We performed a randomized trial comparing the use of quantitative DNAemia versus positive antigenemia for starting preemptive antihuman cytomegalovirus (HCMV) therapy in hematopoietic stem-cell transplantation (HSCT) recipients. In the DNAemia arm, antiviral therapy was initiated on reaching a DNAemia cut-off of 10 000 DNA copies/mL of whole blood, whereas in the antigenemia arm, therapy was started in the presence of a positive antigenemia. The aim of the study was to compare the number of patients treated in the 2 arms. On the whole, 178 patients (89 in each arm), receiving unmanipulated HSCT from either a relative or an unrelated donor, completed the study. Although the incidence of HCMV infection was comparable in DNAemia and antigenemia arms (34% vs 42%, respectively, P = .259), the number of patients treated was significantly lower in the DNAemia arm (18% vs 31%, P = .026). No patient developed HCMV disease. The use of a DNAemia cut-off avoids unnecessary antiviral treatment.
Introduction
Preemptive antiviral therapy, based on the administration of drugs on detection of virus in blood, has been largely used to prevent the occurrence of human cytomegalovirus (HCMV) disease in patients given allogeneic hematopoietic stem-cell transplantation (HSCT).1 This strategy offers the advantage of treating only those patients experiencing HCMV infection and who are, thus, at risk of developing overt disease.2,–4 Two assays have been mainly used during the last decade for early detection of HCMV in blood: antigenemia (detection of HCMV pp65 phosphoprotein in peripheral blood leukocytes)5,,–8 and DNAemia (HCMV DNA detection in either leukocytes, plasma, or whole blood).3 In view of the high risk of fatal HCMV-related pneumonia in HSCT recipients, preemptive therapy for HSCT recipients is currently started on the first confirmed appearance of virus in blood, irrespective of the assay used.3,8,9
We investigated, in a randomized study involving young patients, a different approach based on the comparison for preemptive therapy of a predefined cut-off for DNAemia with the first antigenemia positivity. The DNAemia cut-off was chosen based on a retrospective analysis of DNAemia levels in HSCT recipients undergoing antigenemia-guided HCMV surveillance.10,11
Patients, materials, and methods
From December 2002 to July 2006, a total of 184 patients were given an unmanipulated allogeneic HSCT from either a human leukocyte antigen (HLA)-identical sibling or an unrelated donor (UD) at the Oncoematologia Pediatrica, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Policlinico San Matteo, Pavia, Italy. Inclusion criteria for enrollment of patients in the study were as follows: serologic evidence of past HCMV infection in either donor (D) or recipient (R) or both, and either patient's or parents' written informed consent obtained in accordance with the Declaration of Helsinki. Overall, 178 patients (89 in each arm) were included in the study. Patients' characteristics are detailed in Table 1. The primary end point was the comparison of the number of treated patients in the 2 arms. Approval was obtained from the Fondazione IRCCS Policlinico San Matteo Institutional Review Board and ethics committee for these studies.
The number of patients to be enrolled was calculated by a sample size evaluation method based on the χ2 test. The minimum number of patients to be randomized was 84 per arm, based on a significance level of 0.05, a study power of 0.80, and the hypothesized need for treatment in 40% patients allocated to the antigenemia arm9 and 20% patients allocated to the DNAemia arm.
Patients were randomized using a block random design on the basis of donor type (namely HLA-identical sibling vs UD). In the antigenemia arm, patients were treated upon detection of either 2 or more pp65-positive leukocytes (see below) or first confirmed positivity. In the DNAemia arm, patients were treated upon reaching a DNA level of 10 000 copies/mL of blood, a threshold chosen in view of previously obtained results.10,11
Intravenous ganciclovir (5 mg/kg twice a day) was administered as preemptive therapy in both arms and stopped upon virus clearance from blood. In the case of drug toxicity, foscarnet (90 mg/kg twice a day) was given as replacement for ganciclovir.
All patients were monitored for HCMV infection until 3 months after HSCT by collecting blood samples twice weekly until day + 45 and weekly thereafter. Subsequently, HCMV infection was monitored in all patients at least at scheduled controls at 6, 9, and 12 months and in the presence of clinical signs or symptoms.
Quantitation of viral DNA in whole blood for patients allocated to the DNAemia arm was performed using real-time polymerase chain reaction (PCR).12 The antigenemia assay was evaluated by counting the number of leukocytes positive for pp65/2 × 105 cells examined, according to a previously described procedure13 and more recently standardized procedure.14 In a subgroup of 48 patients, HCMV-specific CD4+ and CD8+ T-cell reconstitution was monitored as reported.15
Data were analyzed as of March 1, 2007. Differences between medians were compared using the Mann-Whitney U test. Differences in percentages were tested using the Pearson χ2 test. All tests were two-tailed. The probabilities of HCMV infection and need for preemptive therapy were calculated and expressed as cumulative incidence, taking into account competing risks.16,17
Results and discussion
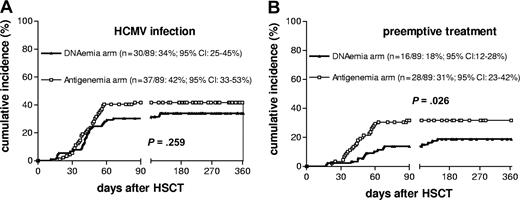
The median follow-up of surviving patients enrolled into this study was 23 months (range, 7-49 months). For at least 1 year after allograft, 129 patients were observed; they represented 88% of patients still alive. Thirty of the 89 HSCT recipients (34%) enrolled in the DNAemia arm and 37 of 89 patients (42%) enrolled in the antigenemia arm developed HCMV infection (P = not significant). The cumulative incidence curves (Figure 1A) showed a probability of HCMV infection at day + 360 of 34% and 42% in the DNAemia and antigenemia arms, respectively. Median time to first HCMV detection was comparable in the 2 arms (43 days, range, 11-118, in the DNAemia arm; and 43 days, range, 19-85, in the antigenemia arm).
Cumulative incidence of HCMV infection and preemptive treatment in the 2 randomization arms. (A) HCMV infection. (B) Preemptive treatment. Numbers in parentheses indicate the actual number of infected (A) or treated (B) patients and the relevant probability at day + 360 with 95% confidence intervals (CI).
Cumulative incidence of HCMV infection and preemptive treatment in the 2 randomization arms. (A) HCMV infection. (B) Preemptive treatment. Numbers in parentheses indicate the actual number of infected (A) or treated (B) patients and the relevant probability at day + 360 with 95% confidence intervals (CI).
In multivariate analysis, the only factor significantly associated with the occurrence of HCMV infection in the whole population was recipient HCMV seropositivity (data not shown).
Twenty-three patients who had DNAemia levels consistently below the cut-off level in the relevant arm, or negative test on a second control after a first positive result in the antigenemia arm (ie, patients with self-resolving infection), were not treated. The remaining 44 patients were treated according to criteria already detailed. As shown in Table 2, the number of patients treated was significantly lower in the DNAemia arm (P = .037). The cumulative incidence curves (Figure 1B) of the probability of starting preemptive treatment at day + 360 was 18% in the DNAemia arm and 31% in the antigenemia arm (P = .026). In addition, treatment onset was significantly delayed in the DNAemia arm compared with antigenemia arm (Table 2). All of the other parameters investigated were comparable in the 2 arms, as well as 100-day cumulative incidence of grade II-IV acute graft-versus-host disease (GvHD) (27% in the DNAemia and 29% in the antigenemia arm) and 1-year transplantation-related mortality (3% in the DNAemia and 7% in the antigenemia arm). No cases of HCMV disease were observed in either arm during the study period.
Upon initiation of treatment, patients allocated to the DNAemia arm had median levels of both viral DNAemia (17 900, range 10 100-62 800 copies/mL of blood) and antigenemia (48, range 0-300 pp65-positive leukocytes) significantly higher (P < .001) than those in the antigenemia arm: 0 (range 0-32800) DNA copies/mL of blood and 3 (range 1-75) pp65-positive leukocytes.
HCMV-specific CD4+ and CD8+ T-cell reconstitution was available for 48 patients,15 equally distributed between the 2 randomization arms. Sixteen were HCMV-seronegative before transplantation and were transplanted from a seropositive donor. None of them developed HCMV infection; HCMV-specific T-cells appeared within the first 3 months in 4 of them. All 32 HCMV-seropositive patients (25 of whom developed HCMV infection) reconstituted HCMV-specific T-cell immunity after a median time of 36 (range 27-153) days for the CD8+ T-cell compartment and 47 (range 27-153) days for the CD4+ T-cell compartment. No difference in time to specific immune recovery was found between the 2 arms.
Results of our study indicate that (i) a DNAemia cut-off can be safely used to guide preemptive therapy for HCMV infections in HSCT recipients, as no case of HCMV disease was observed in the study population; (ii) adoption of a cut-off of 10 000 copies/mL of blood significantly decreases the number of patients candidate to preemptive treatment with respect to antigenemia with advantages in terms of toxic effect and drug cost reduction and no disadvantages in terms of GvHD occurrence and transplantation-related mortality; and (iii) treatment inception was significantly delayed in the DNAemia arm, thus in a situation where hematologic and immunologic recovery is expected to be more advanced.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the technical staff of the Servizio di Virologia for performing the assays, Daniela Sartori for preparing the manuscript, and Laurene Kelly for revision of the English in this manuscript.
This work was supported in part by grants from the Ministero della Salute-Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Policlinico San Matteo (Ricerca Corrente grant 80 541 and Ricerca Finalizzata 2003 grant 89 269) and Fondazione Cassa di Risparmio delle Provincie Lombarde (CARIPLO) (grant 93 005) (G. Gerna) and by grants from Associazione Italiana Ricerca sul Cancro (AIRC), Consiglio Nazionale delle Ricerche (CNR), Ministero dell'Università e della Ricerca Scientifica e Tecnologica (MURST), the European Union (FP6 program ALLOSTEM), and Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Policlinico San Matteo (F.L.).
Authorship
Contribution: D.L., G. Gerna, and F.L. designed and performed the research, collected and analyzed the data, and wrote the paper; M.F., M.E.B., and S.T. collected the data; G. Giorgiani performed the research; F.B. designed the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Giuseppe Gerna, Servizio di Virologia, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Policlinico San Matteo, via Taramelli 5, 27100 Pavia, Italy; e-mail:g.gerna@smatteo.pv.it.