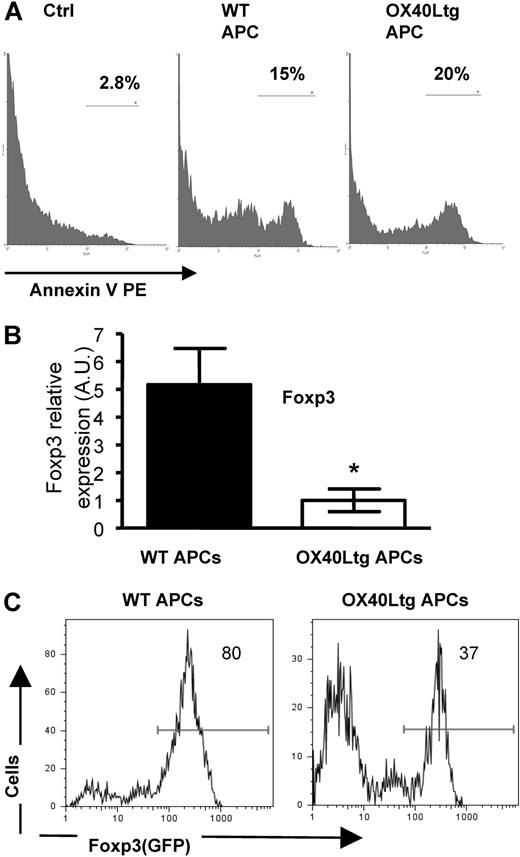
How does OX40 stimulation on Foxp3+ Tregs inhibit their suppressor functions? Clearly, stimulation of OX40 did not induce proliferation of Foxp3+ Tregs (Figure 4), suggesting that the loss of suppressor functions is unlikely due to their altered proliferative responses. To determine whether OX40 stimulation interferes with the survival of Foxp3+ Tregs, we stimulated the FACS-sorted CD4+GFP(Foxp3)+ Tregs in vitro with anti-CD3 plus wt APCs and OX40Ltg APCs, and examined their survival 24 hours later by annexin V staining. As shown in Figure 6A, OX40 stimulation did not significantly alter the viability of Foxp3+ Tregs. In these cultures, there was a small fraction of Tregs (∼ 15%) stained positive for annexin V, and the annexin V-positive fraction remained comparable regardless of the presence or absence of OX40 stimulation (Figure 6A).
Effect OX40 stimulation on survival and Foxp3 expression of CD4+GFP(Foxp3)+ Tregs. (A) Sorted CD4+GFP(Foxp3)+ Tregs were stimulated with anti-CD3 plus wt and OX40Ltg APCs. Cells were stained with PE-annexin V 24 hours later and analyzed by FACS. Freshly prepared Foxp3+ Tregs stained with PE-annexin V were included as a control. The annexin V profile in the GFP+ fraction was shown. (B) Sorted CD4+GFP(Foxp3)+ Tregs were stimulated with anti-CD3 and wt or OX40Ltg APCs. Foxp3 gene transcripts were analyzed 4 days later by real-time PCR. Data shown are mean A.U. of 4 experiments in each group. Error bars represent the SD of triplicate assays. (C) Sorted CD4+GFP(Foxp3)+ Tregs were stimulated with anti-CD3 and wt or OX40Ltg APCs, levels of GFP(Foxp3) expression in the CD4+ fraction were determined by FACS 4 days later and shown. The plot shown is one of 3 experiments. (A,C) Numbers are the relative percentage of cells in each region given by the flow cytometer.
Effect OX40 stimulation on survival and Foxp3 expression of CD4+GFP(Foxp3)+ Tregs. (A) Sorted CD4+GFP(Foxp3)+ Tregs were stimulated with anti-CD3 plus wt and OX40Ltg APCs. Cells were stained with PE-annexin V 24 hours later and analyzed by FACS. Freshly prepared Foxp3+ Tregs stained with PE-annexin V were included as a control. The annexin V profile in the GFP+ fraction was shown. (B) Sorted CD4+GFP(Foxp3)+ Tregs were stimulated with anti-CD3 and wt or OX40Ltg APCs. Foxp3 gene transcripts were analyzed 4 days later by real-time PCR. Data shown are mean A.U. of 4 experiments in each group. Error bars represent the SD of triplicate assays. (C) Sorted CD4+GFP(Foxp3)+ Tregs were stimulated with anti-CD3 and wt or OX40Ltg APCs, levels of GFP(Foxp3) expression in the CD4+ fraction were determined by FACS 4 days later and shown. The plot shown is one of 3 experiments. (A,C) Numbers are the relative percentage of cells in each region given by the flow cytometer.
However, analysis of Foxp3 gene expression by real-time PCR revealed that the effect of OX40 stimulation on Foxp3 gene expression was profound. As shown in Figure 6B, stimulation of CD4+GFP(Foxp3)+ Tregs with anti-CD3 plus OX40Ltg APCs induced marked down-regulation of Foxp3 gene expression compared with those stimulated with wt APCs. Similarly, analysis of GFP expression at the cellular level by flow cytometry also showed a significant down-regulation of GFP expression when GFP(Foxp3)+ Tregs were stimulated with OX40Ltg APCs (Figure 6C). Thus, OX40 appears to be a negative regulator of Foxp3 expression in CD4+GFP(Foxp3)+ Tregs, and the down-regulation of Foxp3 expression triggered by OX40 stimulation may be associated with the loss of their suppressor functions.
OX40 blocks the conversion of T effector cells to new Foxp3+ Tregs
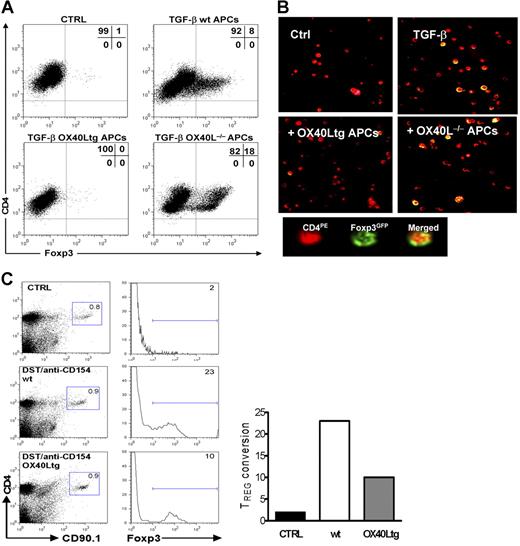
The marked effect of OX40 stimulation on Foxp3 expression in CD4+GFP(Foxp3)+ Tregs prompted us to examine whether OX40 costimulation to T effector cells would prevent the induction of new Foxp3+ Tregs. For this purpose, we stimulated sorted CD4+GFP(Foxp3)− T effector cells with anti-CD3 plus syngeneic APCs in the presence of TGF-β; the induction of GFP(Foxp3)+ Tregs at different time points was determined and compared with that stimulated with OX40Ltg APCs or OX40L−/− APCs. As shown in Figure 7A, addition of TGF-β into the cultures consistently converted a subset of T effector cells into GFP(Foxp3)+ T cells, whereas all the T effector cells remained GFP(Foxp3)− in the absence of TGF-β. Interestingly, the TGF-β-induced conversion of GFP(Foxp3)+ Tregs was markedly inhibited in cultures in which OX40Ltg APCs were used (Figure 7A). In contrast, conversion of T effector cells to new GFP(Foxp3)+ Tregs was enhanced when stimulated with OX40L−/− APCs (Figure 7A).
Effect of OX40 stimulation to T effector cells on the induction of new Foxp3+ Tregs.(A) CD4+GFP(Foxp3)− T effector cells were sorted from foxp3gfpKI mice and stimulated in vitro with anti-CD3 plus wt APCs, OX40Ltg APCs or OX40L−/− APCs in the presence or absence of TGF-β, and induction of GFP(Foxp3)+ cells was determined 4 days later by gating onto the CD4+ fraction. The FACS plot shown is the representative data of 4 individual experiments. (B) The experiments were set up as described in panel A, and the image shown was captured by confocal microscopy 4 days after the culture using a Nikon Eclipse 80i system equipped with E-max software (Nikon Instruments, Melville, NY) (40×/0.75 NA oil immersion lens). Cells were labeled with PE-antimouse CD4. (C) CD4+CD25− T effector cells were sorted from congenic CD90.1 mice and adoptively transferred into wt C57BL/6 and OX40Ltg mice (CD90.2). The host mice were treated with DST and anti-CD154. Induction of Foxp3 expression in the CD90.1+ fraction in the host spleen was determined by intracellular staining for the Foxp3 protein 6 days later. Data shown are representative of 3 experiments. (A,C) Numbers are the relative percentage of cells in each region given by the flow cytometer.
Effect of OX40 stimulation to T effector cells on the induction of new Foxp3+ Tregs.(A) CD4+GFP(Foxp3)− T effector cells were sorted from foxp3gfpKI mice and stimulated in vitro with anti-CD3 plus wt APCs, OX40Ltg APCs or OX40L−/− APCs in the presence or absence of TGF-β, and induction of GFP(Foxp3)+ cells was determined 4 days later by gating onto the CD4+ fraction. The FACS plot shown is the representative data of 4 individual experiments. (B) The experiments were set up as described in panel A, and the image shown was captured by confocal microscopy 4 days after the culture using a Nikon Eclipse 80i system equipped with E-max software (Nikon Instruments, Melville, NY) (40×/0.75 NA oil immersion lens). Cells were labeled with PE-antimouse CD4. (C) CD4+CD25− T effector cells were sorted from congenic CD90.1 mice and adoptively transferred into wt C57BL/6 and OX40Ltg mice (CD90.2). The host mice were treated with DST and anti-CD154. Induction of Foxp3 expression in the CD90.1+ fraction in the host spleen was determined by intracellular staining for the Foxp3 protein 6 days later. Data shown are representative of 3 experiments. (A,C) Numbers are the relative percentage of cells in each region given by the flow cytometer.
To further confirm this finding, we again sorted CD4+GFP(Foxp3)− T effector cells from foxp3gfpKI mice and stimulated them with anti-CD3 plus wt APCs, OX40Ltg APCs, or OX40L−/− APCs in the presence TGF-β, and the induction of Foxp3+ Tregs was visualized by confocal microscopy. As shown in Figure 7B, T effector cells (PE labeled, red) could be converted to GFP(Foxp3)+ Tregs (yellow) by stimulation with anti-CD3/APCs and TGF-β, and such conversion was inhibited by OX40 stimulation using the OX40Ltg APCs. Clearly, OX40 costimulation to T effector cells is antagonistic to the induction of new Foxp3+ Tregs.
To examine the role of OX40 costimulation in the induction of new Foxp3+ Tregs in vivo, we adoptively transferred FACS-sorted CD4+CD25− T effector cells (CD90.1+) into congenic wt C57BL/6 mice and OX40Ltg mice (CD90.2+). The host mice were given DST and anti-CD154 mAb treatment, and induction of Foxp3+ cells in the CD90.1+ fraction was examined by intracellular Foxp3 staining. As shown in Figure 7C, CD90.1+ T effector cells from the spleen of untreated hosts did not show any Foxp3 staining. In contrast, approximately 23% of the CD90.1+ T effector cells became Foxp3+ 6 days after DST and anti-CD154 treatment. Interestingly, the conversion of CD90.1+ T effector cells to Foxp3+ cells in the treated OX40Ltg mice was markedly inhibited (≈10%). Thus, induction of new Foxp3+ Tregs from T effector cells is inhibited by OX40 costimulation both in vitro and in vivo.
Discussion
OX40 is initially identified as a T-cell activation marker, as it is abundantly expressed by activated, but not resting, T cells.26 We now know that OX40 is a T-cell costimulatory molecule and plays an important role in survival and proliferation of activated T cells.13 Thus, blocking OX40 costimulation proves to be highly beneficial in preventing certain autoimmune diseases and in promoting transplant survival.17,27,,,,–32 Conversely, amplified OX40 signaling can markedly enhance the protective immunity against infectious agents and tumors.33 It is always believed that these findings are the sole costimulatory effects of OX40 to the T effector cells.
Here, we have demonstrated that OX40 is highly expressed on both natural and induced Foxp3+ Tregs. Importantly, in contrast to its costimulatory role to T effector cells,14 OX40 is rather a potent negative regulator of Foxp3+ Tregs. Clearly, stimulation of OX40 on CD4+Foxp3+ Tregs using either an agonist anti-OX40 mAb or OX40Ltg APCs consistently abolished their suppressor activities in vitro. Furthermore, stimulation of OX40 on the Foxp3+ Tregs also abrogated the effect of Foxp3+ Tregs in suppressing T effector cell-mediated skin allograft rejection in vivo. The loss of suppressor functions triggered by OX40 stimulation is not due to the altered proliferation of Foxp3+ Tregs or to the death of Foxp3+ Tregs, but appears to be associated with the inhibition of Foxp3 gene expression. Moreover, we showed for the first time that OX40 signaling has marked inhibitory effects on the induction of new inducible Foxp3+ Tregs from activated effector T cells. In our studies, conversion of Foxp3− T effector cells to Foxp3+ Tregs is consistently prevented by OX40 costimulation to T effector cells. These new findings strongly suggest that the overall effects of OX40 on the T-cell response are likely mediated not only by costimulating T effector cells but also by suppressing the Foxp3+ Tregs. Our data also suggest that OX40 likely controls a critical checkpoint where antigen-specific Tregs in the periphery are induced. Clearly, the clinical implication of our finding is likely to be significant. For example, in transplant models in which antigen-specific Tregs are required for tolerance induction, OX40 costimulation is likely to be antagonistic to the acquisition of tolerance, and blocking OX40 costimulation may be critically important in the establishment of donor-specific tolerance.
Apparently, OX40 is not the only cell surface molecule that can alter the suppressor functions of Foxp3+ Tregs. Several other molecules including CD28,34 IL-2/IL-2R,10 GITR,19,35 CTLA-4,36 and Toll-like receptor 237 also can significantly affect the suppressor functions of Foxp3+ Tregs. However, compared with such molecules, OX40 does have several unique features. First, unlike CD28 or the IL-2/IL-2R system where genetic deficiency of such molecules often leads to the reduction or functional impairment of Foxp3+ Tregs,10,38 OX40 is clearly dispensable for the genesis of natural Foxp3+ Tregs. Although reduced numbers of natural Foxp3+ Tregs, as defined by coexpression of CD4 and CD25 markers, has been noticed in young OX40 knockout mice,18 such a reduction is not obvious in our study and that of others in adult OX40 knockout mice.19,38 Also, the OX40 knockout mice are healthy and do not manifest autoimmune abnormalities,38 further suggesting that the Foxp3+ Tregs are unlikely to be defective in these mice. It is important to emphasize that OX40 and CD28 also exhibit opposing effects on the induction of new induced Foxp3+ Tregs from activated T effector cells. In the absence of CD28 costimulation, very few T effector cells can be converted to Foxp3+ Tregs,39 suggesting that CD28 signaling is permissive to the induction of new Foxp3+ Tregs. In stark contrast, OX40 signaling is antagonistic to the conversion of Foxp3+ Tregs. Second, stimulating GITR on the natural Tregs also abrogates their suppressor activities.35 It is believed that the loss of Treg functions by GITR stimulation is associated with the induction of Treg proliferation, followed by apoptotic death.19 A recent study suggests that the principal mechanism by which GITR stimulation mediates the loss of Treg functions is by stimulating the T effector cells, not the Tregs.40 In contrast, OX40 does not appear to affect the survival and proliferation of Foxp3+ Tregs, but rather inhibits their expression of Foxp3 gene transcripts. It remains unclear whether the loss of Treg functions triggered by OX40 stimulation is reversible (ie, the Tregs regain their suppressor functions when OX40 stimulation is stopped) or such a loss is permanent. More studies are clearly warranted to further unravel this issue. Finally, differences between OX40 and CTLA-4 in regulating Foxp3+ Tregs are also striking. The most noticeable difference is that CTLA-4-deficient mice rapidly develop multiorgan autoimmunity and die at 4 weeks of age,41 while OX40-deficient mice are healthy and do not display any autoimmune diseases.20 Also, in certain models, blocking CTLA-4 is detrimental to the suppressor functions of Foxp3+ Tregs,42 while genetic deficiency of OX40 on the Foxp3+ Tregs does not interfere with their suppressor functions. All of those features make OX40 uniquely different from other cell surface molecules in regulating the functions of Foxp3+ Tregs.
Our finding that OX40 prevents de novo generation of new Foxp3+ Tregs from T effector cells is novel and significant. Besides promoting T effector cell proliferation and survival, OX40 is known to direct their differentiation programs. In certain models, OX40 costimulation preferentially supports a Th2 type of immune response, while in others OX40 promotes both Th1 and Th2 responses.43,44 Furthermore, studies using mice deficient for OX40 or OX40L demonstrate that OX40 is also critical to the generation of memory T cells.45 Our study suggests that suppression of de novo generation of new Foxp3+ Tregs by OX40 is likely to be an important mechanism by which OX40 promotes functional differentiation of T effector cells. However, the precise molecular mechanisms by which OX40 directs Th1 and Th2 differentiation, and at the same time turns off Foxp3+ Tregs, especially in the context of T-bet and GATA3 expression, await further investigation. OX40 is known to play an important role in the transition of T effector cells to a memory phenotype.45 Our finding that OX40 costimulation to T effector cells can effectively prevent the induction of new Foxp3+ Tregs suggests a possibility that OX40 is likely involved in reciprocal regulation of antigen-specific Tregs and memory T cells, and suppression the conversion of Foxp3+ Tregs from T effector cells may be an important step by which OX40 promotes the development of memory T cells. More studies are required to further delineate whether OX40 differentially regulates effector to memory transition and effector to Foxp3+ Treg conversion.
Unlike other costimulatory molecules, OX40 appears to play a dual role in the T-cell response; OX40 is undoubtedly a potent costimulatory molecule for T effector cells, but it is also a powerful negative regulator for Foxp3+ Tregs. Recently, OX40 costimulation has been shown to shut off the IL-10-producing regulatory T cells.46 Thus, manipulating OX40 costimulation may have important therapeutic implications. For example, in transplant models in which tolerance requires the induction of donor-specific Tregs,47 blocking OX40 costimulation may be critically important in the induction of long-lasting tolerance. Similarly, dysfunction of Foxp3+ Tregs and the presence of autoantigen-specific memory T cells are key features of certain autoimmune diseases. In such models, blocking OX40 signaling may be required for suppressing autoimmunity and restoring self-tolerance. On the other hand, in tumor models and infectious models, deliberately stimulating the OX40 pathway may turn off the suppressor activity of Tregs and facilitate protective effector and memory T-cell responses.48 In conclusion, our data clearly demonstrate that OX40 costimulation has a diametrically opposing effect on Foxp3+ Tregs and T effector cells, and these new findings may have important therapeutic implications in the clinic.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health and the Juvenile Diabetic Research Foundation International (X.C.L.).
We thank Drs Terry B. Strom, Mohamed H. Sayegh, Douglas W. Hanto, and Vijay Kuchroo for support, guidance, reagents, and helpful discussions.
National Institutes of Health
Authorship
Contribution: M.D.V., X.X., N.D., M.C., and A.K. performed experiments; W.G., N.K., and N.I. provided animal models; and X.C.L. initiated the project and wrote the paper. M.D.V. and X.X. contributed equally to this study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xian C. Li, Beth Israel Deaconess Medical Center, 330 Brookline Ave, HIM-1025, Boston, MA 02215; e-mail:xli@bidmc.harvard.edu.