Abstract
There has been a remarkable explosion of knowledge into the molecular defects that underlie the acute and chronic leukemias, leading to the introduction of targeted therapies that can block key cellular events essential for the viability of the leukemic cell. Our understanding of the pathogenesis of the myelodysplastic syndromes (MDSs) has lagged behind, at least in part, because they represent a more heterogeneous group of disorders. The significant immunologic abnormalities described in this disease, coupled with the admixture of MDS stem or progenitor cells within the myriad types of dysplastic and normal cells in the bone marrow and peripheral blood, have made it difficult to molecularly characterize and model MDS. The recent availability of several, effective (ie, FDA-approved) therapies for MDS and newly described mouse models that mimic aspects of the human disease provide an opportune moment to try to leverage this new knowledge into a better understanding of and better therapies for MDS.
Introduction
When writing a review on the myelodysplastic syndromes (MDSs) for the readers of Blood, the overriding challenge is how to make it interesting for those “outside” the myeloid malignancies field but also provocative enough for those investigating or treating patients with this disease. The availability of several FDA-approved therapies for MDS and of mouse models that recapitulate aspects of the human disorder make this review particulary timely.
Clinical and laboratory features
MDSs are a heterogeneous group of disorders characterized by impaired peripheral blood cell production (cytopenias) and most commonly a hypercellular, dysplastic-appearing bone marrow.1,2 MDS was probably first described in 1900 by Leube as “leukanamie” (a macrocytic anemia progressing to acute leukemia; Berl Klin Wochenschr. 1900;37:851), which at the time was thought to have an infectious etiology. Many decades later, cohorts of patients who developed acute leukemia after having a macrocytic anemia were reported, and the common clinical features described. Patients were given a diagnosis of “pre-leukemia” until the 1970s, when it was realized that many such patients never developed acute leukemia but instead died of complications from the cytopenias. The “pre-leukemia” terminology faded away, and the term “myelodysplastic syndrome” became widely accepted. Signs and symptoms of anemia, accompanied by infectious or bleeding complications, predominate in MDS, with some patients having systemic symptoms or features of autoimmunity, perhaps indicative of the pathogenesis of their disease.
Examination of the bone marrow (aspirate, biopsy, cytogenetics, and flow cytometry) and the peripheral blood should reveal the morphologic features of the disease and exclude other conditions that can lead to cytopenias. Morphologic features of MDS generally include a hypercellular bone marrow with “megaloblastoid changes,” atypical megakaryocytes, erythroid hyperplasia, defective maturation in the myeloid series, and increased blasts or ringed sideroblasts (in some patients). Peripheral blood features may include monocytosis, Pelger Huet-like anomaly, circulating immature myeloid or erythroid cells, and macrocytosis.3,4 Over the past 25 years, diagnostic criteria have been set up to diagnose the MDS: 2 classification systems (French-American-British [FAB] and World Health Organization [WHO]) and several prognostic-scoring systems, the most common being the International Prognostic Scoring System (IPSS), have been widely used.
Classification schemes
The first FAB Cooperative Group meeting identified 2 broad categories of “dysmyelopoietic syndrome,” but in 1982 they expanded their classification to the modern 5 subcategories of MDS: refractory anemia (RA), refractory anemia with ringed sideroblasts (RARS), refractory anemia with excess blasts (RAEB), refractory anemia with excess blasts in transformation RAEB-T), and chronic myelomonocytic leukemia (CMML).5 Although somewhat arbitrary in its definitions, the FAB classification of MDS has provided a common language for physicians to use when describing patients with MDS and also an estimate of survival and the risk of progressing to acute myelogenous leukemia (AML). Despite its obvious limitations, the FAB classification has remained the standard for more than 2 decades. Nonetheless, it has been succeeded by the WHO classification, which records the number of lineages in which dysplasia is seen (unilineage vs multilineage), separates out the 5q− syndrome (a fortuitous situation given the efficacy of lenalidomide in treating this disorder), and changes the threshold maximal blast percentage for the diagnosis of MDS from 30% to 20%.6 Altogether, neither the FAB nor the WHO classification system addresses the pathophysiology of this clonal or oligoclonal disease (for example, does unilineage MDS start in a different cell of origin than multilineage MDS?) and both remain focused on morphology (with little concession to molecular abnormalities or immunophenotypic findings). Thus, we can expect the WHO classification to be revised on an ongoing basis. While the debate rages about how to best classify patients with 20% to 30% blasts, they appear to be distinct in many ways from classic AML patients7 and they are not a uniform group, but rather contain patients with advanced MDS, with “smoldering” AML and likely some patients with an early stage of typical AML.
The IPSS relies on the primacy of the number of cytopenias, cytogenetic profile, and the percentage blasts in the bone marrow to group patients with MDS into one of 4 prognostic categories: low risk, intermediate 1 risk, intermediate 2 risk, and high risk (Tables 1 and 2).8 Unfortunately, this system underweights the clinical importance of severe (ie, life-threatening) neutropenia and thrombocytopenia in determining the need for therapeutic intervention. For example, a patient with normal cytogenetics, 4% blasts in the marrow, a hemoglobin concentration of 9 g/dL, neutrophil count of 200/μL, and a platelet count of 1000/μL has a score of 0.5, whereas a patient with normal cytogenetics, 11% blasts in the bone marrow, a Hgb concentration of 9 g/dL, neutrophils of 2400/μL, and a platelet count of 98 000/μL has a score of 2.0. Yet, the former patient is in a dire clinical situation, whereas the second patient is not. Thus, although prognostically predictive, the great heterogeneity in what variables contribute to the total IPSS score makes it critical to consider the clinical impact of each component of the score, rather than the total score to determine the optimal therapeutic approach (see “Clinical management of MDS patients”). Furthermore, like many scoring systems, it does not take into account the slope of any change in the critical parameters, such as the peripheral blood counts or blast percentage.
Although imperfect in its clinical utility, the IPSS has been very useful in examining and comparing the outcomes of clinical trials. Since its publication, its utility has been confirmed at many institutions; nonetheless, refinements of the IPSS (eg, the WHO classification-based prognostic scoring system, WPSS) continue to be proposed. Whereas the thresholds of 100 000/μL for the platelet count and 1800/μL for the absolute neutrophil count are somewhat arbitrary (as is the cutoff of 10 g/dL for the hemoglobin), the idea of lumping the presence of 2 versus 3 cytopenias together and of not accounting for the severity of the cytopenias is a shortcoming of the IPSS, especially because most patients with MDS, even those with increased bone marrow blasts, die from their cytopenias. Furthermore, although transfusion dependency is associated with a poorer prognosis in MDS,9 this factor is not taken into consideration when calculating the IPSS prognostic score. The WPSS incorporates transfusion dependency, which has been shown to be prognostically important in MDS patients, together with the WHO classification, and the cytogenetics risk group, to separate patients into 5 distinct prognostic groups.10 The assignment of specific cytogenetic abnormalities into the poor risk, intermediate risk, and good risk categories in both classification systems also continues to be reevaluated in large cohorts of MDS patients,11 at times identifying new possible prognostically important abnormalities (eg, those involving 1q).
Clinical management of MDS patients
Previously, the most frequent “treatment” given for patients with MDS was “best supportive care,” meaning red blood cell or platelet transfusions and antibiotics. “Active therapy” was given only when the disease progressed to AML or resembled AML, in terms of severe cytopenias. Now, there are an “arsenal” of therapies available (Table 3) as well as specific guidelines that help guide the choice of therapy for the patient and the treating physician (eg, the National Comprehensive Cancer Network guidelines, which are frequently updated and widely read in the United States, and also earlier guidelines from British and from Italian MDS-focused organizations12-14 ). Given the diverse forms of therapy available to MDS patients, it is important to decide what the goal of therapy is: is it to improve the anemia, thrombocytopenia, or neutropenia, render the patient transfusion independent, achieve a complete remission of the disease, or cure the disease? To codify this issue, an International Working Group of MDS investigators established a set of response criteria to evaluate different forms of therapy for MDS,15 which were recently revised and hopefully improved.16
The choice of therapy and the decision to undergo treatment must be tailored to each patient. The factors that govern these decisions include patient age, performance status, and medical comorbidities as well as the patient's risk-aversion profile and the severity of the disease at presentation. Whereas MDS is not curable without stem-cell transplantation (or aggressive AML-like chemotherapy), the combination of advanced age and limited donor availability renders the majority of patients with MDS ineligible for such therapy.
The clinical approach that I find optimal is to ask 2 questions up front: (1) does the patient have significant neutropenia or thrombocytopenia (or is it imminent); and (2) does the patient have increased bone marrow blasts, and therefore is at risk for progression to AML?
If the answer to both is no, then one is treating the anemia of MDS, and there are many options, including blood transfusions, an erythropoietic agent (erythropoietin or darbepoetin) given alone or with granulocyte colony-stimulating factor, antithymocyte globulin and/or cyclosporin A, lenalidomide (or thalidomide, if lenalidomide is unavailable) or a hypomethylating agent (5-azacytidine or 5-aza-2-deoxycytidine). If the answer to either question is yes, then one must try to alter the natural history of the disease (ie, to improve blood production and delay the progression to AML). Thus far, 5-azacytidine and decitabine appear to improve the outcome for patients with poor risk MDS, and both can ameliorate the cytopenias of MDS and decrease the percentage blasts in the bone marrow. Intensive, AML-like chemotherapy can also accomplish these 2 goals, but studies show that many MDS patients tolerate such treatment poorly. Blood or marrow cell transplantation (and in some patients intensive chemotherapy) can cure this disease, but again many MDS patients are not suitable candidates for this aggressive approach, either. Patients with significant cytopenias, without increased bone marrow blasts, may be candidates for immunomodulatory treatments. Lenalidomide is currently being evaluated for higher risk MDS patients (especially those with a 5q− abnormality), and MDS patients should be encouraged to participate in therapeutic clinical trials.
Hematopoietic growth factors (HGFs) can ameliorate the anemia in a significant fraction of MDS patients, but they do not affect the thrombocytopenia or the propensity of high-risk disease to progress to AML. A recent analysis suggests a possible survival benefit for low-risk patients who respond to HGFs compared with historical controls.17 Long-term “prophylactic” use of granulocyte colony-stimulating factor (or granulocyte-macrophage colony-stimulating factor) in MDS patients has not been beneficial, and thrombopoietic agents, such as interleukin-3 (IL-3), IL-6, thrombopoietin, or IL-11, have generally not been well tolerated, nor have they shown significant efficacy in this disease. Newer thrombopoietic agents are currently being tested in clinical trials. Several excellent reviews describing the clinical results of HGF therapy have been published.18,19
Several types of immunomodulatory therapies have been used in treating MDS, including antithymocyte globulin (ATG) and cyclosporin A. ATG appears to work best in MDS patients with at least some of the following features: (1) refractory anemia subtype, (2) pancytopenia, (3) normal cytogenetics, (4) HLA-DR15 positive, (5) younger age, (6) a marrow that is not hypercellular, and (7) no or a brief history of transfusion dependence. Algorithms exist that can compute the approximate chances of response in a given patient20 ; their reproducibility in other cohorts of MDS patients is unclear. The duration of responses to ATG in MDS is shorter (∼10-12 months) than in aplastic anemia, where this therapy can be curative.21,22
Another “immunomodulatory” agent, lenalidomide, has important erythropoietic activity in red blood cell transfusion-dependent MDS patients, especially those with a 5q− cytogenetic abnormality. After a phase 1/2 clinical trial of lenalidomide in 33 patients with primarily Low/Int-1-risk MDS and significant anemia (Hgb < 9 g/dL or transfusion-dependent) showed an overall erythroid response rate of 64%,23 lenalidomide was tested in 2 phase 2 trials, one in 5q− patients24 and another in non-5q− Low/Int-1 risk patients.25 The potent activity of lenalidomide shown in transfusion-dependent 5q− patients in the first trial was confirmed in the phase 2 trial as fully 67% of the patients with a 5q− abnormality became transfusion-independent, for a median duration of 102 weeks. The median rise in Hgb was 5.3 g/dL, and many of the responding 5q− MDS patients had complete cytogenetic remissions (which occurred with equal frequency whether or not other cytogenetic abnormalities were present). Overall, lenalidomide was well tolerated, although neutropenia and thrombocytopenia were seen and were more severe in the 5q− patients than the non-5q− patients treated on these trials. The exquisite sensitivity of 5q− MDS to lenalidomide is unexplained, as its mechanism of action is largely unknown.26,27 However, the rapid cytopenias that occur after the initiation of lenalidomide therapy suggests a direct inhibitory effect on the 5q− clones, as do reports of lenalidomide-induced changes in gene expression.28 Other effects, such as the restoration of NK T cells found after several months of therapy,29 may or may not be primary.
The efficacy of lenalidomide in this subtype of MDS represents a great opportunity to explore the pathophysiology of this disease (see “5q− and MDS”), perhaps following the example of the myeloproliferative disorder hypereosinophilic syndrome, where a recurring molecular defect (an interstitial deletion that generates a constitutively activated FIP1L-PDGFRA tyrosine kinase) was uncovered once the clinical observation was made that the disease responded to imatinib therapy.30
Hypomethylating agents
Aberrant methylation of cytosines within CpG promoter regions by DNA methyltransferases (DNMT) may silence critical components of the normal cell growth and differentiation programs. DNA methyltransferases, primarily DNMT1 and DNMT3b, faithfully restore DNA methylation immediately after DNA replication. Hypomethylating agents, 5-azacytidine and 5-aza-2′-deoxycytidine, are phosphorylated and incorporated into DNA (as 5-aza-dCTP) during S phase, where they covalently bind to the DNMTs, irreversibly inhibiting their function, leading to the progressive loss of methylation; this process requires cells to go through S phase. Hypomethylating agents promote myeloid differentiation in vitro,31,32 and these effects are seen at doses much lower than those needed for their maximal cytotoxic effect.33
5-Azacytidine was approved for the treatment of MDS in 2004, based on a phase 3 CALGB trial where it was given by subcutaneous injection for 7 days and compared with best supportive care. This trial showed that 5-azacytidine prolonged survival (this was shown using a landmark analysis because of the crossover design of the study), delayed progression to AML, and improved quality of life compared with supportive care.34,35 The complete response (CR) rate to 5-azacytidine was 7%, with 16% partial responses (PRs) and 37% hematologic improvement also seen. A multinational, follow-up study, randomizing patients to either 5-azacytidine or the physician's choice of best supportive care, low dose cytarabine, or AML-induction chemotherapy, has recently been concluded. The data from this non-crossover study presented in abstract form only demonstrated a survival benefit from the use of 5-azacytidine. 5-Aza-2-deoxycytidine (decitabine) was also evaluated in a phase 3 trial, and a 35% overall response rate was seen (9% CR, 8% PR, 18% hematologic improvement) versus no responses in the patients receiving supportive care. Many patients in this trial received 2 or fewer cycles of decitabine, and even responding patients were taken off drug during the course of this study. Indeed, the overall survival of the patients randomized to receive decitabine was not prolonged, but the time to AML or death was significantly prolonged in the high risk/intermediate 2 IPSS subgroup of patients who have the worst prognosis. Given the equal response rates across different FAB subtypes and IPSS categories (intermediate 1 to high), decitabine was FDA approved for the treatment of a broad spectrum of MDS patients in May 2006.
A key aspect of these studies is whether the rate of CRs and PRs, or the impact these drugs have on progression-free survival, is more important. Clearly, overall survival and progression-free survival are critical clinical endpoints; the rates of CR and PR may not translate directly into positive effects on those endpoints, as patients with hematologic improvement or stable disease may also derive benefit from their treatment.
The basis for the effectiveness of 5-azacitidine and decitabine in MDS is not known, and studies examining changes in the expression or promoter methylation of methylated genes (such as the cdk inhibitor p15) have not been very revealing.36 Effects on other cdk inhibitors that regulate the behavior of stem cells (such as p21, p57, or p18) or progenitor cells (such as p27) may be relevant to the clinical response to hypomethylating agents but so may other gene targets that affect cell growth, differentiation, or apoptosis (eg, the p53-related gene p73).37 Generally, it takes many cycles of treatment (ie, many months) to see a response to these agents, making it difficult to document the relevant changes that occur within the hematopoietic cells. Furthermore, it is unclear how these treatments work, whether by restoring gene expression or by creating more chaos in the cell, triggering its death.
The limited efficacy of these drugs in MDS suggests that these agents may only partially relieve aberrant epigenetic silencing of gene expression if that is indeed their mechanism of action. Changes in the dose or schedule of these agents may improve their efficacy; and indeed, the more frequent administration of decitabine (in a different dosing schedule) has been associated with a higher response rate.38 This approach is more myelosuppressive than the approach used in the pivotal phase 3 decitabine trial, and this may also be an important feature for inducing more frequent responses in this disease.
Is there a role for modulating histone acetylation or methylation?
Silencing of gene expression has been largely ascribed to the processes of histone deacetylation, histone methylation, and DNA promoter hypermethylation,39 and combination therapies that target gene silencing are currently being tested in MDS (and AML). Histone deacetylation, which is commonly triggered by leukemia or lymphoma-associated oncogenic proteins (eg, AML1-ETO, PML-RARα, BCL6) that recruit histone deacetylases (HDACs) to regulatory DNA sequences, inhibits the access of activating transcription factors to the DNA. The use of a hypomethylating agent with a histone deacetylase inhibitor has been reported,40,41 as have combinations of hypomethylating agents (alone or with HDAC inhibitors) with compounds that can recruit transcriptional coactivators, such as ATRA or vitamin D analogs.42 Several HDAC inhibitors have been tested as single agents in MDS or AML patients, including phenylbutyrate, valproic acid, depsipeptide, SAHA, MGCD-0103, and MS-275. Remarkably, for many of these drugs, a small number of patients have been reported to have complete remissions. The basis for these responses is unknown.
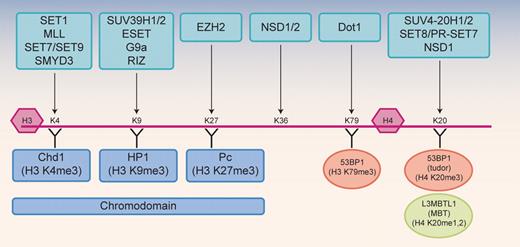
Histone methyltransferases are a component of the epigenetic silencing machinery that have not yet been targeted therapeutically. Some background will be presented here on the lysine methyltransferases (now called KMTs) because they (or the lysine demethylases) represent very promising enzymatic targets. Protein arginine methyltransferases (PRMTs) have also been linked to leukemogenesis and although they are targetable as well, they will not be covered in this review.43 The KMT enzymes methylate specific histone lysine residues, resulting in monomethyl, dimethyl, or trimethyl modifications, which recruit specific chromatin binding proteins (Figure 1).44 Some modifications are found on lysines at the sites of active gene transcription (eg, H3-K4 and H3-K79) and others at sites of gene silencing (eg, H3-K9 and H3-K27). Thus, pharmacologic inhibition of histone methylation will likely require agents with great specificity. Furthermore, the dynamics of these processes need to be considered when trying to optimize therapeutic approaches. Histone acetylation is a rapidly reversible process, whereas DNA methylation is thought to be irreversible. Histone methylation was also thought to be irreversible until demethylases were identified (eg, JMJD6 and LSD1) that act on methyl arginine and methyl lysine, respectively.45,46
Histone lysine methyltransferases. Histone methylation is catalyzed by lysine (K) or arginine (R) methyltransferases that add methyl groups to specific amino acid residues within the histone H3 or H4 tails or globular domains. Several distinct families of lysine methyltransferases (HKMTs) are shown at the top, with the site of methylation in the histones indicated by the arrow. Histone binding proteins are shown below the histones, including chromodomain-containing proteins (indicated in blue) and tudor domain– or MBT domain–containing proteins that recognize the H4K20 methyl mark (53BP1 and L3MBTL1, indicated in orange). Many of the methyltransferases shown have been linked to cancer. The protein arginine methyltransferases (PRMTs) are not shown.
Histone lysine methyltransferases. Histone methylation is catalyzed by lysine (K) or arginine (R) methyltransferases that add methyl groups to specific amino acid residues within the histone H3 or H4 tails or globular domains. Several distinct families of lysine methyltransferases (HKMTs) are shown at the top, with the site of methylation in the histones indicated by the arrow. Histone binding proteins are shown below the histones, including chromodomain-containing proteins (indicated in blue) and tudor domain– or MBT domain–containing proteins that recognize the H4K20 methyl mark (53BP1 and L3MBTL1, indicated in orange). Many of the methyltransferases shown have been linked to cancer. The protein arginine methyltransferases (PRMTs) are not shown.
It is clear that histone lysine methyltransferases play an important role in normal growth control, as they are frequently altered in cancer cells.47 The EZH2 methyltransferase, which methylates H3-K27, has been shown to be overexpressed in lymphoma48 and in a variety of solid tumors (eg, breast cancer and prostate cancer), conferring a poor prognosis.49,50 NSD1 methylates H3-K36, and its gene is fused to NUP98 in the t(5;11) AML-associated chromosomal translocation.51 The Kamps laboratory has recently shown that the NUP98-NSD1 fusion protein transforms hematopoietic cells by deregulating Hox gene expression.52
Two types of methyltransferase domains are found in human genes: the PR domain, which is found in MDS1-EVI1, MEL1, BLIMP1, and RIZ1, and the SET domain (SUVAR39H1, EZH2, Trithorax), which is found in MLL, NSD1, MMSET (NSD3), and other HMTase proteins.53 Whereas RIZ1 contains a PR domain, the nearly identical RIZ2 protein lacks this N terminal PR domain. Both are expressed in normal cells, but only RIZ2 is expressed in tumors, suggesting that RIZ1 may encode a tumor suppressor gene.54 Similarly, MDS1-EVI1 has an N terminal PR domain that is missing in EVI-1. EVI-1 is overexpressed in cancer, whereas MDS1-EVI1 is not. The same holds true for the MDS1-EVI-1-like MEL1 gene (aka PRDM16), where the isoform lacking the PR domain is overexpressed in AML (and MDS) and is transforming in animal models. Thus, in many situations, the methyltransferase containing isoform is lost in cancer and the isoform lacking this domain serves as an oncogene.
MLL, a human homologue of the Drosophila Trithorax protein, is part of a large multiprotein complex that contains histone H3-K4 methyltransferase activity.55 This activity requires the SET domain in the C terminus of MLL; but when MLL is fused to other proteins (by > 30 distinct translocations that involve the 11q23 locus), the SET domain is lost from the fusion protein. Although this would imply loss of activating function, the MLL fusion proteins are generally more potent activators, especially of Hox gene expression. In many cases, the MLL fusion partner is a member of a multiprotein complex that interacts with the Dot1 methyltransferase (including AF4, AF9, and AF10). Dot1 methylates H3-K79, and this activating mark is found on Hox A gene promoters, in association with increased Hox A gene expression. Several excellent reviews of the MLL fusion proteins exist.56-59
Understanding the molecular basis of MDS
The MDSs and myeloproliferative diseases can both be thought of as preleukemic disorders (Table 4). AML, the “full-blown” myeloid malignancy, is characterized by a block in differentiation but also the ongoing ability of the myeloblasts (and leukemic stem cells) to survive and proliferate. In contrast, MDSs have impaired differentiation, making it likely that a second mutation, which allows the blasts to survive and proliferate (indicated by arrows in Table 4), is needed for the disease to progress to AML. Likewise, in the myeloproliferative diseases, where proliferation is enhanced and differentiation is initially normal, the disease most likely progresses to acute leukemia when a second hit impairs differentiation (also indicated by arrows in Table 4).
Given the paucity of specific, identifiable molecular abnormalities in the hematopoietic cells in MDS, the nonhematopoietic cell milieu that supports the MDS process in the bone marrow has also been extensively studied. In addition to demonstrable hematopoietic stem/progenitor cell abnormalities, immune deregulation, abnormal marrow environment, and other factors contribute to the heterogeneous behavior of this disease. Increased macrophage function with increased cytokine secretion, changes in microvessel density, and immunologic abnormalities (which are often found in aplastic anemia patients as well) have been identified in patients with MDS. Recent discoveries, such as the absence of circulating NK T cells in MDS patients,60 may provide insight into new therapeutic approaches, although some abnormalities may simply represent “downstream” effects of the impaired hematopoiesis found in MDS.
The search for the mechanistic basis of MDS has been fueled largely by the identification of recurrent (cyto-)genetic abnormalities that are associated with specific clinical scenarios. The common chromosomal abnormalities found in MDS include abnormalities in 17p, loss of Y, 5q−, 7q− or monosomy 7, trisomy 8, 11q23 abnormalities, del 12p and 20q−, and in half of the patients, a normal chromosome pattern. None of these abnormalities is specifically associated with MDS, as all can be seen in AML and some in the myeloproliferative diseases. The cause of MDS is usually unknown, although it can occur after exposure to radiation, certain environmental toxins, such as benzene, or after treatment of a primary malignancy with alkylating agents or topoisomerase II inhibitors.61
The presence of t(15;17), t(8;21), inv(16), and other specific abnormalities in AML but not MDS seems to reflect true differences in their biology. Recurrent chromosomal translocations in AML often produce fusion transcription factor proteins that act as potent transcriptional repressors (most likely targeting genes that are required for normal hematopoietic cell differentiation), whereas some fusion proteins, such as those that contain the transcriptional regulator MLL, appear to function as potent activators of (Hox) gene expression. Deletions, numerical abnormalities, and unbalanced translocations are more commonly seen in MDS, and translocations specifically associated with MDS are rare.
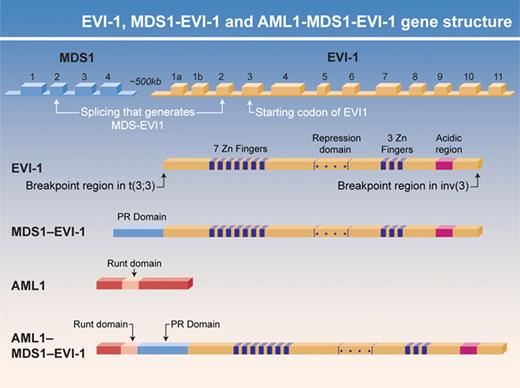
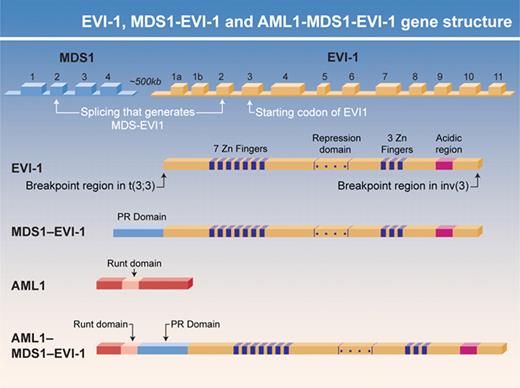
The t(3;21), which is associated with MDS (and CML blast crisis), is one of the first recurrent MDS-associated cytogenetic abnormalities to be molecularly deciphered. The translocation rearranges the AML1 and MDS1–EVI-1 genes, fusing the N terminus of AML1 with a small portion of MDS1 and nearly all of EVI-1 (Figure 2). Both components of the AML1–MDS1/EVI-1 fusion protein appear to play critical roles in deregulating hematopoiesis. AML1 (also known as CBFα or Runx1, for Runt-related protein 1) binds DNA with its non-DNA binding partner CBFβ. Lack of either AML1 or CBFβ is embryonically lethal because of the absence of definitive hematopoiesis and a distinct pattern of central nervous system hemorrhage.62,63 The AML1 gene is involved in numerous translocations, most commonly the t(8;21), which is found in M2, AML, which fuses AML1 to ETO. AML1-ETO function as a dominant inhibitor of AML1 function but has gains of functions as well.64,65
EVI-1 and AML1–MDS1–EVI-1 gene structure. The two protein isoforms generated from the MDS1-EVI-1 gene, MDS1-EVI-1 and EVI-1, differ in their N termini, with MDS1-EVI-1 containing a PR domain that EVI-1 AML1–MDS1–EVI-1 and EVI-1 have oncogenic properties that MDS1–EVI-1 lacks. The gene structure for these isoforms and the location of their functional domains are shown. The role that PR domain– (and SET domain–) containing proteins play in myeloid malignancies is an important area for future investigation.
EVI-1 and AML1–MDS1–EVI-1 gene structure. The two protein isoforms generated from the MDS1-EVI-1 gene, MDS1-EVI-1 and EVI-1, differ in their N termini, with MDS1-EVI-1 containing a PR domain that EVI-1 AML1–MDS1–EVI-1 and EVI-1 have oncogenic properties that MDS1–EVI-1 lacks. The gene structure for these isoforms and the location of their functional domains are shown. The role that PR domain– (and SET domain–) containing proteins play in myeloid malignancies is an important area for future investigation.
The AML1 gene is also mutated at low frequency in AML and MDS, but at much higher frequency in AML, M0, and in treatment-related or radiation-induced MDS.66-68 Whereas the mutations found in M0 AML are often biallelic, those found in MDS are generally monoallelic and result in AML1 insufficiency rather than generating a dominant negative protein. Most of the AML1 mutations in AML cluster in the Runt (DNA binding) domain and impair the binding of AML1 to DNA but not to CBFβ. The AML1 mutations found in MDS, or in patients with MDS to AML, more often occur in the C-terminus of the protein and truncate the protein, eliminating its transactivating domain (and possibly other functional domains).69 AML1 functions as a tumor suppressor gene in individuals with familial platelet disorder/AML; affected individuals with this disorder have only one normal allele of AML1, and have thrombocytopenia and a propensity to develop AML.70 Similarly, AML1+/− mice have defects in megakaryocytic development and an increased frequency of committed progenitors in their marrow71 ; these mice have also been reported to develop a myeloproliferative disorder with time.72
The MDS1–EVI-1 gene (located on 3q26) encodes the MDS1-EVI-1 and EVI-1 zinc finger containing proteins via differential promoter usage. The gene is involved in the t(3;3)(q21;q26), a translocation found in AML, that results in EVI-1 overexpression. EVI-1 lacks a PR domain that is contained in MDS-EVI-1, and EVI-1, but not MDS1-EVI-1 (see Figure 2). EVI-1 is transforming and can block hematopoietic differentiation, especially of the erythroid lineage. AML1-EVI-1 (more accurately called AML1-MDS1-EVI-1 or AME) can transform Rat 1 fibroblasts,73 and both constituent portions of the fusion are required for its many activities. Both AME and AML1-ETO block AML1 function and block TGFβ-signaling pathways74,75 and enhance AP-1 activity. AME also blocks JNK-induced apoptosis.
The constitutively activated TEL-PDGFR fusion tyrosine kinase, generated by the t(5;12) that accompanies CMML with eosinophilia76 is another recurrent translocation seen in MDS. However, this disease fits the model of a myeloproliferative disease better than it does “typical” MDS. CMML can present as either MDS or more like an MPD; this dichotomy has been incorporated into the WHO classifications of those disorders. There are several other “overlap” diseases, which have features of both MDS and MPD: One of these is refractory anemia, ringed sideroblasts with thrombocytosis (RARS-T), a relatively newly defined entity that is characterized by the presence of a JAK2V617F mutation in a large percentage of patients.77 This mutation is generally found in patients with an MPD (in 90% of polycythemia vera and 30%-40% of essential thrombocythemia and primary myelofibrosis), but it has also been found in patients with otherwise unexplainable Budd-Chiari or other hepatic vascular syndromes.78 This mutation probably drives the thrombocytosis of RARS-T; it is unlikely to be the primary event driving the myelodysplasia seen in this disorder.
Using modern molecular biologic techniques, a variety of abnormalities have been documented in patients with AML or MDS, including mutations in the KIT, PDGFRβ, FLT3, FMS, JAK2, and G-CSFR kinases, mutations in the transcription factors RUNX1, CEBPA, PU.1, GATA-1, p53, and MLL, and mutations in ras.79,80 Activated Ras is found in approximately 5% to 20% of MDS patients,79,80 and in particular in patients with CMML.81,82 Ras mutations may be found more commonly in association with RUNX1 point mutations in MDS.83
Loss of NF1, ICSBP, or SMAD4 gene integrity or expression has also been documented in patients with myeloid malignancies. All these genetic abnormalities could impact on the growth of MDS (or AML) cells. Of the many potential targets in these diseases, the farnesyltransferase inhibitors have been evaluated in patients with MDS (and AML), with some success. However, their efficacy does not seem to relate to the presence of mutated ras.84
5q− and MDS
Some MDS patients with an interstitial deletion within the long arm of chromosome 5 (5q−) have the “5q− syndrome,” whereas others do not. The 5q− syndrome, as defined by the WHO, consists of an isolated 5q− cytogenetic abnormality, associated with macrocytic anemia, a normal or elevated platelet count, unilobular megakaryocytes, and a low propensity to develop AML.6 The 5q− syndrome is most commonly seen in females, in contrast to the overall male predominance of MDS. The deletion in the 5q− syndrome most commonly involves the region 5q q13-q31, whereas the most commonly deleted region in other 5q-associated MDS is 5q22 to 5q33.85 Although a good prognostic indicator in MDS, the 5q− conveys a poorer than average prognosis in AML,86 where it is often seen in those exposed to environmental toxins, or to therapeutic chemotherapy (especially alkylating agents).
Despite decades of scientific investigation attempting to identify the critical gene (or genes) deleted by the 5q−, no single gene has been identified where one allele is deleted and the other allele mutated. This finding has given strong support to the idea that gene haploinsufficiency (meaning that if both alleles of a gene are needed to generate sufficient gene product to assure full function in the cell, then having only one allele present leads to insufficient gene product and altered physiology of the cell) can participate powerfully in the process of malignant transformation. Clearly, many biologic abnormalities can result from loss of a single allele of a gene in mice.
Genes on 5q that are expressed in human CD34+ hematopoietic cells and have attracted attention include SPARC, EGR1, CTNNA1 (α-catenin), and NPM1.1,28,87,88 Although some of these genes are outside the commonly deleted region, they may still contribute to the pathogenesis of this disease in some patients. Recently, deletion and decreased expression of the RPS14 gene have been implicated in the 5q− syndrome.89 This gene encodes a protein important for ribosomal biogenesis and is related to the RPS29 gene, which has been implicated in causing Diamond-Blackfan congenital anemia.90 Using individual shRNAs in an elegant strategy to knock down the expression of each of the 41 genes that are in the 5q− syndrome commonly deleted region, the Golub laboratory reported that RPS14 knockdown inhibits erythroid growth and promotes megakaryocytic colony growth, as well as inducing apoptosis and morphologic erythroid abnormalities. Exactly how aberrant ribosomal dysgenesis (which would occur if RPS14 or RPS29 function is deficient) leads to anemia is unclear, but the demand for producing large amounts of globin may trigger a response primarily in cells that commit to the erythroid lineage, even if the lesion is present in the hematopoietic stem cell. How this results in the clonal outgrowth of cells is also unclear at this time.
20q−
Although the presence of 20q− in MDS has a favorable prognosis, the critical genes deleted from this region also remain unknown. My laboratory has been interested in 20q12 deletions since we identified a gene that encodes a histone-binding protein within the commonly deleted region (CDR) on chromosome 20q12. The CDR contains a polycomb group gene, the L(3)MBTL1 gene, which is the human homolog of the Drosophila L(3)MBT (malignant brain tumor) gene.91 In Drosophila, biallelic mutations in L(3)MBT lead to imaginal disk neuronal tumors,92 suggesting that L(3)MBT functions as a tumor suppressor gene. L(3)MBTL1 is expressed in human CD34+ hematopoietic progenitor cells, and we have shown that L(3)MBTL1 functions as a repressor93 and can compact chromatin, preferentially recognizing monomethyl and dimethyl lysine residues in several histone molecules.94 Studies have implicated L(3)MBTL1 in correct mitotic progression,95 and work is ongoing to characterize the function of this repressor molecule in human hematopoiesis. A related gene, L3MBTL3, located on chromosome 6q23, has been implicated in the control of erythropoiesis in mice.96
7q−
Several laboratories have focused on the commonly deleted region on 7q that is associated with MDS. Given the involvement of polycomb and trithorax group genes in AML and MDS and the importance of Hox gene expression in hematopoiesis and leukemogenesis, members of this family continue to be interrogated, such as the MLL5 gene (a member of the MLL family of genes), which is deleted by the 7q− deletion.97 Searches for other candidate genes continue. Furthermore, the search for relevant genes within the commonly deleted regions on chromosomes 5q, 7q, and 20q, has expanded recently to include a new class of genes, those that encode micro RNAs. If the expression of specific micro RNA genes is either lost or reduced by these common cytogenetic abnormalities, the effects of the deletion could be greatly magnified, as micro RNAs bind to the 3′ untranslated regions of many genes in a sequence-specific and cell type-specific manner to block translation of RNAs into protein or to induce RNA degradation.98 Thus far, approximately 230 micro RNAs (which are 17-24 bases long and highly conserved between species) have been identified in mammals, with functional attributes identified for perhaps 10%. Micro RNAs have been shown to regulate a variety of signaling pathways, including ras signaling, and some have potent antioncogenic effects. The identification of specific miRNAs, expressed in specific hematopoietic lineages,99 will allow studies of how their deregulation may contribute to MDS.
Gene expression studies of MDS
Several publication describe the transcript profiles of purified stem cell fractions obtained from the bone marrow of patients with MDS (isolating either CD34+ cells or AC133 expressing cells). Among the overexpressed genes, the Delta-like protein (Dlk1) reported to be up-regulated by several groups,100,101 including Pellagatti et al,101 who found up-regulation of DLK1 in 33 of 55 MDS patients, providing evidence for involvement of the Delta-Notch signaling pathway in MDS. Consistent up-regulation of interferon-γ–inducible genes has also been reported: IFITM1 and IFIT1 mRNA levels, and TRAIL, another interferon stimulated gene are up-regulated in a substantial fraction of the MDS patients. IFN-γ is an important trigger of apoptosis in the erythroid compartment, and it has been strongly implicated in the pathogenesis of aplastic anemia (where it also predicts a response to ATG therapy).102,103 Perhaps expression patterns could predict for a response to specific therapies in certain patients with MDS.
Although down-regulation of the α-catenin gene (CTNN1), which maps to 5q31, is found in some patients with 5q−MDS,104 none of the patients in the Pellagatti report100 had undetectable levels of α-catenin gene expression. Altered expression of genes in the heme biosynthesis pathway has also been reported by Pellagatti et al,101 especially in the RARS patients. The abnormal iron regulation in the mitochondria that leads to the generation of ringed sideroblasts is not well understood; and although a variety of mitochondrial genes are dysregulated in RARS patients, none has been specifically implicated in this disorder.
Mouse models of MDS
The first mouse model for MDS was reported by Buonamici et al,105 who used a retrovirus to overexpress EVI-1 in murine hematopoietic stem cells and then transplant the cells into irradiated recipients. The transplanted mice do not develop leukemia; but approximately 10 months after transplant, they begin to die, and postmortem examination showed pancytopenia with erythroid and megakaryocytic hyperplasia, and dyserythropoiesis. Peripheral blood smears showed no abnormalities for the first 7 to 8 months but then showed marked anisocytosis and poikilocytosis. It is not clear why it takes 10 months to see the MDS phenotype in these mice, given that more than 70% engraftment of the transduced cells was seen (with a ∼20%-30% efficiency of transduction). This suggests that a second hit is likely required for the pancytopenia and the other features of MDS to occur. As a Sca1 promoter-based EVI-1 transgenic mouse developed neither MDS nor AML (although decreased CFU-E was seen and one founder mouse had significant erythroid abnormalities),106 questions remain about the possible retroviral insertional activation of genes that cooperate with EVI-1 to cause the MDS in these mice. In the BMT mouse model, at the later time points, the EVI-1 expressing bone marrow cells did show an impaired response to erythropoietin and an enhanced in vitro response to granulocyte macrophage colony-stimulating factor, which could reflect inhibition of erythroid differentiation by EVI-1. Decreases in EpoR receptor and Mpl (the thrombopoietin receptor) mRNA expression were seen in these mice, 6 months before any hematologic abnormalities become apparent, and enhanced caspase-3 staining of the bone marrow was found, suggesting that apoptosis is increased.105
In a second MDS model, Grisendi et al reported that although the absence of nucleophosmin (NPM) gene expression was embryonically lethal between E11.5 and E12.5, NPM+/− mice had features of MDS.87 NPM plays an important role in ribosome biogenesis; it also regulates the p53/ARF tumor suppressor pathway and plays a role in regulating centrosome duplication. A majority of the NPM+/− heterozygous mice have an elevated MCV and RDW without a reticulocytosis. Furthermore, 8 of 9 NPM+/− mice had abnormal platelet counts (3 greater than normal and 5 less than normal). Although the NPM+/− mice are not particularly anemic, 12 of 16 mice had morphologic erythroid dysplasia, and 7 of 11 had dysplastic megakaryocytes. Overall, this appears to be a reasonable model for MDS. At least at the time of this review, it does not appear that these mice are leukemia prone.
A third model of MDS is the NUP98/Hox D13 transgenic mouse, reported by Lin et al.107 These mice express the t(2;11) associated fusion protein, which contains the N terminus of the nuclear pore complex protein NUP98 fused in frame with the homeobox containing HoxD13 protein, and they seem to represent the most faithful model of MDS thus far. These mice appear healthy for a long time despite cytopenias and bone marrow dysplasia. By 4 to 7 months of age, these mice develop a MDS, characterized by anemia and leukopenia and a trend toward an elevated MCV. Morphologic abnormalities consistent with MDS are found in the circulating white blood cells and red blood cells and the bone marrow morphology of these mice is also consistent with MDS (being somewhat hypercellular and dysplastic with increased apoptosis as well as 12%-17% blasts). None of the mice lived beyond 14 months because, at approximately 10 months, this disorder converts into acute leukemia or the mice develop other complications. Of note, a bone marrow transduction/transplantation model using the NUP98-HoxD13 fusion cDNA resulted in a myeloproliferative disorder that did not evolve into AML.108 Yet expression of NUP98-HoxD13 in murine embryonic stem cells severely impaired their differentiation and promoted their immortalization.109 The NUP98-HOXD13 transgenic mouse model is being used to evaluate therapeutic approaches to MDS and to define what pattern of gene deregulation is causally involved in the disease.
The role of Hox gene deregulation in AML has been widely studied. Its role in MDS is less clear. The role that disrupted nuclear pore proteins play in malignancy is also poorly understood, but the recurrent involvement of the NUP98 (and NUP214) nuclear pore proteins in translocations associated with hematologic disorders suggests that dysregulated nuclear transport can contribute to the aberrant growth of malignant hematopoietic cells. The N-terminus of NUP98 (which is contained in the NUP98 fusion proteins) can also recruit coactivator molecules (eg, p300/CBP) allowing for more potent homeobox gene activation.
Thus far, it has been difficult to model all of the features of MDS in a single mouse model. One aspect is to demonstrate increased apoptosis in the bone marrow cells which has been identified in early-stage MDS and may account for the “ineffective hematopoiesis” that characterizes the disease. The molecular basis for the increased apoptosis is not clear, but increased reactive oxygen species (ROS) generation, mitochondrial membrane dysfunction, and possibly imbalances in the ratios of pro- and antiapoptotic bcl2 family member proteins have all been suggested to play a role.
Unexplained clinical features of MDS
The basis for the selective outgrowth of MDS clones and the decrease in the normal hematopoietic stem/progenitor cells in patients over time are unknown. MDS can occur in patients with an incomplete response to ATG therapy,110 presumably as an escape mechanism, where an abnormal clone takes over because few normal clones are able (or present) to restore hematopoiesis. A similar mechanism may underlie the de novo development of MDS (ie, progressive depletion of normal stem cells accompanied by the outgrowth of MDS clones). The impaired recovery of normal hematopoiesis in MDS patients following intensive chemotherapy supports this contention.
What is the basis for the loss of normal stem cells that occurs over time in a disease that most commonly affects people after age 60? Certainly chronic, low level DNA damage could lead to stem- cell depletion. DNA damage signals activate the ATM (and ATR pathways), but in the absence of ATM, aging mice show a steady depletion of hematopoietic stem cells that can be abrogated by N-acetylcysteine treatment.111 The importance of ROS generation within hematopoietic stem cells (HSCs) is further supported by studies demonstrating the protective role of the bone marrow stem- cell niches in limiting ROS-induced cell damage or death.112 Thus, defects in the bone marrow microenvironment could play a role in MDS, setting up competition between the normal HSCs and the dysplastic ones for occupancy of a limited number of functional stem-cell niches. If the MDS clones are better adapted to life in the stem-cell niche, their occupancy could directly cause loss of normal HSCs. Furthermore, alterations in the balance between symmetric and asymmetric stem cell divisions could lead to stem-cell depletion in MDS. Whereas asymmetric stem-cell divisions result in stem cell maintenance, symmetric cell divisions that generate 2 daughter cells can lead to stem-cell depletion. The fate of dividing stem cells may be regulated by the geography of the stem-cell niche interactions, with divisions occurring perpendicular to the stem-cell niches resulting in asymmetric division, as modeled by the Spradling laboratory in Drosophila.113 This property could be disturbed in patients with MDS.
Premature or enhanced stem-cell senescence could also lead to stem-cell depletion in MDS. Senescence is a safeguard built into cells that can be activated by excessive proliferation (replicative senescence) or by expression of an oncogene (oncogene-induced senescence). Enhanced senescence is frequently observed in premalignant conditions but is absent in “full-blown” malignancies.114 Although activation of oncogenic pathways could lead to depletion of stem cells, a “field effect” would be required for such a mechanism to affect all the normal HSCs.
Aberrant activation of signaling pathways within HSCs could lead to their aberrant growth or their demise. The p38 MAP kinase serine/threonine kinase has been implicated in the stem-cell attrition that accompanies ATM loss and ROS-induced cell damage.112 Clinical trials of p38 inhibitors are under way in MDS patients, and the results are now being reported in abstract form. Aberrant TGFβ signaling could also contribute to MDS, as TGFβ has both growth inhibitory effects and differentiation-inducing effect on HSCs. TGFβ signaling can be blocked by Ras signaling115 and by overexpression of the EVI-1 or AML1–MDS1-EVI-1 proteins.74,75
In conclusion, few recurrent abnormalities have been identified in the bone marrow cells of patients with MDS, but mouse models suggest that dysregulated Hox gene expression, abnormal ribosomal biogenesis, and interference with normal erythroid differentiation may be common underlying themes. Abnormalities in cytokine signaling pathways may also play a role in this disease, and it appears that “epigenetic-directed” therapies may overcome some of these defects in responding patients. While we characterize the molecular lesions in the years ahead, we must efficiently design and test combination therapies using currently available agents that can eliminate the MDS clone. Aggressive therapies, such as stem-cell transplantation, remain the only curative option for MDS patients and fortunately our ability to perform stem-cell transplants for more elderly patients continues to improve.
A burning question that faces the field is: what is missing from our picture of MDS? Whereas this question is relevant for nearly all malignancies that we treat, as we make great strides in understanding hematopoietic stem cell biology, we should gain further insights into MDS. The FDA has approved 3 effective therapies in the past 3 to 4 years; thus, the future looks bright. Hopefully, the study of MDS will attract many talented clinical and scientific investigators in the future, and we can unravel more of the complexities of this group of disorders.
Acknowledgments
The author thanks current and former colleagues at Memorial Sloan-Kettering, UCLA, and the University of Chicago for their collegiality, knowledge, and dedication, and his patients (and their families) at those institutions for their impressive and consistent demonstration of courage, strength of character, and will to live. The author has learned much from these individuals and from many others about MDS; there are too many to acknowledge. Furthermore, the author regrets not being able to cite all of the excellent studies published on MDS because of page limitations. The National Institutes of Health and the Leukemia & Lymphoma Society have been the major sources of funding of the author's work on normal and malignant hematopoiesis over the past 2 decades.
This work was supported by a Leukemia & Lymphoma Society SCOR grant, the Rosemary Breslin Research Fund, the Maynard Parker Research Fund, and the Renny Saltzman Research Fund.
Authorship
Contribution: S.D.N. wrote this paper.
Conflict-of-interest disclosure: The author has consulted for or lectured for several companies who make products used to treat patients with MDS, including Amgen, Celgene, Genzyme, MGI Pharma, Ortho-Biotech, and Pharmion.
Correspondence: Stephen D. Nimer, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, Box 575, New York, NY 10021-6007; e-mail: s-nimer@mskcc.org.