In this issue of Blood, Obara and colleagues report on their use of a GFP transgene to mark kidney Epo-producing cells in mice. These cells in the interstitial space express neuronal markers, and their number correlates with plasma Epo levels and increases with hypoxic induction.
Erythropoietin (Epo), the primary regulator of erythropoiesis, acts in this capacity by binding to its receptor on the surface of erythroid progenitor cells to stimulate cell survival, proliferation, and differentiation. Epo is produced principally in the liver during fetal gestation and in the kidney after birth. Targeted deletion in mice of Epo or its receptor leads to embryonic death due to severe anemia. Epo production is thought to be regulated primarily by Epo gene transcription.1 Using a 180-kb Epo transgene with a green fluorescent protein (GFP) reporter, Obara et al recapitulate tissue-specific, hypoxia-inducible transgene expression in kidney and liver tissue and identify kidney Epo-producing cells as peritubular interstitial cells.
Known Epo gene-regulatory elements include binding sites for hypoxia-inducible factor (HIF) and for hepatocyte nuclear factor-4 (HNF-4). The importance of the HIF protein in regulation of Epo production was recently underscored by the discovery of a gain-of-function mutation in the HIF2α subunit leading to familial erythrocytosis.2 Mutation of the transgene by Obara et al indicates that tissue-specific expression is also regulated, in part, by GATA-box–mediated repression. Previous efforts using transgenes with these regulatory elements were useful in characterizing Epo gene regulation and localizing Epo expression,1 but did not fully recapitulate endogenous Epo expression.
Renal Epo-producing interstitial cells exhibit dendritelike processes and express neural-specific markers. Although the range of Epo expression per cell has not yet been determined, the number of Epo-producing cells correlates with plasma Epo concentration, supporting the hypothesis that Epo production by a subset of interstitial cells is regulated in an on/off fashion.3 Hypoxia and anemia, due to bleeding, increased Epo and GFP expression in the kidney. The number of renal Epo-expressing cells also increased, further indicating that Epo induction was not exclusively through increased Epo expression in those interstitial cells already expressing Epo and marked by GFP. Whether local variation in oxygen tension or some other factor induces Epo-producing cells in the unstressed microenvironment, and the extent of heterogeneity of Epo expression per cell, are issues that remain unresolved. In the liver, Epo-producing hepatocytes lack neural markers, are evident by embryonic day 9.5, persist through birth, and are again detected in adult liver after hypoxia or anemic stress. Further investigation to determine the extent to which endogenous human Epo expression parallels these observations of mouse Epo in the kidney and liver is warranted.
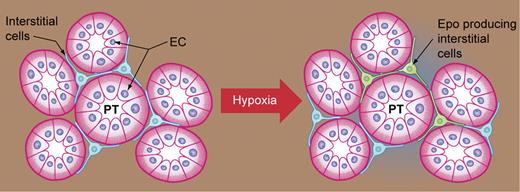
Hypoxia has been found to increase the number of Epo-expressing cells. Peritubular interstitial cells (blue), with long projections between the proximal tubules (PT) formed by epithelial cells (EC), are induced to express Epo (green) by hypoxia or anemic stress. Illustration by Kenneth Probst.
Hypoxia has been found to increase the number of Epo-expressing cells. Peritubular interstitial cells (blue), with long projections between the proximal tubules (PT) formed by epithelial cells (EC), are induced to express Epo (green) by hypoxia or anemic stress. Illustration by Kenneth Probst.
Increasing evidence suggests physiologic function of Epo beyond erythropoiesis. For example, Epo expression in the brain, and its apparent neuroprotective activity in culture and animal models, have led to investigation of the use of Epo therapy for ischemic stroke.4 Localization and enumeration of Epo-expressing cells in the brain under normal and ischemic conditions would offer insight into endogenous Epo activity in this organ. Obara and colleagues observe GFP-expressing cells only in the kidney and liver: either additional sequences are required for Epo production in other tissues, or GFP expression is below the level of their detection method. Increased understanding of the nature and function of Epo-producing cells in the kidney, liver, and other organs may be useful in broadening cell-type specificity for new therapeutic strategies designed to enhance endogenous Epo expression.5 Examination of the GFP transgenic mouse may also provide new insight into factors that affect endogenous Epo production, such as the fate of kidney Epo-producing cells in renal disease.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health