In this issue of Blood, Quintarelli and colleagues demonstrate that cytotoxic T cells engineered with cytokines, a selectable marker, and a suicide gene exhibit improved growth, enhanced antitumor functions, and an increased safety profile suitable for clinical immunotherapy.
The most effective form of immunotherapy currently available for patients with solid tumors is adoptive-cell transfer (ACT).1 ACT involves the isolation of tumor-specific T cells, their activation and expansion to large numbers ex vivo, and their readministration to the patient. The addition of a lymphodepleting preparative regimen and exogenous cytokine support such as interleukin (IL)–2 improves the antitumor effects of the transferred lymphocytes.2 About half of patients with refractory metastatic melanoma respond to ACT therapy, and nasopharyngeal carcinomas and some lymphomas of viral etiology are also responsive to this treatment.3 The 2 major hurdles to the wider application of ACT therapy are an inability to produce lymphocytes that react with common epithelial tumors, and the poor in vivo function and persistence of lymphocytes after extensive in vitro culture.
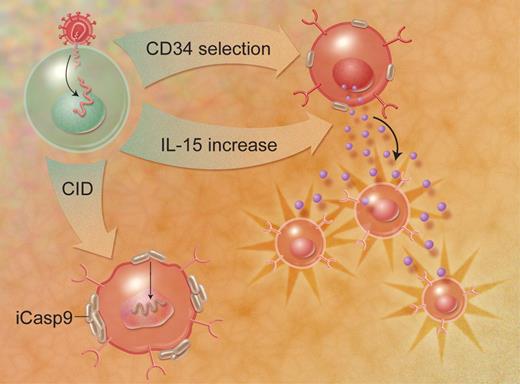
Quintarelli and colleagues sought to improve the function of cultured lymphocytes for ACT by engineering tumor-reactive T cells to express the cytokines IL-2 or IL-15. Previous investigators have demonstrated an in vitro growth advantage of human lymphocytes transduced with cytokine genes, but Quintarelli and colleagues added 2 clever twists, in the form of a selectable marker gene and an inducible suicide gene (refer to accompanying figure). All 3 genes were encoded by a single retroviral transcript and expressed as individual proteins using a 2A polypeptide “ribosomal skip” mechanism,4 ensuring that all 3 gene products were expressed in roughly equal proportions. By combining 3 functional genes in 1 retroviral payload, the authors make this a sophisticated and clinically relevant gene-therapy package with novel implications for use in clinical trials.
Three-gene transduction allows selection of a homogeneous CD34+ population, enhanced growth via autocrine IL-15 production, and elimination of aberrant cells through an inducible caspase-9 gene. Illustration by A. Y. Chen.
Three-gene transduction allows selection of a homogeneous CD34+ population, enhanced growth via autocrine IL-15 production, and elimination of aberrant cells through an inducible caspase-9 gene. Illustration by A. Y. Chen.
Epstein-Barr virus (EBV)–specific human T cells were transduced with retroviruses encoding the 3 genes. The marker gene was a truncated version of CD34, which is expressed on the cell surface but does not transmit an intracellular signal. After transduction, a pure population of EBV-specific, CD34+ lymphocytes was obtained (using clinically applicable reagents) and the transduced cells were then tested in a variety of in vitro and in vivo functional assays. The growth of the IL-2– or IL-15–transduced cells was cytokine independent when stimulated with cognate antigen in vitro. Furthermore, the transduced T cells were more effective in a mouse model of tumor therapy, with increased proliferation at the tumor site and persistence for extended periods in vivo in the absence of exogenous cytokines. Finally, a third transduced gene was the inducible caspase-9 (iCasp-9) gene, which could be used to ablate the transduced cells in the event that T-cell mutants with uncontrolled growth arose. Administration of a pharmacological dose of a chemical inducer of dimerization (CID) caused the aggregation and activation of the iCasp-9 gene product, leading to apoptosis of the transduced cells and the elimination of cytokine production in vitro and in vivo.
The article concludes by advocating for clinical trials to evaluate the use of cytokine gene-transduced lymphocytes in ACT. Indeed, such clinical trials are already approved and underway for retroviral vectors encoding a single IL-2 gene, but this elegant combination of 3 genes expressed from a single retroviral transcript brings a new level of research interest and clinical safety to this gene-therapy approach. This multigene strategy could also be applied to other genetic payloads like T-cell receptors (TCRs). TCR-transduced lymphocytes can be retargeted to recognize tumor antigens, and can mediate the regression of metastatic cancer when administered to patients.5 Cytotoxic T cells that are cotransduced with a selectable marker, iCasp-9, and TCR genes could be retargeted to react with self-proteins that are overexpressed by tumors but also expressed by normal tissues—antigens like p53, Her2/neu, telomerase, or CEA—and might be used for the development of safe and effective ACT for common epithelial cancers.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■