Abstract
Although chemotherapy can induce complete responses in patients with chronic lymphocytic leukemia (CLL), it is not considered curative. Treated patients generally develop recurrent disease requiring additional therapy, which can cause worsening immune dysfunction, myelosuppression, and selection for chemotherapy-resistant leukemia-cell subclones. Cellular immune therapy promises to mitigate these complications and potentially provide for curative treatment. Most experience with this is in the use of allogeneic hematopoietic stem-cell transplantation (allo-HSCT), in which graft-versus-leukemia (GVL) effects can be observed and shown responsible for long-term disease-free survival. However, use of allo-HSCT for CLL is limited because of the lack of suitable donors and the treatment-related morbidity/mortality for elderly patients, who constitute the majority at risk for developing this disease. The GVL effect, however, suggests there are specific CLL-associated antigens that could be targeted in autologous cellular immune therapy. Effective strategies for this will have to overcome the disease-related acquired immune deficiency and the capacity of the leukemia-cell to induce T-cell tolerance, thereby compromising the activity of even conventional vaccines in patients with this disease. We will discuss the different strategies being developed to overcome these limitations that might provide for effective cellular immune therapy of CLL.
Introduction
Chronic lymphocytic leukemia (CLL) is a CD5+ B-cell malignancy that is considered incurable. Historically, the first-line treatment consisted of alkylating agents, which resulted in response rates in up to 70% of patients, but did not improve survival.1 Treatment regimens with the nucleoside (purine) analogs, such as fludarabine monophosphate (F-ara-A) or pentostatin, were found to yield higher response rates and to provide for longer progression-free survival,2 especially in combination with cyclophosphamide.3 However, despite effecting increased complete response rates, treatment with purine analogs alone does not appear to improve survival.2 Newer treatment combinations have incorporated use of monoclonal antibodies to chemotherapy.4,5 Although treatment with such combinations might provide for a first-time-observed survival benefit,6 such therapy still is not considered curative
Although most patients initially respond to these chemotherapeutic approaches, many develop therapy-resistant disease with repeated therapy. Patients with refractory disease more frequently have leukemia cells that harbor deletions in the short arm of chromosome 17 (17p−), which often is associated with loss of functional p53, or in the long arm of chromosome 11 (11q−) in and/or around the gene encoding the ataxia telangectasia (ATM), which is a kinase required for p53 function.7,8 Moreover, many chemotherapy-refractory patients have leukemia cells that have lost functional p53. Because the cytoreductive activity of most chemotherapy agents requires functional p53,9,10 loss of p53 is thought to result from selection of chemotherapy-resistant leukemia-cell subclones.11,12 This has stimulated interest in treatment regimens that are cytotoxic for leukemia cells via mechanisms independent of p53 function.
Allo-HSCT
Autologous hematopoietic stem-cell transplantation (auto-HSCT) following high-dose chemotherapy can result in molecular remissions in more than two-thirds of patients with CLL. However, these remissions are not durable.13,14 In contrast, long-term clinical remissions can be achieved following allogeneic HSCT (allo-HSCT), In allo-HSCT, the relapse incidence decreases over time, not only following myeloablative conditioning,15,16 but also following nonmyelotoxic reduced intensity conditioning.17-19 Evidence that this clinical effect is due to an ongoing graft-versus-leukemia (GVL) effect in CLL is obtained from the following observations: (1) the relapse risk is reduced in the presence of chronic graft-versus-host disease (GVHD),20 (2) the relapse risk is increased when T-cell–depleted allografts are used,21 and (3) donor lymphocyte infusions (DLIs) following allo-HSCT can effect reductions in tumor burden.17,21,22
The existence of a GVL effect is further supported by a study from Ritgen and colleagues, who measured the kinetics of minimal residual disease (MRD) following reduced intensity conditioning allo-HSCT.23 Although the conditioning regimen did not eliminate overt disease, durable remissions were observed between 100 and 200 days following allo-HSCT in patients who had GVHD or who received DLI following transplantation.
Donor-derived cytotoxic T cells (CTL), the main effectors of the GVL effect, kill target cells both through death receptor ligation and exocytosis of cytotoxic granules. Although sensitivity to ligation of death receptors depends partly on the function of p53, cytotoxic granules can effect leukemia-cell killing in a p53-independent manner.24,25 This might explain why patients with CLL cells that lack functional p53 apparently can experience a significant therapeutic benefit from the GVL response following allo-HSCT.19,23,26 Based upon these findings, a consensus guideline initiated by an international allo-HSCT expert panel states that all eligible patients who have CLL cells lacking functional p53 should be considered for allo-HSCT, preferably as early as possible.27
Although allo-HSCT, which can be considered the most clinically advanced approach of cellular immunotherapy, is a very effective tool in the treatment of CLL, widespread use is hindered by treatment-specific limitations. First, the treatment-related mortality (TRM) of allo-HSCT is considerable. Following myeloablative conditioning, the TRM varies between 17% and 44%,15,16,28,29 and although reduced intensity conditioning significantly lowered the TRM, it still varies between the 15% and 22%.17-20 Since the mean age of patients in these studies varied between 53 and 56 years, it might be expected that transplantation-related toxicity would be higher in the majority of (mostly elderly) patients with CLL. Besides mortality, many patients suffer from transplantation-related morbidity, mainly a result of GVHD.17-19 Furthermore, there is a lack of suitable donors for many patients, particularly the elderly, who constitute the majority of patients with CLL.
Nevertheless, the GVL effect implies that CLL can be effectively targeted in a cellular immune response. This has inspired development of strategies that obviate the use of allogeneic donor cells and allow for stimulation of autologous immune cells against putative CLL-associated antigens. There are several cellular immunotherapeutic strategies under development that will be reviewed in this article (Table 1).
Obstacles for cellular immunotherapy
The CLL cells from each patient share expression of a monoclonal immunoglobulin protein, which bears idiotypic determinants that can be targeted by the immune system. In addition, there are several tumor-associated antigens, including fibromodulin, MDM2 (murine double minute 2), survivin, and KW-13,33,38-40 which are expressed in CLL. However, most of these potential CLL-associated antigens have a low immunogenicity. For example, although cytotoxic T-cell responses can be generated against idiotypic peptides, cytotoxic immune responses generally are weak and/or have limited capacity to effect tumor-cell clearance.33,41
Another problem is that of inducing CTL antitumor responses in CLL. First, the CLL cells lack expression of immune costimulatory molecules and hence function poorly at antigen presentation. In addition, CLL cells can induce changes in T cells that can mitigate their capacity to respond to cellular antigens. Compounding this is the disease-related immune deficiency of patients with CLL, which potentially will be impaired further by chemotherapy, rendering patients less able to marshal an immune response to even conventional vaccines, let alone vaccines intended to elicit immune responses against weak tumor-associated antigens. A variety of strategies are being developed to overcome these limitations (Table 1).
Improving the capacity of CLL to function as effective antigen-presenting cells
Although CLL cells express major histocompatibility complex (MHC) class I and II, CD54 (ICAM-1), CD27, and CD40, they have a low to absent expression of important immune costimulatory molecules, such as B7–1 (CD80) and B7–2 (CD86).42 A little over a decade ago, it was first discovered that interaction of CD40 with its ligand, CD154 (normally expressed on T cells), induces expression of immune costimulatory molecules and enhances the antigen-presenting capacity of normal and neoplastic B cells both in mouse and in man.43 This has formed the basis of studies to detect whether the antigen-presenting function of CLL cells could be optimized by CD40/CD154 interactions. In summary, CD40 triggering increased the expression of a variety of immune accessory molecules, such as adhesion molecules (CD54) and costimulatory molecules (CD80, CD86, CD70) (Figure 1).42-44 CD40-activated CLL B cells could induce proliferation of allogeneic or autologous CD4+ and CD8+ T cells,42,45,46 and different groups showed cytotoxicity of both primed allogeneic or autologous T cells against CD40-activated CLL cells in vitro.42,45-47
Effects of CD40 activation of CLL cells on susceptibility to apoptosis induction. CD40 stimulation results in increased expression of adhesion and costimulatory molecules, thereby activating autologous T cells, which results in increased expression of death receptor ligands and IL-21. Next, CD40-mediated expression of death receptors and IL-21 receptor results in increased susceptibility to nonspecific immune effector–mediated apoptosis, possibly by cleavage of the proapoptotic protein Bid. Bid expression on CD40 activation is mediated by c-Abl–dependent expression of p73. CD40-activated CLL cells are more sensitive to chemotherapy, which could be mediated by p73.
Effects of CD40 activation of CLL cells on susceptibility to apoptosis induction. CD40 stimulation results in increased expression of adhesion and costimulatory molecules, thereby activating autologous T cells, which results in increased expression of death receptor ligands and IL-21. Next, CD40-mediated expression of death receptors and IL-21 receptor results in increased susceptibility to nonspecific immune effector–mediated apoptosis, possibly by cleavage of the proapoptotic protein Bid. Bid expression on CD40 activation is mediated by c-Abl–dependent expression of p73. CD40-activated CLL cells are more sensitive to chemotherapy, which could be mediated by p73.
To date, 2 clinical studies based upon CD40 activation of CLL cells have been reported. In the first study, a replication-defective adenovirus (Ad) vector was used to transduce CLL B cells to express the mouse CD40-ligand (CD154). Following single reinfusion of autologous, AdCD154-transfected tumor cells, both the transduced cells and bystander CLL cells were stimulated to express immune costimulatory antigens, such as CD80 and CD86, indicating that CD40/CD154 interactions had occurred in vivo. There were no dose-limiting toxicities. Although none of the treated patients experienced a complete remission following a single infusion of autologous, transfected CLL cells, several of the treated patients had significant clinical responses, including rapid falls in leukemic cell counts and reductions in the sizes of lymph nodes and spleen. Also, treatment increased the numbers of CD4 and CD8 T cells found in the blood.31
In a separate study, 8 patients received subcutaneous injections of autologous CLL B cells that had been transfected to express CD154 and interleukin-2 (IL-2) using an Ad construct. The investigators noted that the treated patients had a transient rise in the numbers of T cells capable of producing interferon-gamma (IFN-γ), granzyme B, and/or IL-5. More importantly, 3 of the 8 treated patients experienced reductions in the sizes of affected lymph nodes by more than 50%.30 Collectively, these studies indicate that CD40-based immunotherapy might be effective in CLL.
Since both studies used adenoviral vectors, it could be possible that part of the inflammatory response is caused by pre-existing immunity against Ad proteins. Indeed, it has been shown that host immune responses against adenoviral vectors include the production of proinflammatory cytokines such as IFN-γ48 and the expansion of (adenoviral-specific) CTLs.49 Although an in vivo antiadenoviral response could have contributed to the clinical effects of both studies, enzyme-linked immunospot (ELISPOT) assays and autologous mixed lymphocyte reactions (MLRs) indicated that the infusion of Ad-CD154-CLL cells also increased the numbers of T cells that produce IFN-γ in vitro in response to autologous CLL cells.31 These in vitro studies used CD40-activated CLL stimulator cells that were not infected with Ad, indicating that infusion of Ad-CD154-CLL cells induced immune responses against leukemia cells independent of Ad antigens.
Another possible explanation for the clinical efficacy of AdCD154 gene therapy could be that the cells were transfected with potentially immunogenic murine CD154. In a current phase 1 clinical trial a novel replication-defective Ad encoding a humanized, functional, membrane-stable CD154 is used.50 Although the results are preliminary, the infusion of autologous CLL cells transfected with the human CD154 construct seems as effective as infusion of tumor cells transfected with the murine construct.
Besides up-regulation of immune costimulatory molecules, CD40 activation acutely can enhance the survival of normal or neoplastic B cells by induction of expression of antiapoptotic proteins,51,52 including Bfl-1, Bcl-xL, and Mcl-1.53-58 Since in vitro studies showed that CD40 activation also could enhance the resistance of CLL cells to chemotherapeutic drugs,59-61 some concerns were raised about the safety of CD40-based immunotherapy. However, several recent studies showed a multifaceted influence of CD40 activation on apoptosis regulation of CLL cells, paradoxically enhancing the sensitivity of the leukemia cells to apoptosis induced by a variety of different mechanisms (Figure 1).
When measured over time, the induced expression of genes encoding antiapoptotic proteins following CD40 ligation are transient.62 In contrast, CLL cells are induced to express death receptors and the proapoptotic BH3-only molecule Bid for weeks after CD40 ligation.58,63 Bid is a key regulator of apoptosis by facilitating the cross-talk between mitochondrial-dependent, apoptosis-inducing pathways and death receptors.64 Also, expression of Bid in other cancers has been associated with increased sensitivity to anticancer drugs.65-67
We found that the induced expression of Bid following CD40 ligation is mediated by the induced-expression of the alpha-isoform of p73 (TAp73alpha), a member of the p53 family (Figure 1).68 The DNA-binding regions of this protein have greater than 60% amino-acid sequence homology with that of p53. Under physiologic conditions, p73 protein levels are kept low by rapid degradation via the ubiquitin-proteasome pathway. However, the transactivating full-length (TA) p73 rapidly accumulates in response to genotoxic stress.69 Overexpression of TAp73 results in increased expression of genes encoding death receptors70 and proapoptotic proteins,71 thereby sensitizing cells to chemotherapeutic agents.72,73 Importantly, TAp73 can mediate apoptosis independent of functional p5374,75 and can sensitize p53-deficient tumors to chemotherapy.76-79
In line with these observations, we showed that the cytotoxic activity of various anticancer drugs, such as F-ara-A, was enhanced for CLL cells following CD40 ligation, particularly for CLL cells that lacked functional p53. Specific blocking of c-Abl tyrosine kinase, a kinase that stabilizes TAp73 by phosphorylation,80,81 inhibited the capacity of CD40 ligation to induce high-level expression of TAp73 as well as Bid. This also was associated with the inhibition of the capacity of CD40 ligation to enhance the sensitivity of CLL cells to anticancer drugs. That TAp73 was involved in enhancing the sensitivity of CLL cells to chemotherapeutic agents was supported by studies showing that transduction of CLL cells with an Ad encoding TAp73 also could enhance the sensitivity of CLL cells to F-ara-A, particularly in CLL cells lacking functional p53.68 These results indicate that CD40 activation can sensitize leukemia cells to apoptosis via induction of TAp73 and restore the sensitivity of p53-deficient CLL cells to anticancer drugs that ordinarily require functional p53 for their cytotoxic activity. It follows that patients with dysfunctional p53 might benefit from AdCD154 gene therapy prior to treatment with cytotoxic agents that require functional p53 and/or p73.
CD40 activation also can sensitize CLL cells to clearance by innate immune effector mechanisms. A role for the innate immune system in the effects of CD154 gene therapy was suspected by the very rapid reductions in leukemia-cell counts and lymph node size observed in patients several days after intravenous infusion of CD154-expressing autologous leukemia cells.31 The rapidity of this response makes it appear unlikely that the early clinical effects of this treatment reflect an adaptive immune response. Instead, it appears that the rapid clearance of CLL cells following infusion of CD154-expressing cells might be due to innate immune effector mechanisms. Important in this regard was the observation that CD40-ligation induces CLL cells to express the extrinsic death receptors Fas (CD95) and death receptor 5 (DR5).44,63 In addition, autologous, activated T cells could mediate killing of CD40-activated CLL cells in a non-MHC–restricted fashion through a mechanism that could be inhibited by anti-Fas antibodies.53 The fact that anti-Fas antibodies could inhibit such killing was curious in that nascent activated CLL cells appeared to be resistant to apoptosis mediated solely by ligation of CD95.53,58-60 However, activated T cells and natural killer (NK) cells often coexpress both the ligand for CD95 (CD178) and tumor necrosis factor apoptosis-inducing ligand (TRAIL), the ligand for DR5. We found that coligation of both CD95 and DR5 on CD40-activated CLL cells could induce apoptosis of CLL cells that had been activated less than 24 hours earlier via CD40 ligation.63 Over time, CLL cells become increasingly sensitive to CD95- and/or DR5-mediated apoptosis following CD40 ligation. The enhanced latent sensitivity to Fas-mediated apoptosis appears linked to the induced expression of Bid.53,82 Of note, we could show that both NK cells and activated CD4+ T cells of patients with CLL expressed ligands for these death receptors (Figure 1),63 allowing them to kill autologous, CD154-transduced leukemia cells in vitro and potentially also in vivo in patients treated with CD154-expressing CLL cells.31
Another mechanism that might account for the rapid clearance of leukemia cells following CD40 activation could be caused by IL-21.83 Following CD40 activation, CLL cells express the receptor for IL-21 and become increasingly sensitive to the cytotoxic effects of this cytokine (Figure 1). Moreover, IL-21 could induce Bid-dependent apoptosis in CLL cells following CD40 ligation. Since CD4+ T cells produce IL-21 upon activation84 IL-21 could induce apoptosis of CD40-activated CLL cells and thereby also play a role in mediating the acute cytoreduction of leukemia cells following CD154 gene therapy.
Although these findings point to nonspecific effector mechanisms, there is also evidence that CLL cells transduced to express CD154 can induce an autologous antigen-specific T-cell response.38 Since some patients included in the phase 1 clinical trial experienced a long-lasting remission (J. E. Castro, and communications, September 2004), the therapeutic potential of this approach could involve recruitment of both innate and adaptive immune effector mechanisms.
These data encourage further development of CD154-based immunotherapy. An important question to be answered is whether combination with other molecules/drugs will further optimize its clinical effectiveness. In vitro, expression of CD154 (an early costimulatory molecule) and OX40L (a later-acting costimulatory molecule) could enhance development of antitumor responses over that induced by expression of either of these molecules alone.85 Another strategy to boost the effectiveness of this approach could be use of CD154 in combination with treatment with exogenous IL-2 (a cytokine that stimulates expansion and maturation of activated T cells).30,86
A different method to increase efficacy of (CD154-based) immunotherapy might be to combine such treatment with drugs that specifically target proteins responsible for the resistance of tumor cells to apoptosis. This approach conceivably could enhance the therapeutic activity of weak immune effector mechanisms while still relying on the development of an immune response for antitumor specificity. A promising target protein is the X-linked inhibitor of apoptosis protein (XIAP). XIAP blocks the execution phase of apoptosis through direct inhibition of the effector caspase-3 and/or caspase-7 and prevents initiation of the intrinsic caspase activation cascade by directly inhibiting the apical caspase-9.87 CLL cells express high levels of XIAP,88,89 rendering them relatively resistant to death receptor–mediated90 or granzyme-mediated apoptosis.91 We observed that inhibitors of XIAP could work synergistically with death receptor ligation to kill CD40-activated CLL cells in vitro.92 Conceivably, such XIAP inhibitors could enhance the effectiveness of CD154-immune gene therapy.
Current clinical approaches based on infusion of ex vivo transfected CLL cells are complicated and require access to large numbers of circulating tumor cells. An alternative approach is to inject virus vectors directly into the lymph nodes that are typically effaced by infiltrating leukemia/lymphoma cells. This could result in transduction of neoplastic B cells in vivo, allowing for the in situ formation of leukemia/lymphoma vaccines, thereby obviating ex vivo transduction. Encouraging results were observed in murine models,93 and clinical studies currently are being planned at the University of California, San Diego.
Improving T-cell function and activation
A number of well-characterized defects have been described in the circulating T cells of patients with CLL. CLL cells express immune-modulating factors, including transforming growth factor (TGF)–β, IL-10, and IL-4.94-96 These cytokines skew the immune system toward a T helper 2 (Th2)–type response, thereby suppressing T-cell activation, expansion, and effector functions by HLA class I–presented antigens.94,96-98 Furthermore, the T cells of patients with CLL express significantly lower levels of CD154 than the T cells of healthy individuals.99 Microarray analyses of CD4+ and CD8+ T cells of patients with CLL revealed that they had altered expression of genes compared with that of normal T cells. The CD4+ T cells had aberrant expression of genes involved in cell differentiation. The CD8+ T cells had aberrant expression of genes encoding proteins involved in cytoskeleton formation, vesicle trafficking, or cell-mediated cytotoxicity.100 An active role for the malignant cells in this process was suggested by the observation that coculture of T cells from healthy donors with (allogeneic) CLL cells induced the T cells to have similar gene expression profiles as the T cells of patients with CLL.100
With the goal of achieving a specific antitumor response, several attempts have been made to overcome T-cell dysfunction in CLL. One such approach is based on ex vivo activation of autologous T cells. In this system, called Xcellerate,32 T cells from patients with CLL were obtained by leukapheresis and cultured in the presence of magnetic beads coated with mAbs against CD3 and CD28 in the presence of IL-2 (reviewed by Wierda and collegues101 ). A phase 1/2 dose-escalation clinical trial with Xcellerated T cells in patients with CLL resulted in dose-dependent increases in blood T-cell counts and improvements in absolute neutrophil and platelet counts and hemoglobin levels. However, reductions in blood leukemia cell counts were not seen.32
A more specific approach to activate T cells against tumor-associated antigens involves the use of “heteroclytic peptides” that mimic the peptides of defined tumor-associated antigens. Heteroclytic peptides have binding activities for the MHC class I molecule that are greater than those of the native peptides derived from a target tumor-associated antigen. As such, these peptides can be presented by a given MHC molecule more effectively than the native peptide. Such peptides can be engineered so that the MHC-bound heteroclytic peptide appears the same to T cells as do the MHC-bound peptide derived from the tumor-associated antigen. As such, instead of the low immunogenicity of the native peptides derived from tumor-associated antigens,102 heteroclytic peptides can be strongly immunogenic and capable of inducing CTLs that have activity not only for cells presenting the heteroclytic peptide but also tumor cells presenting the native peptide derived from the tumor-associated antigen.41,103 Promising peptides to be used for this approach in CLL might be the shared Ig framework region (FR)–derived peptides. Since subsets of patients with CLL share similar antigen receptors,104,105 peptides corresponding to these shared idiotypic determinants could be used as a shared idiotypic vaccine that could be used in more than 1 patient. Recent studies showed that autologous CTLs stimulated with FR-derived heteroclytic peptides efficiently kill CLL cells expressing even very weakly binding native peptides,33,34 and hold high promise for vaccination strategies.
A third approach is to induce a tumor-specific T-cell response using dendritic cells (DCs). DCs can activate the immune system against antigens that usually fail to elicit a protective immune response in the host. This applies in particular to tumor-associated antigens for which there might not be many reactive T cells either because of deletion or anergy (reviewed by Rivoltini106 ). For CLL, several reports described potent in vitro activation and expansion of T cells by coculture with DCs pulsed with either apoptotic tumor cells, tumor cell lysate, tumor cell RNA, or idiotype antigens.107-111 So far, 1 clinical trial using DCs in CLL have been reported.35 In this study, 9 patients with CLL in early-stage disease were vaccinated by intradermal injections with allogeneic monocyte–derived DCs. The DCs were pulsed with either tumor cell lysates or apoptotic bodies prior to injection. This treatment resulted in a transient decrease in lymphocyte numbers soon after injection in most patients. Almost 2 years after treatment, no significant changes were found in lymphocyte counts and/or sizes of lymph nodes, spleen, or liver.35
Although injection of patients with ex vivo–stimulated autologous T cells or antigen-pulsed blood-derived DCs caused the blood T-cell counts to increase, patients who were treated with either approach did not have sustained reductions in blood lymphocyte counts or lymph node size. This is despite the fact that the latter study showed expansion of T cells specific for the putative tumor-associated antigen RHAMM/CD168.35 As such, it seems that the activity of these T cells and/or their induced numbers were insufficient to sustain a clinical benefit.
A promising approach might be not to focus on tumor-specific T cells, but to take advantage of virus-specific CTLs. The immune system has developed means with which to detect and reject virus-infected cells. Specifically, cytomegalovirus (CMV) infection induces expansions of CMV-specific CD8+/CD45RA+/CD27−/CD57+ T cells with cytotoxic activity for autologous CMV-infected cells.112 In the elderly, such CMV-specific T cells can comprise up to 15% of the total blood T-cell population.113 We demonstrated that patients who are seropositive for CMV and who have CLL have increased relative and absolute numbers of CD8+ T cells with this phenotype relative to that of age-matched control subjects.114 This was independent of any evidence for active viremia. As such, it appears that patients with CLL have functional CMV-specific effector CTLs even though they have the T-cell defects described at the beginning of this paragraph.
Recently, we found that these CMV-specific CD8+ cells could be cytotoxic for autologous CLL cells pulsed with the appropriate HLA class I–restricted CMV peptide.36 Importantly, such T cells could kill even CLL cells that lacked functional p53 (R. Mous, A.P.K., unpublished data). These CD8+ cells do not require ex vivo (re)stimulation and are active against peptide-pulsed autologous CLL cells immediately after their isolation from blood.36 Conceivably, such T cells could be used for the immunotherapy of CLL.
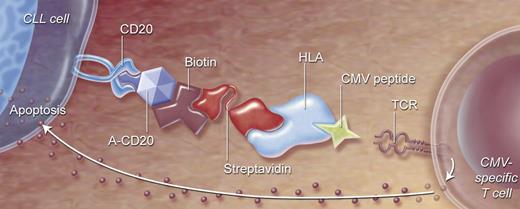
One way of directing T cells against autologous CLL cells is to link effector cells and target CLL cells using recombinant HLA class I/viral peptide complexes coupled to an antibody against tumor-associated cell-surface antigens. Already, studies have demonstrated that CD20-expressing tumor cells incubated with anti-CD20 viral HLA class I/peptide complexes could be lysed very efficiently by the blood mononuclear cells of healthy donors, in vitro prestimulated with viral antigen.115 In vivo studies in mice showed that anti-CD20–HLA-A2/influenza peptide complexes that targeted lymphoma cells could provide protective antilymphoma activity when inoculated together with influenza-specific CTLs.116 In a recent study, we targeted CLL cells with complexes consisting of a streptavidin fused anti-human CD20 single-chain variable fragment coupled to CMV peptide–loaded biotinylated HLA class I (Figure 2). CLL cells coated with this targeting complex (TC) were lysed by autologous CMV-specific CTLs with efficiency similar to that of CLL cells directly loaded with CMV peptide.37 Furthermore, we demonstrated that TC-coated CLL induced both proliferation and production of IFN-γ, tumor necrosis factor alpha (TNF-α), and macrophage inflammatory protein-1β in CMV-specific CD8+ T cells.37
Directing CMV-specific T cells to CLL cells by a 2-step antibody targeting system. (1) Anti-CD20 construct and HLA class I molecules presenting viral peptide will be connected by strong streptavidin-biotin interaction, forming a targeted complex (TC). (2) Anti-CD20 will bind to CD20-expressing cells (like CLL), and HLA/CMV peptide will bind to specific T-cell receptors. CMV-specific T cells will be activated, resulting in specific killing of target cells.
Directing CMV-specific T cells to CLL cells by a 2-step antibody targeting system. (1) Anti-CD20 construct and HLA class I molecules presenting viral peptide will be connected by strong streptavidin-biotin interaction, forming a targeted complex (TC). (2) Anti-CD20 will bind to CD20-expressing cells (like CLL), and HLA/CMV peptide will bind to specific T-cell receptors. CMV-specific T cells will be activated, resulting in specific killing of target cells.
CMV-specific CD8+ cells are promising effector cells for use in clinical cellular immunotherapy studies. They are proven to be cytotoxic, not autoreactive and capable of homing in a broad variety of tissues.117 Since 70% to 90% of healthy adults are CMV+, CMV-based approaches should be widely applicable.
Conclusions
Although CLL cells apparently can suppress immune function, this disease should be amenable for immune therapy. Encouraging, long-term disease-free survival has been observed for patients who have received allo-HSCT, due in part to the capacity of the allograft to recognize CLL-associated antigens, accounting for the GVL effect. The presence of such antigens indicates that it might be possible to induce effective autologous cellular immune therapy. Such therapy will have to overcome factors responsible for the acquired immune deficiency of patients with this disease and the capacity of leukemia cells to effect cellular immune tolerance. Strategies have been developed for overcoming these factors, which already have yielded encouraging biologic effects and clinical responses. Conceivably, CLL, a disease noted for its capacity to cause immune deficiency, may one day yield to strategies that activate the immune system to effect curative cellular immune therapy of this disease.
Acknowledgments
This work was supported by a “Veni” grant from ZonMw (The Netherlands Organization for Health Research and Development) to A.P.K.
Authorship
Contribution: A.P.K., M.H.J.v.O., and T.J.K. wrote the paper.
Conflict-of-interest disclosure: T.J.K. is a scientific advisor to Memgen. The other authors declare no competing financial interests.
Correspondence: Arnon P. Kater, Department of Hematology (F4–224), Academic Medical Center, University of Amsterdam, Meibergdreeg 9, 1105 AZ Amsterdam, the Netherlands; e-mail: a.p.kater@amc.uva.nl.