It has been proposed that heterogeneity in natural killer (NK)–cell phenotype and function can be achieved through distinct thymic and bone marrow pathways of NK-cell development. Here, we show a link between Notch signaling and the generation of intracellular CD3ϵ (cyCD3)–expressing NK cells, a cell population that can be detected in vivo. Differentiation of human CD34+ cord blood progenitors in IL-15–supplemented fetal thymus organ culture or OP9-Delta-like 1 (DL1) coculture resulted in a high percentage of cyCD3+ NK cells that was blocked by the γ-secretase inhibitor DAPT. The requirement for Notch signaling to generate cyCD3+ NK cells was further illustrated by transduction of CD34+ cord blood (CB) cells with either the active intracellular part of Notch or the dominant-negative mutant of mastermind-like protein 1 that resulted in the generation of NK cells with respectively high or low frequencies of cyCD3. Human thymic CD34+ progenitor cells displayed the potential to generate cyCD3+ NK cells, even in the absence of Notch/DL1 signaling. Peripheral blood NK cells were unable to induce cyCD3 expression after DL1 exposure, indicating that Notch-dependent cyCD3 expression can only be achieved during the early phase of NK-cell differentiation.
Introduction
Natural killer (NK) cells are CD3−CD56+ large granular lymphocytes that can kill infected or malignantly transformed cells and produce cytokines, such as IFN-γ, to regulate the immune response. NK cells develop in the bone marrow from hematopoietic stem cells. However, NK precursors can also be isolated from the thymus. It is unclear whether this signifies a closer relationship to the T-cell lineage. Recently, Di Santo and colleagues1 established the existence of a developmentally and functionally distinct thymus-derived NK subset in mice. These NK cells are functionally distinct from bone marrow–derived NK cells in that they show reduced cytotoxicity but stronger cytokine production. These properties correspond to human peripheral CD56brightCD16− NK cells that are also less cytotoxic but that are stronger cytokine-producing cells compared with their CD56dimCD16+ counterpart. The mouse data suggest that thymic NK cells are generated through a pathway involving GATA-3 and CD127.
As a result, analysis of human NK-cell differentiation must be reconsidered in view of both a bone marrow and a thymic differentiation pathway. It has been shown that a subset of human NK cells shows similarities with T cells. Besides FcϵRIγ and CD3ζ, these NK cells may also express other components of the CD3–T-cell receptor (TCR) complex, such as CD3ϵ, CD3γ, and CD3δ.2,–4 In particular, it has been reported that fetal and thymic NK cells express intracellular CD3ϵ chain (cyCD3),2 raising speculation that these cyCD3+ NK cells are related to thymus-derived NK cells, and that their precursors received a Notch signal.
Notch signaling regulates commitment and early development of T lymphocytes. The 4 mammalian Notch proteins (Notch1-Notch4) comprise a family of transmembrane receptors conserved throughout evolution that are involved in various cell-fate decisions (see Lai5 and Ehebauer et al6 for review). Upon binding with 1 of 5 ligands (Jagged-1 and Jagged-2 and Delta-1, Delta-3, and Delta-4), Notch undergoes a series of proteolytic cleavages. One of them is mediated by presenilin, a member of the γ-secretase complex, and this releases the Notch intracellular domain (ICN), the activated form of Notch. ICN translocates to the nucleus and binds CSL, a transcriptional repressor. The binding of ICN converts CSL into a transcriptional activator through recruitment of several proteins, such as the proteins of the mastermind-like (MAML) family, resulting in initiation of Notch target gene expression. Several lines of research reveal an essential, nonredundant role of Notch 1 signaling in the commitment of progenitors to the T-cell lineage in the thymus (for review, see Rothenberg et al7 and Maillard et al8 ).
Here, we studied whether Notch signaling is involved in the development of human NK cells. Our results show that cyCD3ϵ expression is linked to Notch/Delta-like 1 (DL1) signaling, but not with the recently described thymic pathway for NK-cell development.
Materials and methods
Cells and sorting
Pediatric thymi, bone marrow (BM), cord blood (CB), and peripheral blood (PB) samples were obtained and used according to the guidelines of the Medical Ethical Commission of Ghent University Hospital (Belgium). Informed consent was obtained in accordance with the Declaration of Helsinki. After isolation of mononuclear cells by gradient centrifugation on Lymphoprep (Axis-Shield PoC AS, Oslo, Norway), CD34+ CB or BM cells were purified by CD34 magnetic-activated cell-sorting (MACS) beads (Miltenyi Biotec, Bergisch Gladbach, Germany). Cells were then stained with CD34-APC, CD19-FITC, and CD56-PE (all monoclonal antibodies [mAbs] from BD Immunocytometry Systems [BDIS], Mountain View, CA) to sort CD34+Lin− cells (FACSVantage; BDIS). CD34+ thymocytes were purified with CD34 MACS beads, stained with CD34-APC and CD1-PE, and sorted for CD1−CD34++ progenitors. Purity of the sorted cells was always at least 98%. PB NK cells were purified by CD4, CD14, and CD19 depletion using sheep anti-mouse–coupled Dynalbeads (Dynal, Invitrogen, Merelbeke, Belgium). Subsequently, cells were stained with CD56-PE and CD3-FITC and sorted for CD56+CD3− NK cells.
Cell transduction
Transduction of CB cells with retroviral supernatant of transiently transfected Phoenix-A cells with a vector containing the dominant-negative (DN) MAML1-GFP fusion construct9 (a gift from W. S. Pear, Philadelphia, PA) or cDNA encoding the intracellular constitutively active form of Notch (ICN; amino acids 1770-2555) was done as described previously.10
FTOC and stromal cell coculture
The mixed human-mouse fetal thymic organ culture (FTOC) was performed as described previously.10,,–13 Severe combined immunodeficiency–nonobese diabetic (SCID-NOD) mice were housed and bred under specific pathogen–free conditions in our breeding facility and were treated and used in agreement with the guidelines of the local ethical committee. FTOCs were cultured in presence of 10 ng/mL IL-15 (R&D Systems, Abingdon, United Kingdom) with different concentrations of γ-secretase inhibitor DAPT (7 N-[N-(3,5-difluorophenyl-L-alanyl)]-S-phenyl-glycine t-butyl ester; Peptides International Inc, Louisville, KY) or with 0.05% dimethyl sulfoxide (DMSO) as control. Half the medium was changed weekly.
Coculture on stromal cells was performed by seeding 5 × 103 to 10 × 103 progenitors/well into 24-well tissue-culture plates (Becton Dickinson, Erembodegem, Belgium) containing either a confluent monolayer of MS-5 cells (a gift from L. Coulombel, Hôpital Paul Brousse, Villejuif, France) or OP9-control or OP9-DL1 cells (a gift from J. C. Zuniga-Pflücker,University of Toronto, Canada).14 All cocultures were performed in α-MEM (Invitrogen, Merelbeke, Belgium) supplemented with 10% (MS-5) or 20% fetal calf serum (Perbio, Erembodegem, Belgium) in the presence of 5 ng/mL recombinant human IL-7 (rhuIL-7; a kind gift from Immunex, Seattle, WA), 5 ng/mL rhu Flt-3L (Peprotech, Princeton, NJ), 5 ng/mL rhu stem cell factor (SCF; R&D Systems), and 10 ng/mL rhuIL-15 (R&D Systems). Cocultures were refreshed every 3 to 4 days by recovering the cells with vigorous pipetting followed by filtering the cells through a Falcon cell strainer with a mesh of 70 μm (VWR International, Leuven, Belgium) and centrifugation (200g at room temperature for 5 minutes). The cell pellet was transferred to a fresh confluent monolayer of stromal cells.
Flow cytometry of NK-cell cultures
After 17 to 20 days of FTOC, harvested thymocytes were incubated with anti-mouse FcRγII/III (clone 2.4.G2)15 and human immunoglobulin G (IgG; FcBlock; Miltenyi Biotec) to avoid nonspecific staining of mouse and human cells. Subsequently, cells were stained with anti-mouse CD45-CyChrome (BD PharMingen, San Diego, CA) in combination with 1 or more of the following antihuman mAbs: CD56-APC (Coulter, Miami, FL), CD34-APC, CD3-APC or CD3-FITC, CD7-FITC, CD56-FITC or CD56-PE, or the appropriate control mAb (IgG1- and IgG2a-FITC, APC, or PE; BDIS). Human viable cells, gated by exclusion of propidium iodide and mouse CD45+ cells, were examined for the expression of the antigens on a FACScalibur using CellQuest Pro software (BDIS). For intracellular staining, cells were fixed and permeabilized using Fix and Perm (Imtec, San Francisco, CA) according to the manufacturer's instructions and stained with CD3-APC or granzyme B–FITC (BDIS).
For flow cytometric analysis of stromal cocultures, cells were harvested by forceful pipetting at the indicated time points and were stained as described for FTOC, without mouse CD45-CyChrome. When enhanced green fluorescent protein (EGFP) was used as a reporter (ICN or LZRS-IRES-EGFP [LIE]) on MS-5 stromal cells, the stromal cells were gated out by scatter properties, and human cells were identified by the green fluorescence of the reporter gene. OP9 murine stromal cells were gated out according to their high scatter properties and green fluorescence. The validity of this gating strategy was verified by staining the cells with anti human CD45. The following monoclonal antibodies were used: CD335-PE and CD127-PE (Coulter); NKG2D-PE and CD94-PE (eBiosciences, San Diego, CA); CD117-PE, CD45-PE, or APC (Miltenyi Biotec); CD158-APC (R&D Systems); and CD16-FITC, CD25-PE, CD62L-PE, CD69-FITC, CD122-PE, and CD161-PE (BDIS).
CD107 (lysosome-associated membrane protein 1) expression assay
CD107a expression was used to measure NK-cell degranulation, as described.16 OP-9– or OP9-DL1–cocultured cells were incubated with K562 target cells at different effector-target (E/T) ratios as indicated. CD107a-FITC antibody (BD Biosciences) was added at the start of the culture, and the cells were spun down (5 minutes at 300g). Following 2 hours of stimulation with K562 cells at 37°C, cells were washed with EDTA buffer and stained with CD56-PE antibody for membrane labeling. Cells were washed, fixed, permeabilized with cytofix-cytoperm according the guidelines of the manufacturer (BDIS), and stained intracellularly with CD3-APC antibody prior to flow cytometric analysis on gated CD56+ NK cells.
Intracellular staining of cytokines in activated NK cells
OP-9– or OP9-DL1–cocultured cells were stimulated for 2 hours with 10 ng/mL rhuIL-12 and 50 ng/mL rhuIL-18 (both from R&D Systems) at 37°C in complete medium. Brefeldin A (5 μg/mL; BD Biosciences) was added, and the cells were incubated for another 4 hours at 37°C. Cells were washed and stained with CD56-FITC. For simultaneous intracellular staining, the fixation, permeabilization, and staining was performed as described for intracellular staining in the CD107 assay using anti-IFNγ–PE (BDIS) and CD3-APC.
Results
Comparative phenotypic analysis of NK cells generated on OP9-DL1 or OP9 stromal cells
Notch is important for regulating the T- versus B-lineage decision, but is also permissive for NK-cell development10 . To investigate the role of Notch in NK-cell development, we generated NK cells from CD34+CD56− CB progenitor cells on OP9 stromal cells (OP9-control) and OP9 cells transfected with the Delta-class Notch ligand Delta-like 1 (OP9-DL1). Upon addition of IL-15 to the cytokine mix of Flt-3L, SCF, and IL-7, NK cells are efficiently generated on both OP9 stromal cell lines instead of B lymphocytes on OP9-control cells, or T cells on OP9-DL1 stromal cells when CB cells are cultured in absence of IL-15.14 As shown in Figure 1A, CD56+ NK cells lacking surface CD3 expression were generated in both conditions, corroborating the view that Notch/DL1 signaling is not essential for NK-cell development.17 Part of the OP9-DL1–derived NK cells showed lower CD56 expression compared with NK cells generated in OP9-control cocultures after 3 weeks of culture. Further culture of sorted CD56−, CD56dim, and CD56bright cells on OP9-DL1 stromal cells shows that CD56 expression is gradually up-regulated, indicating that the CD56dim population represents an intermediate developmental stage (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article). In the OP9-control cocultures, we see a CD56− cell subpopulation that is mainly composed of monocytes, whereas in the OP9-DL1 cocultures, the CD56− subset is composed of a mixture of dendritic/monocytic cells (data not shown) and early precursors that still have NK-cell potential (Figure S1). Exposure to Notch/DL1 increased NK-cell numbers 2- to 3-fold in comparison with OP9-control cocultures (Figure 1A). After 3 and 5 weeks of culture, a comparative analysis of cell membrane marker expression and intracellular CD3ϵ (cyCD3) was done on CD56+ gated cells. There was a striking difference in cyCD3 expression. The vast majority (> 90%) of OP9-DL1–derived NK cells expressed cyCD3 after 3 weeks (Figure 1B) or 5 weeks (Figure 1C) in contrast to the low percentage (< 20%) in OP9-control–derived NK cells. After 3 weeks, there was an up-regulation of the cytokine receptors CD25 (IL-2Rα) and CD117 (c-kit), but both markers were more pronounced for the OP9-DL1–cocultured NK cells. CD122 (IL-2/15Rβ) could not be detected by flow cytometry, but was clearly detected by quantitative reverse transcription–polymerase chain reaction (RT-PCR) and showed increased expression in OP-DL1–derived NK cells (Figure S2). CD127 (IL7-Rα) was nearly absent on both populations, and RT-PCR analysis confirmed low expression that was not obviously different between both NK-cell populations (Figure S2). In both coculture conditions, the NK-cell activation marker CD69 was strongly expressed. NK cells generated on OP9-DL1 stromal cells expressed higher levels of CD7 and lower levels of CD62L (L-selectin), an adhesion marker mediating early interaction with vascular endothelium. After 3 weeks of culture, the expression of the activating C-type lectin receptor NKG2D, the natural cytotoxic receptor CD335 (NKp46), and the activating coreceptor CD161 (NKR-P1A) was increased in NK cells that were generated on OP9-DL1 stromal cells. At that time of culture, the inhibitory KIR (CD158) was not expressed, consistent with the observation that expression of activating receptors precedes up-regulation of inhibitory receptors. At 3 weeks of coculture, all the NK cells showed dim CD16 expression. After 5 weeks, CD56 was homogeneously and strongly expressed on NK cells obtained in both coculture conditions (Figure 1C). There was an up-regulation of CD16 and CD158 on a subset of the NK cells generated on OP9-DL1 cultures (Figure 1C). At that time, the NK cells generated on OP9-control did not express those markers, but part of the cells did express CD94 and CD7, which were already homogeneously expressed by OP9-DL1–derived NK cells. Taken together, the profile of surface marker expression indicates that NK-cell differentiation was accomplished on both stromal cell types. A striking difference was the high frequency of cyCD3+-expressing NK cells obtained in coculture with OP9-DL1 stromal cells. This cyCD3 expression has already been observed in fetal NK cells,2 in NK cells derived from thymic progenitors,18 and in in vitro–generated T/NK-cell progenitors as a result of excess Notch signaling.10
NK-cell number and flow cytometric analysis of surface-marker and cyCD3ϵ expression on in vitro–generated NK cells. (A) Dot plot analysis of CD56 versus membrane CD3 expression in cells generated from CD34+ CB progenitor cells in the presence of Flt-3L, SCF, IL-7, and IL-15 on a coculture with OP9 or OP9-control stromal cells after 3 weeks of culture. Graph shows the kinetics of the CD3−CD56 + NK-cell number starting from 1000 CD34+ CB progenitor cells generated on OP9-DL1 (◇; full line) or OP9-control (■); dotted line) at different time points of culture as indicated on the x-axis. Data are shown as the means (± SD) from 3 independent experiments. There is a consistently higher number of NK cells when cultured on OP9-DL1 stromal cells. (B,C) Flow cytometric analysis of the NK cells obtained after 3 weeks (B) or 5 weeks (C) with OP9-control or OP9-DL1 stromal cells. The histograms represent the fluorescence intensity of the cells that were gated on CD56+ NK cells; the fluorescence of the isotype control of the cells cultured on OP9DL1 is shown as a thin dotted line; the fluorescence of the indicated antigen on cells cultured on OP9-DL1 or OP9-control stromal cells is shown as a thick line or as a gray-shaded area, respectively. The isotype control of the cells cultured on OP9-control stromal cells is not shown as it did not differ from the isotype control of the cells cocultured on OP9-DL1 stromal cells.
NK-cell number and flow cytometric analysis of surface-marker and cyCD3ϵ expression on in vitro–generated NK cells. (A) Dot plot analysis of CD56 versus membrane CD3 expression in cells generated from CD34+ CB progenitor cells in the presence of Flt-3L, SCF, IL-7, and IL-15 on a coculture with OP9 or OP9-control stromal cells after 3 weeks of culture. Graph shows the kinetics of the CD3−CD56 + NK-cell number starting from 1000 CD34+ CB progenitor cells generated on OP9-DL1 (◇; full line) or OP9-control (■); dotted line) at different time points of culture as indicated on the x-axis. Data are shown as the means (± SD) from 3 independent experiments. There is a consistently higher number of NK cells when cultured on OP9-DL1 stromal cells. (B,C) Flow cytometric analysis of the NK cells obtained after 3 weeks (B) or 5 weeks (C) with OP9-control or OP9-DL1 stromal cells. The histograms represent the fluorescence intensity of the cells that were gated on CD56+ NK cells; the fluorescence of the isotype control of the cells cultured on OP9DL1 is shown as a thin dotted line; the fluorescence of the indicated antigen on cells cultured on OP9-DL1 or OP9-control stromal cells is shown as a thick line or as a gray-shaded area, respectively. The isotype control of the cells cultured on OP9-control stromal cells is not shown as it did not differ from the isotype control of the cells cocultured on OP9-DL1 stromal cells.
NK-cell functional competence and cyCD3 phenotype
To address whether the expression of cyCD3 in NK cells identifies a subpopulation with different functional properties, we examined both cytolytic and cytokine production capacities of the cells. Expression of CD107 (LAMP-1), which is transiently expressed on the cell surface upon stimulation of the cytotoxic activity,16 was analyzed upon coculture with the NK target cell line K562. The flow cytometry–based analysis of CD107 as a marker for cytotoxic function enables fixation and permeabilization of the cells, which is necessary to stain and gate for cyCD3+ cells. The NK cells generated in OP9-DL1 coculture were more cytolytic and produced more IFN-γ than the NK cells that are generated on OP9 stromal cells (Figure 2A-B; left panel). In addition, OP9-DL1–derived NK cells expressed higher levels of granzyme B compared with NK cells generated on OP9 stromal cells (Figure 2C; left histogram). These assays did not show any differences between cyCD3+ or cyCD3− NK subsets from OP9-DL1 cocultures (Figure 2A-C; right panel).
Functional analysis of NK cells generated on coculture with OP9-DL1 or OP9 stromal cells. NK cells were generated on OP9-DL1 (□) or OP9-control stromal cells (▩) in the presence of Flt-3L, SCF, IL-7, and IL15 for 20 days and either (A) incubated with K562 target cells and analyzed for CD107 expression, (B) stimulated with IL-12 and IL-18 and analyzed for IFN-γ expression, or (C) without preincubation stained for granzyme B. (A) Percentages of CD107a+ NK cells after incubation with K562 target cells for 2 hours with different E/T ratios as indicated in the x-axis. Data are shown as the means (± SD) from 2 independent experiments. The left bar diagram shows the comparative analysis of NK-cell activity of NK cells generated on OP9-control (□) or OP9-DL1 stromal cells (▩). The right bar diagram shows the comparative analysis of NK-cell activity of gated cyCD3+ (■) and cyCD3− (□) NK cells generated on OP9-DL1. (B) Frequencies of IFN-γ+ NK cells on cytokine-activated NK cells. After 3 weeks of coculture, the cells were stimulated with rhuIL-12 and rhuIL-18. Data are shown as the means (± SD) from 2 independent experiments. The left bar diagram shows the comparative analysis of the frequency of IFN-γ+ NK cells generated on OP9-DL1 (□) or OP9-control stromal cells (▩). Right bar diagram shows the comparative analysis of the frequency of IFN-γ+ NK cells of gated cyCD3+ (■) and cyCD3− (□) NK cells generated on OP9-DL1. (C) Granzyme B expression as detected by intracellular flow cytometry of cells obtained on OP-control or OP9-DL1 stromal cells. The left panel of histograms shows the fluorescence of the isotype control of the cells cultured on OP9-DL1, the fluorescence of the granzyme mAb staining of NK cells obtained on OP-9DL1 or on OP9-control stromal cells as labelled. The right panel of histograms shows the fluorescence of the isotype control of the cells cultured on OP9-DL1 and the fluorescence of granzyme B on the gated cyCD3+ or cyCD3− NK cells cultured on OP9-DL1. The same picture, but with lower intensity for granzyme B, is observed for the OP9-control–generated NK cells (data not shown).
Functional analysis of NK cells generated on coculture with OP9-DL1 or OP9 stromal cells. NK cells were generated on OP9-DL1 (□) or OP9-control stromal cells (▩) in the presence of Flt-3L, SCF, IL-7, and IL15 for 20 days and either (A) incubated with K562 target cells and analyzed for CD107 expression, (B) stimulated with IL-12 and IL-18 and analyzed for IFN-γ expression, or (C) without preincubation stained for granzyme B. (A) Percentages of CD107a+ NK cells after incubation with K562 target cells for 2 hours with different E/T ratios as indicated in the x-axis. Data are shown as the means (± SD) from 2 independent experiments. The left bar diagram shows the comparative analysis of NK-cell activity of NK cells generated on OP9-control (□) or OP9-DL1 stromal cells (▩). The right bar diagram shows the comparative analysis of NK-cell activity of gated cyCD3+ (■) and cyCD3− (□) NK cells generated on OP9-DL1. (B) Frequencies of IFN-γ+ NK cells on cytokine-activated NK cells. After 3 weeks of coculture, the cells were stimulated with rhuIL-12 and rhuIL-18. Data are shown as the means (± SD) from 2 independent experiments. The left bar diagram shows the comparative analysis of the frequency of IFN-γ+ NK cells generated on OP9-DL1 (□) or OP9-control stromal cells (▩). Right bar diagram shows the comparative analysis of the frequency of IFN-γ+ NK cells of gated cyCD3+ (■) and cyCD3− (□) NK cells generated on OP9-DL1. (C) Granzyme B expression as detected by intracellular flow cytometry of cells obtained on OP-control or OP9-DL1 stromal cells. The left panel of histograms shows the fluorescence of the isotype control of the cells cultured on OP9-DL1, the fluorescence of the granzyme mAb staining of NK cells obtained on OP-9DL1 or on OP9-control stromal cells as labelled. The right panel of histograms shows the fluorescence of the isotype control of the cells cultured on OP9-DL1 and the fluorescence of granzyme B on the gated cyCD3+ or cyCD3− NK cells cultured on OP9-DL1. The same picture, but with lower intensity for granzyme B, is observed for the OP9-control–generated NK cells (data not shown).
Thus, NK cells that matured in the presence of Notch/DL1 signaling seem to be more potent at both the level of cytoxicity and immune regulation, independent of cyCD3 expression.
Unlike circulating adult PB NK cells, NK cells from CB or thymus express a high frequency of cyCD3
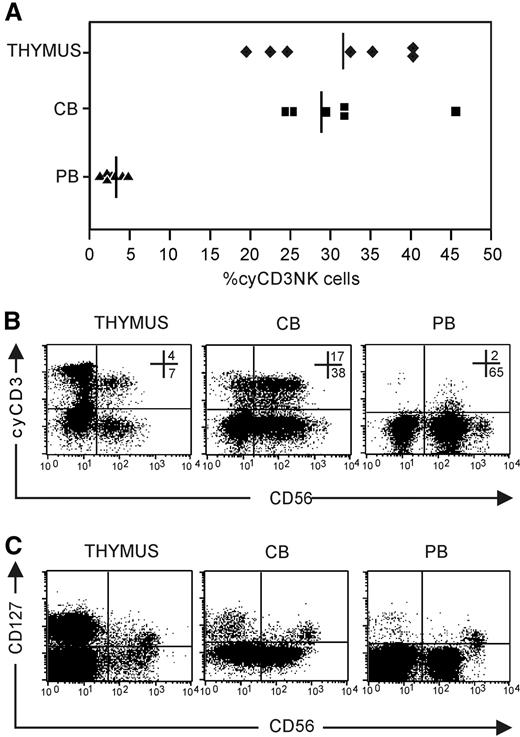
To address the in vivo relevance of the cyCD3+ NK-cell phenotype, we analyzed the presence of cyCD3+ NK cells in CB, PB, and the thymus. The frequency of cyCD3+CD56+ NK cells is significantly higher in thymus (n = 7; 31.4 ± 7.6) and CB (n = 6; 29.2 ± 8.1) compared with the frequency found in PB (n = 10; 3.0 ± 1.2; P < .01) (Figure 3A). In addition, the expression level of cyCD3 in PB is weaker compared with that found in CB or the thymus (Figure 3B). In view of the recent delineation of NK cells according to the thymic origin, wherein it was shown in mice that CD127 serves as a molecular marker which identifies a population that originates in the thymus,1 we looked to see if the expression of cyCD3 in NK cells correlates with CD127 expression. We also detected CD127 expression on CD56brightCD16− thymic, CB, and PB NK cells (Figure 3C), but since most cyCD3+ cells are present in the CD56dim population, it is obvious that intracellular cyCD3 does not correlate with CD127 expression (Figure 3B).
Expression of cyCD3+ in NK cells in vivo. NK cells were enriched by depleting membrane CD3+ cells with CD3-biotin and streptavidin Dynalbeads. The NK-cell–enriched cell fraction was analyzed for CD56 and cyCD3 expression after carefully gating out membrane CD3+ cells to avoid contamination with peripheral T cells and NKT cells that are cyCD3+. (A) Frequencies of NK cells that express cyCD3 in thymus (♦), CB (■), and PB (▴); solid lines represent the means of 6 to 8 independent samples. (B) Dot plot analysis of cyCD3 expression on gated CD56+CD3− NK cells. Note the difference in the intensity of cyCD3 expression between PB (low) and CB (high) and thymus (high). Numbers in dot plots show the frequency of cells in the respective top right and bottom right quandrants. (C) Dot plot analysis of CD127 versus CD56 expression on gated CD3− thymus, CB, and PB cells. Note that the CD127+ subset is present in the CD56bright subpopulation, whereas most cyCD3 cells are present in the CD56dim population.
Expression of cyCD3+ in NK cells in vivo. NK cells were enriched by depleting membrane CD3+ cells with CD3-biotin and streptavidin Dynalbeads. The NK-cell–enriched cell fraction was analyzed for CD56 and cyCD3 expression after carefully gating out membrane CD3+ cells to avoid contamination with peripheral T cells and NKT cells that are cyCD3+. (A) Frequencies of NK cells that express cyCD3 in thymus (♦), CB (■), and PB (▴); solid lines represent the means of 6 to 8 independent samples. (B) Dot plot analysis of cyCD3 expression on gated CD56+CD3− NK cells. Note the difference in the intensity of cyCD3 expression between PB (low) and CB (high) and thymus (high). Numbers in dot plots show the frequency of cells in the respective top right and bottom right quandrants. (C) Dot plot analysis of CD127 versus CD56 expression on gated CD3− thymus, CB, and PB cells. Note that the CD127+ subset is present in the CD56bright subpopulation, whereas most cyCD3 cells are present in the CD56dim population.
Generation of cyCD3+CD56+ NK cells is highly dependent on Notch/DL1 signaling
Human NK cells develop when fetal thymi from day-14 to -15 SCID-NOD mice are seeded with human CB CD34+ progenitors and cultured with IL-15. These NK cells express CD56 but not CD3 on the cell membrane (data not shown). However, a significant percentage of those NK cells express cyCD3 (Figure 4A). When the FTOCs are cultured in presence of the γ-secretase inhibitor DAPT, the potential to generate NK cells with IL-15 is not significantly altered (Figure 4A), consistent with previous results12 and with the hypothesis that Notch signaling is not essential for NK-cell development. However, the involvement of Notch signaling in cyCD3 expression is evidenced by the observation that the frequency and absolute number of cyCD3+ NK cells drops in a dose-dependent way in the presence of DAPT (Figure 4A).
Notch signaling favors the differentiation of NK cells with cyCD3 expression. Numbers in the dot plots show the frequency of cells in the respective top right and bottom right quadrants. R% indicates the frequency of cyCD3+ cells within the CD56+ cell population. (A) The generation of cyCD3+ NK cells in FTOC can be inhibited by DAPT. Dot-plot analysis of cyCD3 (y-axis) versus CD56 expression (x-axis) in human CD56+ cells generated in FTOC starting from human CD34+Lin− CB cells after 14 days of culture with different concentrations of DAPT. Note the dose-dependent decrease in both the frequency and the absolute number of cyCD3+CD56+ cells when the Notch pathway is inhibited by the γ-secretase inhibitor. The left bar diagram shows the frequencies of cyCD3+CD56+ NK cells; the far right bar diagram shows the absolute number of CD56+ NK cells (□) or cyCD3+CD56+ NK cells (■) obtained in FTOC with different concentrations of DAPT as indicated on the x-axis. Bars represent means and SD of 3 independent experiments. (B) Notch signaling by DL1 favors the generation of cyCD3+ NK cells on stromal cell coculture. Dot-plot analysis of cyCD3 (y-axis) versus CD56 expression (x-axis) in human CD56+ cells generated on stromal cell line OP9-control or OP9-DL1 as indicated starting from human CD34 CB cells after 14 days of culture. Note the high frequency and absolute number of cyCD3+CD56+ cells when the Notch pathway is triggered by DL1 (OP9-DL1), and the low frequency when the DL1 signal is absent (OP9-control) or inhibited by the γ-secretase inhibitor DAPT. Left bar diagram shows the frequencies of cyCD3+CD56+ NK cells; the far right bar diagram shows the absolute number of CD56+ NK cells (□) or cyCD3+CD56+ NK cells (■) in different culture conditions as described on the x-axis. Bars represent means and SD of 3 independent experiments. (C) ICN1-transduced CB cells generate cyCD3+ NK cells on coculture with the MS-5 stromal cell line. Dot-plot analysis of cyCD3 (y-axis) versus CD56 expression (x-axis) in human CD56+ cells generated on MS-5 stromal cells starting from human CD34+ CB cells either transduced with LZRS-IRES-EGFP (LIE) or ICN1 after 14 days of coculture on the MS-5 stromal cell line with the mix of Ftl-3L, SCF, IL-7, and IL-15. Note the high frequency and total numbers of cyCD3+CD56+ NK cells when the Notch pathway is triggered by ICN1. Left bar diagram shows the frequencies of cyCD3+CD56+ NK cells; far right bar diagram shows the absolute number of CD56+ NK cells (□) or cyCD3+CD56+ NK cells (■) for the cells generated from either ICN1- or LIE-transduced cells as described on the x-axis. Bars represent means and SD of 2 independent experiments. (D) DNMAML1 inhibits the generation of cyCD3+ NK cells starting from CB on OP9-DL1 cells. Dot-plot analysis of cyCD3 (y-axis) versus CD56 expression (x-axis) in human CD56+ cells generated on OP9-DL1 stromal cells starting from human CD34+ CB cells either transduced with control EGFP murine stem cell virus vector (MSCV) or DNMAML1 after 18 to 20 days of coculture with Flt-3L, SCF, IL-7, and IL-15. Note the low frequency and numbers of cyCD3+CD56+ NK cells when the Notch-CSL complex is inhibited by DNML1. Left bar diagram shows the frequencies of cyCD3+CD56+ NK cells, and the 2 far right bar diagrams show the absolute number of CD56+ NK cells (□) or cyCD3+CD56+ NK cells (■) generated from control (MSCV) or DNMAML1-transduced CB cells as described on the x-axis. Due to the large interindividual variation of the 2 independent experiments, the results are shown separately.
Notch signaling favors the differentiation of NK cells with cyCD3 expression. Numbers in the dot plots show the frequency of cells in the respective top right and bottom right quadrants. R% indicates the frequency of cyCD3+ cells within the CD56+ cell population. (A) The generation of cyCD3+ NK cells in FTOC can be inhibited by DAPT. Dot-plot analysis of cyCD3 (y-axis) versus CD56 expression (x-axis) in human CD56+ cells generated in FTOC starting from human CD34+Lin− CB cells after 14 days of culture with different concentrations of DAPT. Note the dose-dependent decrease in both the frequency and the absolute number of cyCD3+CD56+ cells when the Notch pathway is inhibited by the γ-secretase inhibitor. The left bar diagram shows the frequencies of cyCD3+CD56+ NK cells; the far right bar diagram shows the absolute number of CD56+ NK cells (□) or cyCD3+CD56+ NK cells (■) obtained in FTOC with different concentrations of DAPT as indicated on the x-axis. Bars represent means and SD of 3 independent experiments. (B) Notch signaling by DL1 favors the generation of cyCD3+ NK cells on stromal cell coculture. Dot-plot analysis of cyCD3 (y-axis) versus CD56 expression (x-axis) in human CD56+ cells generated on stromal cell line OP9-control or OP9-DL1 as indicated starting from human CD34 CB cells after 14 days of culture. Note the high frequency and absolute number of cyCD3+CD56+ cells when the Notch pathway is triggered by DL1 (OP9-DL1), and the low frequency when the DL1 signal is absent (OP9-control) or inhibited by the γ-secretase inhibitor DAPT. Left bar diagram shows the frequencies of cyCD3+CD56+ NK cells; the far right bar diagram shows the absolute number of CD56+ NK cells (□) or cyCD3+CD56+ NK cells (■) in different culture conditions as described on the x-axis. Bars represent means and SD of 3 independent experiments. (C) ICN1-transduced CB cells generate cyCD3+ NK cells on coculture with the MS-5 stromal cell line. Dot-plot analysis of cyCD3 (y-axis) versus CD56 expression (x-axis) in human CD56+ cells generated on MS-5 stromal cells starting from human CD34+ CB cells either transduced with LZRS-IRES-EGFP (LIE) or ICN1 after 14 days of coculture on the MS-5 stromal cell line with the mix of Ftl-3L, SCF, IL-7, and IL-15. Note the high frequency and total numbers of cyCD3+CD56+ NK cells when the Notch pathway is triggered by ICN1. Left bar diagram shows the frequencies of cyCD3+CD56+ NK cells; far right bar diagram shows the absolute number of CD56+ NK cells (□) or cyCD3+CD56+ NK cells (■) for the cells generated from either ICN1- or LIE-transduced cells as described on the x-axis. Bars represent means and SD of 2 independent experiments. (D) DNMAML1 inhibits the generation of cyCD3+ NK cells starting from CB on OP9-DL1 cells. Dot-plot analysis of cyCD3 (y-axis) versus CD56 expression (x-axis) in human CD56+ cells generated on OP9-DL1 stromal cells starting from human CD34+ CB cells either transduced with control EGFP murine stem cell virus vector (MSCV) or DNMAML1 after 18 to 20 days of coculture with Flt-3L, SCF, IL-7, and IL-15. Note the low frequency and numbers of cyCD3+CD56+ NK cells when the Notch-CSL complex is inhibited by DNML1. Left bar diagram shows the frequencies of cyCD3+CD56+ NK cells, and the 2 far right bar diagrams show the absolute number of CD56+ NK cells (□) or cyCD3+CD56+ NK cells (■) generated from control (MSCV) or DNMAML1-transduced CB cells as described on the x-axis. Due to the large interindividual variation of the 2 independent experiments, the results are shown separately.
Further evidence for a direct link between Notch signaling and cyCD3 expression in NK cells was found by comparative analysis of NK-cell differentiation on stromal cells. CB CD34+-derived NK cells from OP9-DL1 stromal cells display a significant higher frequency and absolute number of cyCD3+CD56+ NK cells compared with NK cells from OP9-control cells (n = 3; P < .02) or from OP9-DL1 cells in the presence of 5 μM DAPT (P < .02) (Figure 4B). This argues for Notch triggering as the underlying mechanism for the generation of cyCD3+ NK cells on OP9-DL1 cocultures.
Using molecular techniques, we further addressed the involvement of the Notch pathway in the cyCD3 expression in NK cells. ICN1, the active part of Notch 1, was retrovirally introduced in CB CD34+ progenitor cells; afterward, NK cells were generated on MS-5 stromal cells. ICN1-transduced progenitors generated a higher frequency and absolute cell number of cyCD3+CD56+ NK cells compared with cells transduced with the LIE control vector (Figure 4C) and with untransduced control cells (data not shown). This shows that ICN, which forms an active complex with CSL, is linked with cyCD3 expression in human NK cells.
To further show the importance of the ICN1-CSL complex in the generation of cyCD3+CD56+ human NK cells, we transduced the progenitor cells with DNMAML1, which inhibits activation of the ICN1-CSL-MAML1 ternary complex by displacing the active form of MAML1. Despite strong Notch stimulation during coculture with OP9-DL1 stromal cells, the progenitor cells wherein the Notch pathway was inhibited with DNMAML1 did express a significantly lower percentage of cyCD3 compared with cells transduced with a control vector (Figure 4D) or the untransduced control cells (data not shown). A similar drop in absolute numbers of cyCD3+CD56+ NK cells was observed. Data are shown separately for 2 independent experiments (Figure 4D), as the interindividual properties of the CD34 progenitor cells resulted in a large variation of generated cell numbers in both experiments.
Collectively, these data show that cyCD3 expression in human NK cells is dependent on Notch signaling.
BM CD34+ generate cyCD3+ NK cells in the presence of OP9-DL1, which is abrogated by DAPT treatment
Most PB NK cells in adults do not express cyCD3 and are thought to be derived from bone marrow–differentiated cells.19 Thus, these NK cells matured in a microenvironment that contains mainly Jagged-class Notch ligands but almost no Delta-family ligands that are responsible for T-cell development in the thymus.20 Here, we addressed the question of whether the CD34+ precursor pool from BM can be induced to generate cyCD3+ NK cells upon Notch/DL1 signaling. When cultured on OP9-DL1 stromal cells, a high frequency of cyCD3+CD56+ NK cells was generated (n = 4; 30.0 ± 17.3). Inhibition of Notch signaling on OP9-DL1 by DAPT interferes with the generation of these cyCD3+ NK cells (8.2 ± 4.6). Likewise, a low number of cyCD3+CD56+ NK cells (9.5 ± 7.5) was obtained after coculture on OP9-control cells. These results show that CD34+ progenitor cells from bone marrow have the capacity to generate cyCD3+ NK cells upon Notch signaling. The low percentage of PB cyCD3+ NK cells strongly suggests that most cells do not encounter Notch/DL signaling during their development.
Generation of cyCD3+CD56+ NK cells is dependent on transient Notch/DL1 signals during early stage of development
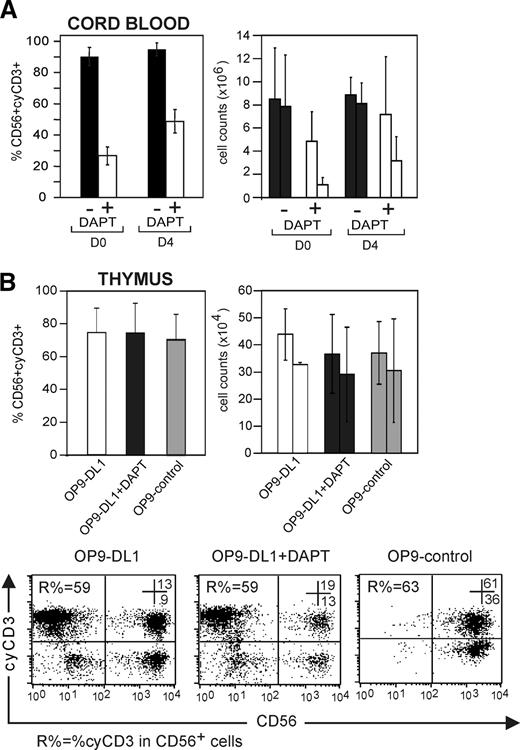
To address whether the expression of cyCD3 in NK cells is dependent on continuous Notch/DL signaling, CD34+ CB cells were either cultured on OP9-DL1 cells with SCF, Flt-3L, IL-7, and IL-15 with or without 5 μM DAPT or were precultured on OP9-DL1 stromal cells with SCF, Flt-3L, and IL-7 without IL-15 for 4 days prior to addition of IL-15 with or without DAPT. Progenitor cells that were not precultured generated cyCD3+ NK cells with an average of 90.2% ± 5.7%, which dropped to 26.7% ± 5.7% in the presence of DAPT (n = 4) (Figure 5A; right panel). Progenitor cells that were precultured for 4 days on OP9-DL1 before the addition of IL-15 generated cyCD3+ NK cells with an average of 91.9% ± 4.1%, which only dropped to 48.7% ± 7.5% in the presence of DAPT (n = 4). A similar picture was observed for the absolute number of cyCD3+CD56+ NK cells, whereas the absolute number of CD56+ NK cells was not significantly affected (Figure 5A).
Transient Notch stimulation allows generation of cyCD3+CD56+ NK cells. (A) Notch receptor triggering of CB progenitor cells by DL1 for 4 days is sufficient to generate cyCD3+ NK cells. Comparative frequencies of and numbers of cyCD3+CD56+ NK cells derived from CD34+ progenitor cells that did or did not receive a Notch signal in preculture. CD34+ CB progenitor cells received a Notch signal in vitro by preculture on OP9-DL1 cells during 4 days with Flt-3L, SCF, and IL-7. Both CD34+ CB cells without preculture (D0) and CD34+CB cells precultured on OP9-DL1 for 4 days (D4) were used to generate NK cells by coculture for 22 or 18 days, respectively, on OP9-DL1 stromal cells with Flt-3L SCF, IL7, and IL-15 in the absence or presence of DAPT. Left bar diagram shows the frequency of cyCD3+ NK cells generated from CB cells without preculture (D0) or precultured (D4) and further cultured on OP9-DL1 in the absence (■) or presence (□) of 5 μM DAPT. The right bar diagram shows the influence on the absolute number of NK cells obtained in the absence (■) or presence (□) of DAPT as indicated. Each time, the left bar indicates the absolute number of all CD56+ NK cells, whereas the right bar indicates the absolute number of cyCD3+CD56+ NK cells. Results represent means (+SD) of 2 independent experiments. (B) NK cells derived from thymic precursors express cyCD3, even without further Notch signaling. CD34+ progenitors from the thymus were cultured on OP9-DL1, OP9-DL1 in the presence of 5 μM DAPT, and OP9-control. The left bar diagram represents the frequencies of cyCD3+CD56+ NK cells. The right bar diagram represents the absolute number of NK cells in different stromal coculture conditions as described on the x-axis. Each time, the left bar indicates the number of all CD56+ NK cells, whereas the right bar indicates the absolute number of cyCD3+CD56+ NK cells. Dot plots in lower panel represent the flow cytometric analysis of cyCD3 (y-axis) versus CD56 (x-axis) of cells generated from thymic progenitor cells under different stromal coculture conditions as indicated. Numbers in the dot plots show the percentage of cells in the respective top right and bottom right quadrants. R% indicates the frequency of CD56+CyCD3+ NK cells within the CD56+ cell population. Note the high number of cyCD3+CD56+ NK cells even when the Notch pathway is not triggered by DL (OP9-control) or inhibited by the γ-secretase inhibitor DAPT. The bars represent means (+ SD) of 3 independent experiments, of which 1 is shown as a representative dot plot.
Transient Notch stimulation allows generation of cyCD3+CD56+ NK cells. (A) Notch receptor triggering of CB progenitor cells by DL1 for 4 days is sufficient to generate cyCD3+ NK cells. Comparative frequencies of and numbers of cyCD3+CD56+ NK cells derived from CD34+ progenitor cells that did or did not receive a Notch signal in preculture. CD34+ CB progenitor cells received a Notch signal in vitro by preculture on OP9-DL1 cells during 4 days with Flt-3L, SCF, and IL-7. Both CD34+ CB cells without preculture (D0) and CD34+CB cells precultured on OP9-DL1 for 4 days (D4) were used to generate NK cells by coculture for 22 or 18 days, respectively, on OP9-DL1 stromal cells with Flt-3L SCF, IL7, and IL-15 in the absence or presence of DAPT. Left bar diagram shows the frequency of cyCD3+ NK cells generated from CB cells without preculture (D0) or precultured (D4) and further cultured on OP9-DL1 in the absence (■) or presence (□) of 5 μM DAPT. The right bar diagram shows the influence on the absolute number of NK cells obtained in the absence (■) or presence (□) of DAPT as indicated. Each time, the left bar indicates the absolute number of all CD56+ NK cells, whereas the right bar indicates the absolute number of cyCD3+CD56+ NK cells. Results represent means (+SD) of 2 independent experiments. (B) NK cells derived from thymic precursors express cyCD3, even without further Notch signaling. CD34+ progenitors from the thymus were cultured on OP9-DL1, OP9-DL1 in the presence of 5 μM DAPT, and OP9-control. The left bar diagram represents the frequencies of cyCD3+CD56+ NK cells. The right bar diagram represents the absolute number of NK cells in different stromal coculture conditions as described on the x-axis. Each time, the left bar indicates the number of all CD56+ NK cells, whereas the right bar indicates the absolute number of cyCD3+CD56+ NK cells. Dot plots in lower panel represent the flow cytometric analysis of cyCD3 (y-axis) versus CD56 (x-axis) of cells generated from thymic progenitor cells under different stromal coculture conditions as indicated. Numbers in the dot plots show the percentage of cells in the respective top right and bottom right quadrants. R% indicates the frequency of CD56+CyCD3+ NK cells within the CD56+ cell population. Note the high number of cyCD3+CD56+ NK cells even when the Notch pathway is not triggered by DL (OP9-control) or inhibited by the γ-secretase inhibitor DAPT. The bars represent means (+ SD) of 3 independent experiments, of which 1 is shown as a representative dot plot.
To address whether NK cells in a later stage of differentiation can also up-regulate cyCD3 after Notch/DL1 stimulation, purified NK cells from PB were cultured in suspension or in OP9-control and OP9-DL1 cocultures. Since the frequency of the cyCD3+ NK cells did not increase in any culture condition (n = 2; 2.5 ± 0.4 [before culture]; 3.4 ± 1.1 [without stroma]; 3.5 ± 0.9 [OP9-DL1 coculture]; and 2.7 ± 0.3 [OP9-control coculture]), this implies that cyCD3+ NK cells are derived from precursor cells that even transiently received Notch signaling, but not from mature NK cells.
Thymic CD34+CD1− progenitor cells, which could have been triggered by Notch/DL in vivo, generated cyCD3+ NK cells on OP9-DL1 stromal cells (53.7% ± 5.6%; n = 3), as expected (Figure 5B). Importantly, those progenitor cells did not require further Notch/DL signaling to generate cyCD3+ NK cells, since their frequency on OP9-DL1 in presence of the γ-secretase inhibitor DAPT (56.4 ± 16.2; n = 3) or in OP9-control cocultures was not reduced (Figure 5B,C). The same goes for the absolute number of cyCD3+CD56+ NK cells generated (Figure 5B right bars). This indicates that cyCD3 expression in NK cells could serve as a marker for Notch/DL1-primed progenitor cells, as is the case in a thymus microenvironment.
Discussion
Our study demonstrates that cyCD3+ human NK cells are derived from progenitor cells that received Notch/DL signaling in the early stages of their development. This is evident from our observation that triggering of the Notch receptor on early CD34+ progenitors, either by the thymic microenvironment, where Delta-1 and Delta-4 is expressed,21 or by interaction with OP9 Delta-1–expressing stromal cells, results in a significantly higher frequency of cyCD3-expressing NK cells. In addition, this effect is inhibited by the γ-secretase inhibitor DAPT, which strongly implicates the Notch signaling pathway in the generation of NK cells expressing cyCD3. Moreover, CD34+ hematopoietic progenitor cells retrovirally transduced to overexpress ICN1 also generated a higher frequency of cyCD3+CD56+ NK cells. As expected, this is no longer inhibited by DAPT (data not shown), which acts upstream the signaling pathway by inhibiting the formation of ICN and indicates that ICN1 is directly involved. Finally, inhibition of the Notch signaling pathway by DNMAML1, which acts at the level of interaction of ICN1 with the transcription factor CSL, shows the involvement of the CSL activation complex in the cyCD3+ expression. However, the precise mechanism by which Notch activation up-regulates the expression of intracellular CD3 during NK-cell differentiation is not known. We do not think that CD3ϵ is a Notch target gene, as we were not able to induce intracellular CD3ϵ expression in mature NK cells by coculture on OP9-DL1, despite the expression of significant levels of Notch1 and Notch2 transcripts in CD56dimCD16+ and, more abundantly, in CD56brightCD16− NK cells.22 Furthermore, CD3ϵ up-regulation was shown to be part of the T-lineage specification process in mouse fetal liver precursor cells and was not directly up-regulated, as was the Notch target gene HES1.23 An alternative possibility could be that NK cells that express cyCD3 represent a population that has developed along a lymphoid pathway wherein Notch signaling is involved.
We and others have previously shown that Notch signaling facilitates human T/NK-cell precursor development and blocks B lymphopoiesis.10,12,24,25 It has been shown that human thymic lympho-myeloid precursors can develop into NK cells, but that ICN1 transduction inhibits their NK-cell potential.26 However, we have shown that partial inhibition of the Notch signaling pathway with intermediate doses of the γ-secretase inhibitor DAPT allows the generation of NK cells.12 There is considerable evidence that part of NK cells and T cells are closely related and arise from a common T/NK-cell progenitor.27 In this way, activation of the Notch pathway in early progenitors skews their differentiation toward the T/NK lymphoid lineage and allows in the presence of IL-15 the generation of NK cells that express intracellular cyCD3ϵ. In this respect, it was recently shown in mice that pro-B cells from Pax5 knock-out (KO) mice could not differentiate efficiently to NK cells on OP9 stromal cells unless they had received a transient Notch signal.28,29 These results suggest that progenitor cells that are already engaged along the B lymphoid pathway do not readily produce NK cells, even in the presence of IL-15. However, a Notch signal allows for the redirection of those cells by repressing the B-cell fate, and induces progression toward a T/NK progenitor cell.
Intracellular CD3ϵ protein has been found in freshly isolated fetal human NK cells and in NK cells derived from cultured human fetal liver and fetal thymus precursors.2,4 This is in agreement with our observations that NK-cell progenitors from the thymus or from cord blood cultured for 4 days on OP-9DL1 cells are able to produce a high number of cyCD3+ NK cells in the subsequent culture period, also in the absence of further Notch signaling. Interestingly, it has been reported by others that in murine fetal liver pluripotent hemato-lymphoid progenitors, CD3ϵ expression is induced after 3 days of Notch/DL1 signaling, concurrent with the appearance of lineage-specified cells.23 In this respect, the previous reports of cyCD3+ NK-cell clones established from human fetal liver progenitor cells4 are compatible with the presence of Notch ligands Delta-1 and 430 in the fetal liver, which could therefore deliver a Notch signal to allow the generation of cyCD3 NK cells.
The functional relevance of CD3ϵ expression in NK cells is unknown. NK-cell development and function is normal in CD3ϵ KO mice.31 Interestingly, a high copy number of a CD3ϵ transgene disrupts both T- and NK-cell development.32 In these mice, uterine NK cells are absent.33
Human NK cells are now divided into immunoregulatory CD56bright and cytotoxic CD56dim cells.34 We were not able to link these 2 subsets to the different coculture conditions on OP9 or OP9-DL1. In both conditions, we obtained CD56bright cells. The fact that after 3 weeks of culture on OP9-DL1 cells we obtained a mixture of CD56bright and CD56dim NK cells reflects the capacity of DL1 to support the expansion and maintenance of CD34 progenitor cells. Therefore, instead of a single wave of NK cells that have already become CD56bright as seen on OP9 cells, the OP9-DL1 culture represents more a mixed population of a first wave of NK cells that has already become CD56bright, and a successive population that starts later to develop and has therefore only reached the CD56dim stage. This was shown by the progressive increase of the CD56 expression when OP9-DL1–cocultured CD56dim NK cells were sorted and recultured on OP9-DL1 cells (Figure S1). When we compared the surface-marker profiles of the CD56bright and CD56dim NK cells obtained after 3 weeks of culture on OP9-DL1 cells, we noticed that the frequency of CD16, CD94, CD158, and CD335 expression is increased on the CD56bright NK cells, whereas CD161 expression is lower. This is compatible with the view that CD56bright cells have already progressed further in the NK-cell differentiation pathway and the delineation of NK-cell developmental changes within secondary lymphoid tissues as decribed.19 Although the cells we obtained are CD56bright, the NK cells display both a stronger cytolytic and cytokine-producing capacity. This argues for the fact that in OP9-DL1 conditions the NK cells become more strongly activated, as shown by CD69 expression.35 However, we were not able to correlate differences in surface expression profile, cytolytic activity, and cytokine production with the expression of cyCD3. This argues for the fact that cyCD3 reflects a DL-Notch triggering early in NK-cell development but does not influence the functional capacities. However, we cannot exclude that our culture conditions differ from the conditions in vivo, with regard to the cytokine milieu and the microenvironment, so that the cells we generated do not display the characteristics that are observed for the NK cells in vivo. In this respect, it was shown recently that CD56bright human NK cells differentiate into CD56dim cells on coculture with peripheral fibroblasts.36 Nevertheless, the existence of cyCD3+ NK cells in human fresh thymus, CB, and PB implies that the generation of cyCD3+ NK cells is not an in vitro artifact but also occurs in vivo.
It is tempting to use cyCD3 as a molecular marker of a pathway of human NK-cell development that originates in the thymus. In this respect, Di Santo et al1 has described recently a thymic pathway of mouse NK cells and identified the transcription factor GATA-3 and CD127 as molecular markers of this pathway. Those thymus-derived murine CD127+ NK cells showed, with their reduced cytotoxicity but considerable cytokine production, characteristics that are reminiscent of the human CD56brightCD16− NK-cell subset, which was also found to express CD127 and have more GATA-3 expression than human CD56dimCD16+ NK cells.1 Therefore, the authors concluded that similarly to the mouse, the human CD56brightCD16− NK-cell subset could originate in the thymus1 . However, we were not able to correlate the expression of CD127 with intracellular cyCD3 expression in NK cells that we generated on stromal coculture, nor were we able to show cyCD3 expression in CD127+ NK cells in the PB of healthy donors. Therefore, we were not able to provide evidence for a separate thymic dependent developmental NK pathway in the human CD56bright subset.
Overall, we have shown that there is an obvious link between cyCD3 expression in NK cells and Notch signaling. Nevertheless, it is possible that Notch signaling also occurs extrathymically and that cyCD3 therefore does not distinguish thymic-dependent NK cells exclusively. In this regard, NK cells develop in secondary lymphoid tissues,19 where Delta ligands are also present.8
Importantly, part of the NK-cell malignancies37 are characterized by the expression of cyCD3. In view of our results, the expression of cyCD3 could reflect that the malignant transformation occurs either in a niche which expresses DL ligands, or that oncogenic activation of Notch is involved, as is the case for most human T-cell acute lymphoblastic leukemia (T-ALL).38
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank C. Collier for animal care, C. Deboever for artwork, and B. Vandekerckhove for reading the manuscript.
This work was supported by GeconcerVeerda Onderzoeksacvie (GOA) and Fonds Wetenschappelijk Onderzoek-Vlaanderen (FWO-Vlaanderen) as well as a Belspo fellowship (to T.T.).
Authorship
Contribution: M.D.S. designed the research, performed research, and analyzed data; T.T. performed research and analyzed the data; I.V.d.W. performed research; G.D.S. performed research; G.L. designed research and analyzed the data; and J.P. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence address: Jean Plum, Ghent University, Ghent University Hospital, Department of Clinical Chemistry, Microbiology & Immunology, 4BlokA, De Pintelaan 185, B-9000 Ghent, Belgium; e-mail:jean.plum@ugent.be.