Allogeneic hematopoietic stem-cell transplantation (HSCT) is the only curative treatment for sickle cell disease (SCD); nevertheless, its use has been limited by the risk of transplantation-related mortality (TRM). Between November 1988 and December 2004, 87 consecutive patients with severe SCD ranging from 2 to 22 years of age received transplants in France. Cerebral vasculopathy was the principal indication for transplantation (55 patients). All the patients received grafts from a sibling donor after a myeloablative conditioning regimen (CR). The only change in the CR during the study period was the introduction of antithymocyte globulin (ATG) in March 1992. The rejection rate was 22.6% before the use of ATG but 3% thereafter. With a median follow-up of 6 years (range, 2.0 to 17.9 years), the overall and event-free survival (EFS) rates were 93.1% and 86.1%, respectively. Graft versus host disease (GVHD) was the main cause of TRM. Importantly, cord blood transplant recipients did not develop GVHD. No new ischemic lesions were detected after engraftment, and cerebral velocities were significantly reduced. The outcome improved significantly with time: the EFS rate among the 44 patients receiving transplants after January 2000 was 95.3%. These results indicate that HLA-identical sibling HSCT after myeloablative conditioning with ATG should be considered as a standard of care for SCD children who are at high risk for stroke.
Introduction
In recent years, progress has been made in the management of sickle cell disease (SCD) such as the prevention of pneumococcal infection, establishment of transfusion programs, and the introduction of hydroxyurea (HU) therapy.1,,,,,,–8 Studies have shown that HU has significant efficacy in reducing the frequency of painful vaso-occlusive crises (VOCs) and acute chest syndrome (ACS) episodes in some adults2 and children,3,4 although it does not significantly reduce the risk of stroke.1,2,7,8 Further advances in the management of SCD came about with the greater availability of new technologies such as transcranial Doppler (TCD) ultrasonography that can be used to identify patients at high risk for overt stroke9,,,,,–15 or magnetic resonance imaging (MRI) that can detect the presence of silent infarcts, which have been proven to have a negative effect on cognitive functioning.12 Nevertheless, patients remain at high risk for significant morbidity and early death
Hematopoietic stem cell transplantation (HSCT) is the only curative therapy for SCD, and more than 200 SCD patients have now received transplants worldwide.16,,,,,,,,–25 The French transplantation experience in this setting began in 1988,17,20,22,23 following grafting in a patient with SCD and leukemia in the United States25 and in 5 patients with SCD in Belgium.16 Here, we report the results of familial allogeneic HSCT after myeloablative conditioning in 87 consecutive young patients with severe SCD.
Patients, materials, and methods
Setting
Following the first report of 5 successful transplantations but prior to the publication of large studies of transplantation in SCD, the French group Société Française de Greffe de Moelle (SFGM) established consensual treatment guidelines to be followed by all major French transplantation centers. It was decided that transplantation would be recommended to young patients with severe SCD.
The results reported here span a period of 16 years, during which the indications for transplantation have evolved. Between 1988 and 1992, the SFGM group defined severe SCD as a history of stroke, frequent vaso-occlusive crises and/or acute chest syndrome episodes (more than 3 per year), multiple osteonecrosis, or red cell alloimmunization (more than 2 antibodies). Patients with severe residual functional neurologic impairment were excluded, but patients with hemiplegia alone were eligible. Twelve patients received transplants during this period and, to improve quality of care, the results were discussed at a workshop organized in Créteil in February 1992. Given the high rejection rate observed in the first 12 patients, addition of antithymocyte globulin (ATG) to the conditioning regimen was adopted, as was the monitoring of patients with serial magnetic resonance imaging (MRI) and chimerism studies. The transplantation criteria were also further defined; thus, after 1992 HU was proposed as the first-line treatment for patients with frequent vaso-occlusive crises and/or acute chest syndrome (VOC/ACS) episodes while transplantation was only offered in case of HU partial efficacy (ie, reduction but persistence of frequent VOC/ACS) or failure (ie, no reduction of the rate of VOC/ACS or osteonecrosis occurrence). Between 1992 and 1996, transcranial Doppler (TCD) was only used at the Créteil Sickle Cell Center. As TCD and magnetic resonance angiography (MRA) became more readily available in most centers, patients with no stroke history but with cerebral stenoses or abnormal velocities despite long-term transfusion were also considered for transplantation.
Informed consent was obtained from recipients, donors, and their parents or guardians before transplantation. The benefit-risk ratio of the procedure was thoroughly discussed with the parents (and when possible with the patients) with the help of written documents. Moreover, since 1995, families were systematically informed by a specific committee to ensure their complete comprehension of this complex matter, as required by French laws. This committee included pediatricians and psychologists who were not involved in the transplantation programs to eliminate any possible conflict of interest. In addition, a form authorizing data retrieval and analysis through computer statistical programs was completed and signed by the parents/guardians before transplantation, as mandated by the French legislation (Informatique et Liberté Act). The data were obtained in the context of consensual treatment guidelines among French transplantation centers in accordance with the Declaration of Helsinki and the French laws and regulations protecting human subjects that were in operation between 1988 and 2004.
Characteristics of the patients
Patients with SCD (hemoglobin genotype S/S or S/βo) were considered for transplantation when an HLA-identical sibling donor was available. Between November 1988 and December 2004, 87 consecutive patients (40 female, 47 male) received transplants in 14 centers (Table 1). Three of these patients had previously been included in an international study.19 The mean patient age was 9.5 years (range, 2 to 22 years). The indications for transplantation were history of stroke (35 overt strokes and 1 transient ischemic attack [TIA]), arterial stenoses detected by TCD and confirmed by conventional arteriography or MRA (6 cases), and persistent abnormal high arterial velocities despite a transfusion program (2 cases). Thirteen patients also received transplants because of severe anemia associated with cognitive deficiency and/or silent infarcts on MRI. Transplantation was indicated for multiple antierythrocyte antibodies in 2 cases. The other patients (n = 28) received transplants because of recurrent vaso-occlusive crises (more than 3 per year) and/or acute chest syndrome episodes. Seven patients who had been initially treated effectively with HU were also offered transplantation after secondary failure of HU therapy and/or development of osteonecrosis under HU. Forty-eight patients had been receiving monthly red cell transfusions before transplantation (36 for stroke, 8 for abnormal arterial velocities [6 also had stenoses], and 4 for frequent VOC/ACS or osteonecrosis). Eleven patients were receiving iron chelation. The serum ferritin values in the 87 patients ranged from 13 to 3820 μg/L (mean, 911 μg/L). Cerebral MRI showed ischemic lesions in the 35 patients with a history of overt stroke, no lesions in the patient with TIA, and silent infarcts in another 15 patients (4 also had stenoses).
Donors and stem-cell sources
The hemoglobin genotypes of the donors were A/A in 24 cases, A/S in 61 cases, and heterozygous β-thalassemia in 2 cases. Sibling donors were HLA-identical in 83 cases and, in 4 cases, had 1 HLA antigen mismatched with the recipient. ABO incompatibility was present in 24 donor-recipient couples. Stem cell source was bone marrow in 74 cases, cord blood in 10 cases, bone marrow and cord blood in 2 cases, and peripheral blood stem cells in 1 patient with the U-erythroid phenotype and alloimmunization to reduce transfusion requirements.
Transplantation procedure
Before transplantation, all patients who were not receiving long-term transfusion therapy underwent several transfusions or partial exchange transfusion to achieve a peripheral hemoglobin S level below 30%.
The first 12 patients were prepared for transplantation with a combination of busulfan and cyclophosphamide at different doses.17 In February 1992, given the observed high rate of unstable mixed chimerism and subsequent rejection (4 of 12 patients), rabbit antithymocyte globulin (ATG) (Thymoglobuline; Genzyme, Saint-Germain en Laye, France) was added to the conditioning regimen (total dose, 20 mg/kg administered in 4 equal fractions from day −6 to day −3). Furthermore, after considering the results of busulfan pharmacokinetics performed in some patients, the busulfan dose was adjusted to body area (485 mg per square meter, never less than 16 mg/kg of body weight, administered orally every 6 hours from day −10 to day −7 for a total of 16 doses). Cyclophosphamide was given at the total dose of 200 mg/kg administered at a dose of 50 mg/kg daily from day −5 to day −2. Intravenous corticosteroids (1 mg/kg) were always administered 24 hours before ATG administration. Six patients were treated in centers that opted out of ATG conditioning. After 2001, 6 patients received intravenous busulfan.
Prophylaxis of acute graft versus host disease (aGVHD) consisted of either a combination of methotrexate and cyclosporine (62 patients) or of cyclosporine alone (25 patients). Either regimen was given for at least 6 months and up to 12 months after transplantation. Due to a high incidence of neurologic complications after transplantation, the following guidelines were adopted in June 1993: anticonvulsant prophylaxis by treatment with clonazepam was initiated during busulfan administration and continued during cyclosporine therapy; arterial hypertension was strictly controlled; magnesium deficiency was promptly corrected; the hemoglobin concentration was maintained above 90 g/L and the platelet count above 50×109/L. After 2002, cyclosporine was replaced by mycophenolate mofetil in case of GVHD requiring steroid therapy. All patients received oral penicillin, given twice daily, for at least 2 years after transplantation
Chimerism studies
Hematopoietic cell chimerism was studied in 77 patients by means of restriction fragment length polymorphism or tandem repeat analysis after polymerase chain reaction amplification of DNA obtained from whole blood. In 2 centers, when the donor and recipient were sex-mismatched, in situ hybridization with a Y chromosome–specific probe was used. Chimerism was studied 1, 3, 6, and 12 months after transplantation and every year thereafter.
Statistical analysis
Overall survival (OS) and event-free survival (EFS) rates (“events” defined as death, nonengraftment, or graft rejection) were estimated by using the Kaplan-Meier method. A cumulative incidence curve was constructed for graft rejection, with deaths unrelated to rejection being considered as competing events. The following variables were included in univariate analyses: sex, age (more or less than 15 years), stem cell source, HLA matching, use of ATG, GVHD grade II or above, and date of transplantation (before or after January 15, 2000, the median transplantation time). All variables with P values below .1 were introduced in multivariate analyses. Comparisons between Kaplan-Meier probabilities used the log-rank test, while cumulative incidence curves were compared by the Gray test. Multivariate analysis was based on the Cox model with stepwise regression.
Statistical analyses were performed with the SAS 8.2 (SAS, Cary, NC) and R (R Foundation for Statistical Computing, Vienna, Austria) software packages.
Results
Engraftment
Engraftment was successful in all but 1 case. The time to absolute neutrophil count above 0.5×109/L was significantly shorter after bone marrow transplantation (BMT) compared with cord blood transplantation (CBT; mean ± SD, 21.7 ± 7.7 versus 34 ± 11, respectively; P < .001). Similarly, the platelet count reached 0.5 × 109/L sooner after BMT (day 29.4 ± 14.9) than after CBT (day 48 ± 17.8; P = .001). The only case of nonengraftment involved a girl with an HLA-identical cord blood donor who underwent gradual autologous reconstitution despite a second transplantation with bone marrow from the same donor (then 13 months old).
Rejection
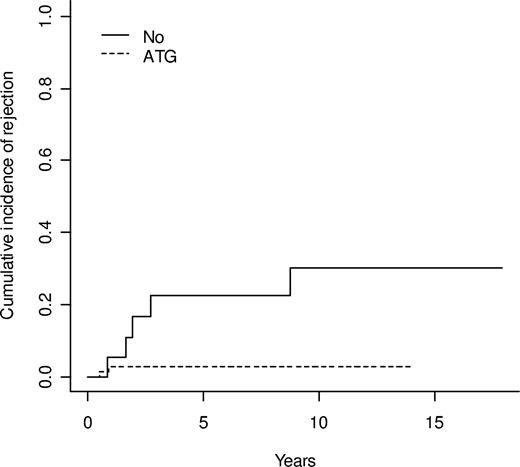
Six rejections occurred at 5, 11, 15, 23, 28, and 100 months after transplantation and 1 nonengraftment was also observed. The overall cumulative incidence of rejection was 7.0% at 5 years (Figure 1). The conditioning regimen had a significant effect on the 5-year cumulative incidence of rejection, which was 2.9% in patients who received ATG and 22.6% in those who did not (P = .002, Gray test) (Figure 2). Five of the 18 patients who did not receive ATG as part of their conditioning had mixed unstable chimerism. One patient, with 50% mixed chimerism, developed aplastic anemia 15 months after transplantation and successfully underwent a second transplantation while the other 4 patients had a gradual decrease in the percentage of donor cells, resulting in autologous reconstitution. One of these patients successfully received a retransplantation. Among the 69 patients who received ATG, 2 rejections with autologous reconstitution were observed; 1 occurred very early and was considered as nonengraftment (see above) while the other occurred 11 months after transplantation in a patient with mixed chimerism and a low level of donor cells (30%) 2 months after transplantation.
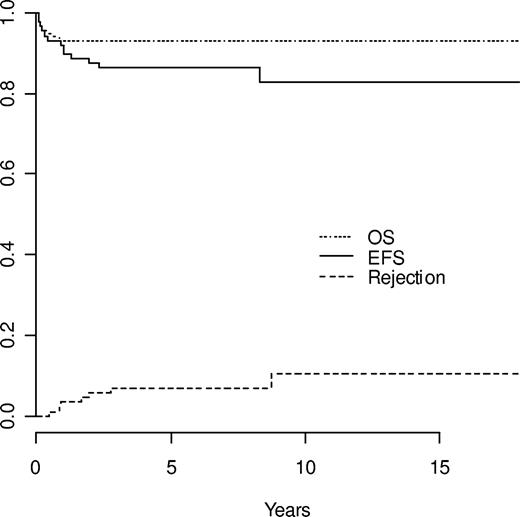
Estimated outcomes: OS, EFS, and cumulative incidence of rejection in the 87 patients.
Estimated outcomes: OS, EFS, and cumulative incidence of rejection in the 87 patients.
Comparison of the cumulative incidence of rejection in patients conditioned with and without antithymocyte globulin (ATG)-at 5 years 2.9% with ATG versus 22.6% without (P = .002).
Comparison of the cumulative incidence of rejection in patients conditioned with and without antithymocyte globulin (ATG)-at 5 years 2.9% with ATG versus 22.6% without (P = .002).
Survival
Median follow-up of survivors was 6 years (range, 1.6 to 17.5 years). The 6 transplantation-related deaths that occurred were due to sepsis during aplasia in 1 patient, cerebral hemorrhage following successful engraftment on day 32 in a patient with pretransplantation Moya-Moya disease, and GVHD in 4 patients at 2, 4, 12, and 12 months after transplantation. The estimated 5-year transplantation-related mortality (TRM) rate was 6.9%, with no events occurring after the first 12 months after transplantation (Figure 1). No death was observed after the 40th transplantation or after CBTs. In univariate analysis, the only factors significantly influencing survival were the date of transplantation (before or after the median transplantation time [January 15th 2000]; P = .01), age (more or less than 15 years; P = .002), ATG (P < .001), and aGVHD (grade II or above; P < .001). In multivariate analysis, date of transplantation (P < .001), ATG (P < .001), and aGVHD (hazard ratio, 11.7; 95% confidence interval [CI], 1.36 to 100.59; P = .05) remained as significant independent prognostic variables.
Graft versus host disease
Among the 86 assessable patients, 17 (20%) developed aGVHD grade II or above (10 grade II, 11.6%; 5 grade III, 5.8%; and 2 grade IV, 2.3%). In univariate analysis, the incidence of aGVHD was increased in patients older than 15 years (P = .003) and in recipients of HLA-mismatched grafts (P = .007). The incidence of aGVHD grade II or above was 23% in bone marrow recipients (median age, 9.3 years) and 0% in CB recipients (n = 12) (median age, 6.7 years), but the difference was not significant (P = .16). Cumulative incidence of chronic GVHD (cGVHD) was 12.6%. Among the 83 patients assessable for cGVHD, mild forms were observed in 9 patients (11%) and extensive forms were observed in 2 patients (2.4%). None of the CB recipients experienced cGVHD (P = .23).
Event-free survival
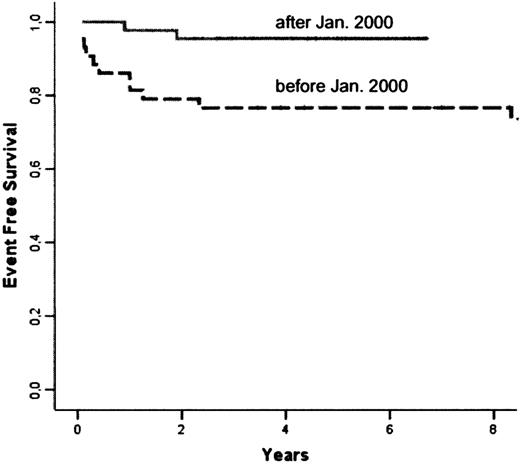
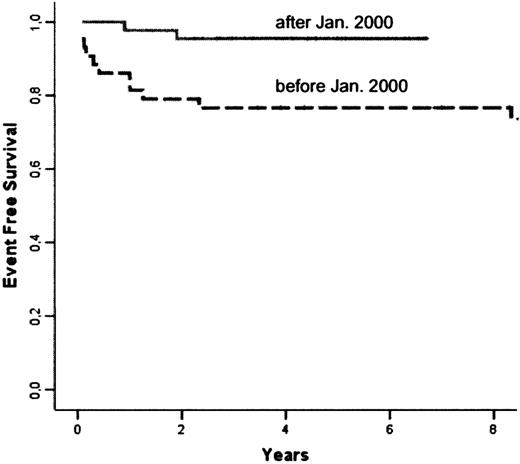
The following events occurred: 6 deaths, 1 case of nonengraftment, and 6 cases of rejection. The Kaplan-Meier estimate of overall EFS at 5 years was 86.1% (Figure 1). Univariate analysis showed a significant influence of the date of transplantation (before or after January 2000; P = .026), grade II or above aGVHD (P = .008), and cGVHD (P < .001). Multivariate analysis showed that the date of transplantation was the only significant risk factor, while other variables did not add any further information. The 5-year EFS rate was 95.3% in patients receiving transplants after January 15, 2000 compared with 76.7% in patients receiving transplants before (hazard ratio after 2000 as compared with before, 0.177; 95% CI, 0.039 to 0.809; Figure 3).
Comparison of Kaplan-Meier estimates of EFS in patients receiving transplants before (n = 43) and after January 2000 (n = 44): respectively 76.7% and 95.3% at 5 years (P = 0.26).
Comparison of Kaplan-Meier estimates of EFS in patients receiving transplants before (n = 43) and after January 2000 (n = 44): respectively 76.7% and 95.3% at 5 years (P = 0.26).
Nucleated cell chimerism
Nucleated cell chimerism was assessable in 77 patients (59 and 18 patients conditioned with and without ATG, respectively). Chimerism was categorized as follows: total donor chimerism (more than 95% donor cells), rejection (less than 5% donor cells), or mixed chimerism subdivided into low (5% to 49% donor cells) or high (50% to 95% donor cells). The use of ATG significantly influenced the degree of chimerism observed in the patients (Figure 4; P = .03 at 12 months after transplantation, P = .002 at 24 months after transplantation). Low mixed chimerism was significantly more frequent in patients prepared for transplantation without ATG (P = .04 at 12 months and P = .002 at 24 months after transplantation), whereas high mixed chimerism was more frequently observed in patients prepared with ATG (P = .03 at 12 months and P = .001 at 24 months after transplantation) and remained stable over time (Figure 4). No significant influence of ABO incompatibility was found on the rate of rejections or mixed chimerisms.
Chimerism data. The percentage of patients in each category of chimerism (0% to 5%D, 5% to 50%D, 50% to 95%D, 95% to 100%D) with comparison of patients prepared without ATG (left) or with ATG (right).
Chimerism data. The percentage of patients in each category of chimerism (0% to 5%D, 5% to 50%D, 50% to 95%D, 95% to 100%D) with comparison of patients prepared without ATG (left) or with ATG (right).
Organ function following transplantation
Spleen function.
Howell-Jolly bodies disappeared from peripheral blood smears of 70 patients of the 81 still alive 2 years after transplantation and remained present in the other patients (4 with splenectomy, 4 with autologous reconstitution, and 3 with donor engraftment). Splenic uptake was seen on colloid scintigraphy in all 4 patients who underwent this test after transplantation.
Osteonecrosis.
Transplantation-related neurologic toxicity.
Sixteen patients (24%) had seizures. Factors associated with seizures were the use of steroids to treat GVHD (11 of 16 patients; P = .003) and arterial hypertension (10 of 11 assessable; P < .001). However, because a strong interaction between steroids, aGVHD, and arterial hypertension was found, multivariate analysis could not be performed. A history of cerebrovascular disorders, age, and the date of transplantation were not significant risk factors. Reversible posterior leukoencephalopathy due to cyclosporine toxicity was observed in 7 patients— with seizures (6 of 7), cortical blindness (5 of 7), headache, coma or transient loss of conscience, and typical posterior abnormalities on CT or MRI. None of these patients had sequelae.
Outcome of cerebral vasculopathy.
Among the 36 patients with a pretransplantation history of stroke, only 2 had a recurrence of stroke. One patient experienced a TIA on day 10 after transplantation while the other, who had severe cerebrovascular disorders with Moya-Moya disease, had fatal intracranial hemorrhage on day 32 after transplantation. With a median follow-up of 6 years, the risk of recurrence was 5.6%. Vascular occlusions persisted, and the outcome of stenoses was variable: 5 stenoses resolved, 16 remained unchanged, and 2 stenoses continued to progress (data not shown). However, no strokes or silent ischemic lesions occurred in patients with successful engraftment, including those with progressive cerebrovascular disease. Cortical atrophy worsened in 2 cases. Arterial velocities were significantly reduced (P < .001) 1 year after transplantation in the 49 assessable patients (from 138 (± 50) cm/sec at baseline to 100 (± 34) cm/sec in the right middle cerebral artery [MCA] and from 138 (± 46) cm/sec to 103 (± 40) cm/sec in the left MCA). Arterial velocities normalized 3 months after transplantation in 2 patients who had abnormal velocities before transplantation, despite a long-term transfusion program.
Growth and development.
Two years after transplantation, the mean height was unchanged (0.5 ± 1.2 SD), whereas weight had significantly improved (0.05 ± 1.6 SD). Seven female patients (range, 13 to 22 years of age) who were postpubertal at the time of transplantation developed amenorrhea with low serum estradiol and elevated luteinizing hormone (LH) and follicle-stimulating hormone (FSH) levels during the year following transplantation, necessitating hormone replacement therapy. Most of the girls who were not pubertal at transplantation required hormone therapy to develop secondary sexual characteristics when they reached a bone age of 13 years. However, 2 girls receiving transplants at 6.4 and 8.3 years of age spontaneously underwent normal puberty. Pretransplantation ovarian cryopreservation was performed by celioscopy in 6 cases. All the male patients had testosterone and FSH and LH levels in keeping with their bone age and pubertal status.
Present situation.
The patients' mean age is 16.2 years (range, 6.1 to 28 years). Among the 6 patients who rejected their grafts, 2 have received a successful second transplantation. The other 4 patients with autologous reconstitution had high, protective hemoglobin F levels for several months or years,17,20 and only 2 of them have had SCD symptom recurrence and are now on HU therapy. Among the 81 patients still alive, 77 have the same hemoglobin profile as their donor by electrophoretic analysis (ie, 0% HbS in the case of an A/A or heterozygous β–thalassenic donor and 30% to 50% HbS in the case of an A/S donor), and 75 of 77 have hemoglobin levels above 100 g/L. The mean Hb levels at baseline before and 1 year after transplantation were 72 (± 12) g/L and 116 (± 15) g/L, respectively, and the corresponding white blood cell counts were 14.7 × 109 (± 4.9 × 109) and 6.9 × 109 (± 3.1 × 109)/L. Only 2 patients, who are siblings and have low but stable donor chimerism (20%), have moderate anemia (85 and 90 g/L), with the same hemoglobin profile by electrophoretic analysis as their donor and no SCD symptoms. These 2 siblings had different sibling donors. Seventy-nine patients have not experienced vaso-occlusive crises or acute chest syndrome episodes and have not been given transfusions since engraftment. Seven patients have mild cGVHD with a 90% Karnofsky or Lansky score, and none has extensive cGVHD. The 2 patients who rejected the grafts and are currently on HU therapy have an 80% Karnofsky or Lansky score. The Lansky score was 80% in 5 patients and 90% in 9 others because of stroke-related hemiparesis or hemiplegia sequelae. All other 58 patients have a 100% Karnofsky or Lansky score.
Discussion
This series of 87 patients represents the largest cohort of SCD patients having received transplants and has the longest follow-up time (at least 2 years). The OS and EFS rates are 93.1% and 86.1%, respectively. The EFS probability in this series is similar to the values reported by Vermylen et al21 and Walters et al24 (82% and 84%, respectively) (Table 2). Notably, our cohort included higher-risk patients, such as 10 patients older than 15 years and 4 patients who received a graft from a familial donor with 1 HLA antigen mismatch. The most important new finding is that our results improved markedly in recent years, with a 95.3% EFS rate among our last 47 patients. The overall mortality rate among our patients (7%) must be interpreted with respect to the prognosis without transplantation.27,28 A recent study of SS/Sβ0 children28 showed an overall mortality rate of 0.4 per 100 patient-years, with 6.4% of patients dying before the age of 18 years. Thus, HSCT does not expose children to a higher risk of death than SCD itself while offering radically improved quality of life.
Contrary to HSCT for hematologic malignancies, the risk of mixed chimerism, rejection, and nonengraftement is high in SCD because of the patients' normal immunocompetence, highly proliferative bone marrow, and immunization by multiple transfusions. The addition of rabbit ATG to the conditioning largely prevented graft rejection, reducing the 5-year cumulative incidence of rejection from 22.6% to 2.9%. It is also notable that the 7 patients who rejected their graft or experienced nonengraftment nonetheless survived the procedure and that the 5 patients who did not receive a second transplantation benefited from high HbF levels for long periods. The use of ATG was associated with an increased frequency of mixed but stable chimerism. As previously reported,29,30 low levels of donor chimerism were sufficient to improve severe SCD. These results are in keeping with recently published data31 showing that, compared with AS erythroblasts, SS erythroblasts are at a competitive disadvantage and that low levels of donor (AS) erythroid engraftment result in donor cell predominance among erythroblasts and erythrocytes.
The conditioning regimen was well tolerated. Among the 6 deaths, only 1, due to sepsis during aplasia, was related to conditioning, and no cases of veno-occlusive disease occurred. Considering the very high risk of rejection after nonmyeloablative procedures,32 we believe that only myeloablative transplantations should be used in children without organ failure. The principal complication was GVHD, which caused 4 deaths. There was a significant relation between GVHD and older age, suggesting that HSCT should be performed before the age of 15 years. This also points to the need for procedures that minimize the risk of GVHD without affecting engraftment. The use of CB is compatible with these requirements.33,34 The absence of severe aGVHD grade II or above and cGVHD in the 12 patients who received related CBT is noteworthy. Thus, like others,35 we think that sibling cord blood cryopreservation should be systematically offered to families. Preimplantation genetic diagnosis of SCD and HLA typing could be useful in this setting.36
This series confirms and extends previous reports on cerebral vasculopathy.20,22–24 Early transplantation-related neurologic toxicity has been reported in this setting.37 In our study, despite preventive measures, seizures and posterior leukoencephalopathy, albeit reversible, remained a particularly frequent adverse effect of cyclosporine and steroid therapy. In contrast, the outcome of preexisting cerebrovascular disorders was highly favorable after transplantation. In particular, there were no cases of ischemic stroke and only 1 intracranial hemorrhage, whereas the risk of stroke recurrence is about 10% among patients on a transfusion program.38,39 Among our patients with successful donor engraftment, including those with persistent stenosis or thrombosis, no new silent ischemic lesions were observed on MRI after transplantation, showing that the most important risk factor for stroke is SCD itself and not the associated vascular disease. The rapid normalization of arterial velocities in 2 patients whose values had remained abnormal despite a 3-year transfusion program is the first such report and suggests that HSCT is more effective than transfusion for cerebrovascular disorders. Several phenomena might contribute to this effect, such as a different rheology of AA/SS double populations compared with AS red cells, persistent hemolysis during transfusion, and endothelial repair by transplanted stem cells.
Two girls receiving transplants before 8 years of age had spontaneous functional ovarian recovery. Nevertheless, as ovarian failure remains a concern after myeloablative conditioning, female patients should be offered ovarian cryopreservation when possible, because this procedure has recently been shown to be effective.40,41 Six of our female patients had this procedure without complications. All boys had normal testosterone and FSH and LH levels after transplantation.
Since 2000, no deaths and only 2 graft rejections have occurred among 44 patients receiving grafts (1 in a patient who did not receive ATG). These excellent results can be attributed to several factors, such as greater experience, the use of cord blood, and blood product filtration. Transplantation was also offered sooner to patients who were identified early by TCD as having cerebrovascular disorders. The favorable effect of HU therapy in some patients, and the improvement in the results of HSCT over the years, gradually led us to modify our indications for transplantation. Overt stroke causes irreversible lesions that compromise motor and cognitive function.12 The possibility of diagnosing cerebrovascular disorders by means of transcranial Doppler has improved the early detection of stenosis and primary stroke prevention by transfusion programs (Stroke Prevention trial by transfusions in sickle cell disease, [STOP 1] study).11 It therefore seems reasonable to propose HSCT when stenosis is detected, before stroke occurs.14 Furthermore, the STOP 2 study showed that, even in patients with normalized velocities after a 30-month transfusion program, discontinuation of transfusion resulted in a high rate of stroke and reversion to abnormal velocities15 while HSCT prevents stroke recurrence and, as shown in this study, significantly reduces abnormal velocities. This also encourages the use of HSCT as soon as abnormal velocities are detected by transcranial Doppler examination.
Since January 2000, 5 patients over 15 years of age have successfully received transplants; although this experience is limited, it suggests that stem cell grafting could also be proposed to young adults42 with nonsevere disease during childhood but developing severity criteria such as a tricuspid regurgitant jet velocity of at least 2.5 m per second43 during adulthood.
For SCD patients without related donors, the use of unrelated cord blood would be a logical perspective.44 However, a recent review of experience in the United States, in which unrelated 4 HLAs out of 6 HLA-matched cord bloods were used, reports rather disappointing results in terms of GVHD and rejection rates.45 Cord blood banking must therefore be encouraged in this setting to increase the possibility of finding a well-matched unrelated donor, which currently is very low.46 Alternative donors such as HLA-haplomismatched family members, with T-cell depletion of the graft,47 have been used in 5 children with a history of stroke and multiple alloimmunization, hindering the use of a transfusion program. Young patients qualifying for transplantation but lacking an HLA-identical donor are likely to be the first candidates for gene therapy in the near future.48
Considering the hope to cure 95% of SCD children with genoidentical HSCT, this therapeutic approach should be discussed early with families, especially as soon as a long-term transfusion program becomes necessary.
Presented in part at a plenary session of the 44th annual meeting of the American Society of Hematology, Philadelphia, PA, December 8, 2002.
An Inside Blood analysis of this article appears at the front of this article.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We sincerely thank all the patients and their families for their participation in this study, all the nurses on the various transplantation units and outpatient clinics for their dedicated work, and the practitioners who referred patients for transplantation. We thank Drs Martine Torres and Thomas D. Coates for their help in reviewing the manuscript.
Authorship
Contribution: As corresponding author, F.B. designed and initiated the study; had full access to all the data in the study; participated in selection of the patients, the transplantations, and follow-up of patients; had final responsibility for the decision to submit for publication; and participated in the analysis, interpretation of results, manuscript writing, review, and submission. G.S., M.K., and E.G. were involved in study design, the transplantations, and writing of the manuscript. S.C. contributed to data analysis, carried out the statistical analysis with F.B., and assisted in the writing the manuscript. M.D. was involved in study design and the transplantations and contributed to data analysis and the writing of the manuscript. Y.B., J.-P. Vannier, K.Y., I.T., P.B., A.F., P.L., J.-L.S., H.E., N.D., E.P., L.C., G.M., and J.-P. Vernant were involved in the study design and the transplantations. S.V. was involved in MRI/MRA, TCD data analysis, and interpretation and writing the manuscript. D.B. was involved in chimerism data analysis and interpretation. All the authors participated in the review of the manuscript.
All participating institutions in the Société Française de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC) study group are listed in the affiliations note on the title page of this article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Françoise Bernaudin, Centre de Référence de Drépanocytose, Hôpital Intercommunal de Créteil, 40 avenue de Verdun, 94010, Créteil, France; e-mail:francoise.bernaudin@chicreteil.fr.