Abstract
Blood cell interactions with the vessel wall were first documented almost 170 years ago. Modern advances have revealed that leukocyte and platelet interactions with the endothelium are at the nexus of complex, dynamic cellular and molecular networks that, when dysregulated, may lead to pathological inflammation and thrombosis, which are major sources of morbidity and mortality in the Western world. In this review, we relate the history of blood cell interactions with the vasculature, discuss recent progress, and raise some unresolved questions awaiting the field.
Historical overview
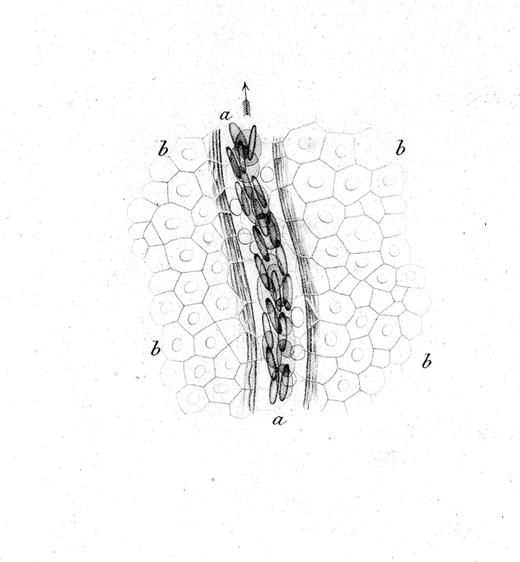
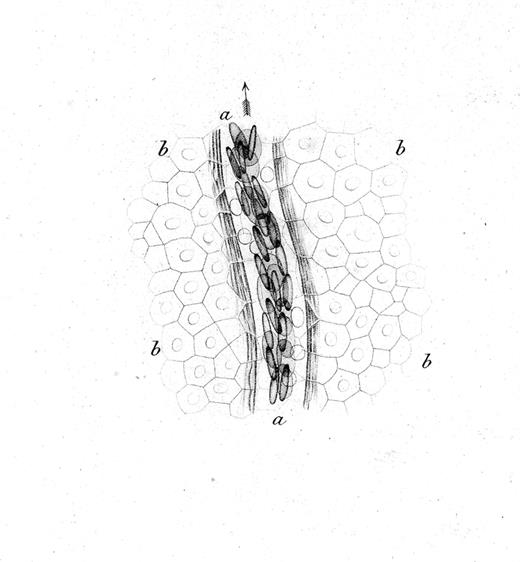
The interactions of leukocytes and platelets with the vessel wall, such as occur in inflammation, are highly dynamic and often transient. The development of intravital microscopy was the spark that allowed real-time observations of blood cells in live animals, and led to the detection and molecular analyses of their interactions with the vessel wall. Rudolph Wagner first published in 1839 a description of leukocytes interacting with the vessel wall.1 In blood vessels of the webbed feet of a grass frog, he observed that leukocytes (then called lymph-corpuscles) in venules were moving in close contact with the vessel wall, and more slowly than other blood cells. The drawing in Figure 1 likely represents the first depiction of rolling leukocytes; we now know that leukocytes roll constitutively in venules of the skin.
Drawing by Rudolph Wagner; legend translated from the German.1 Small venous branch a of the webbing of Rana temporaria at 350× magnification and close to the surface of the epidermis, whose cobblestone-like, mostly hexagonal, flattened, and for the most part nucleated cells b,b,b,b go over the vessel. Blood corpuscles are seen in multiple rows partially on the flat side, partially standing on the edge; in the light area between the flow of blood corpuscles and the vessel wall surrounded by several parallel filaments, one can see the round, bright, much slower moving lymph-corpuscles. The whole image was prepared at low illumination.
Drawing by Rudolph Wagner; legend translated from the German.1 Small venous branch a of the webbing of Rana temporaria at 350× magnification and close to the surface of the epidermis, whose cobblestone-like, mostly hexagonal, flattened, and for the most part nucleated cells b,b,b,b go over the vessel. Blood corpuscles are seen in multiple rows partially on the flat side, partially standing on the edge; in the light area between the flow of blood corpuscles and the vessel wall surrounded by several parallel filaments, one can see the round, bright, much slower moving lymph-corpuscles. The whole image was prepared at low illumination.
A few years later the frog—this time its spread tongue—was again central to another milestone microscopic observation. Augustus Waller noted that the trauma caused by the pins and the long exposure to ambient air produced a tongue irritation that triggered the adhesion of lymph-corpuscles onto the vessels of the microcirculation. He also noted that as time passed more of them “escaped” into the surrounding tissue while scarcely any red blood cells (RBCs) could be seen outside the vessel. Waller remarked on “the restorative power of blood which immediately closes the aperture” formed by the extravasating leukocytes and proposed that pus has its origin from these colorless extravasated corpuscles.2 It took 40 years for defined theories to attempt to explain what initiates these inflammatory responses. In his Lectures on General Pathology, Julius Cohnheim stated “we have here to deal with a molecular change of the vessel walls… comprised under the notion and name of inflammation.”3 Cohnheim attributed everything to alterations in the endothelium and noted that inflammatory changes did not affect blood as the vessel wall could be modified even if blood were replaced by saline before ligation. In contrast, at about the same time, Elie Metchnikoff concentrated on “the fundamental importance of phagocytosis in inflammation.” In this phagocytic theory of inflammation, he suggested that phagocytes were crucial to destroy the “irritant bodies.”4 Although Metchnikoff stated that “inflammation may occur without any intervention of the blood vessels,” he hinted at the need for leukocyte activation as “these cells are in the first place affected by various substances which posses an attraction for them.” Clearly, these brilliant scientists were equally right as it is now known that both endothelial and leukocyte activation are key to the inflammatory response.
Following vascular injury, the rapid coalescence of blood platelets into forming thrombi was observed by intravital microscopy in the late 1800s (reviewed in Jackson5 ). The adhesion of platelets to presumably intact but stimulated endothelium was documented only in 1970 by Begent and Born.6 After ADP application to the outside of a venule in a hamster cheek pouch preparation, platelets adhered and small unstable thrombi formed. Single platelet rolling on a stimulated venule of the mesentery was detected relatively recently, likely due to the small size of the platelet.7 This mouse mesentery preparation was actually introduced by the Born laboratory in the 1970s to measure the velocity of leukocyte rolling and quantify leukocyte adhesion under various experimental conditions (Atherton and Born8 ). In their work, the authors postulated the existence of specific adhesive interactions between blood cells and the vessel wall. The unraveling of the major molecular players activating and mediating such adhesive interactions occupied this field for the next 25 years.
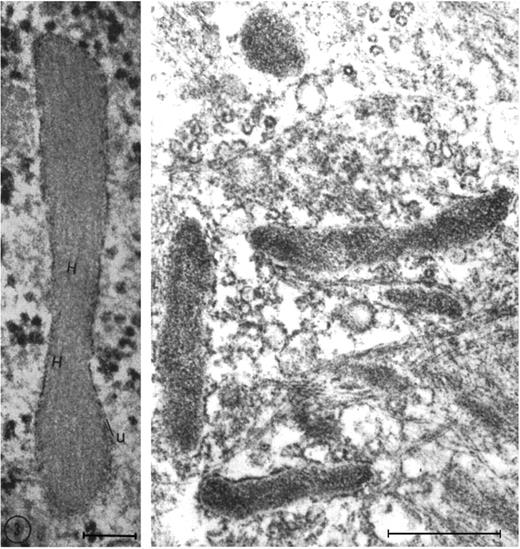
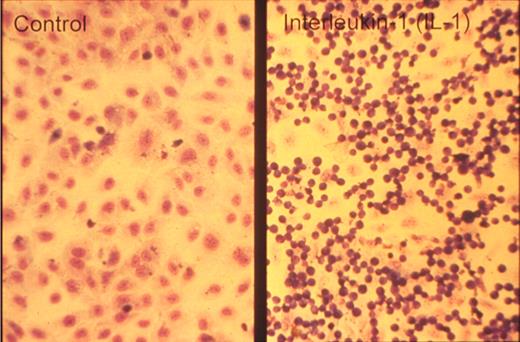
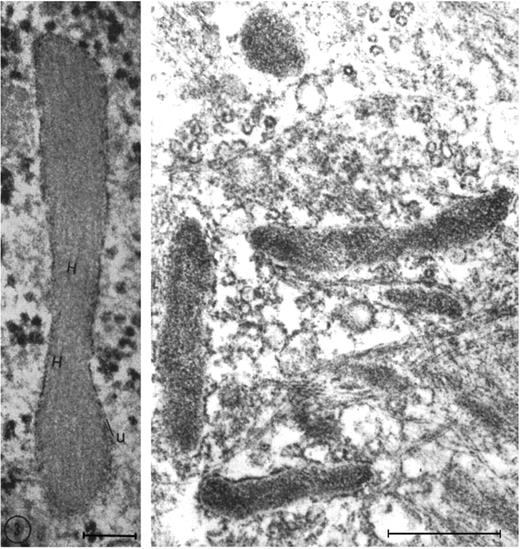
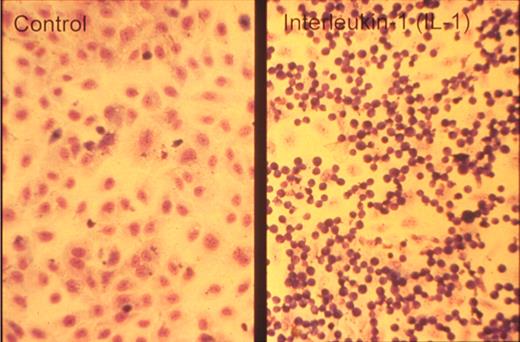
One of the key developments was the isolation and in vitro culture of endothelial cells in the early 1970s by Jaffe et al9 and Gimbrone et al10 who taught many, including one of us (D.D.W.), this art. The presence of a unique organelle discovered by Weibel and Palade in 196411 turned out to be an important marker to identify bona fide endothelial cells (Figure 2 left). This organelle, now called the Weibel-Palade body (WPB), was found in 1982 by Wagner et al12 to represent the storage granule for von Willebrand factor (Figure 2 right), a molecule mediating platelet adhesion. A few years later, a receptor for leukocytes (P-selectin) was found in its membrane.13,14 Thus, the secretion of these organelles provides a very rapid way for the activated endothelium to become adhesive for platelets and leukocytes. Treatment of endothelial cultures with cytokines by Bevilacqua et al showed another way to augment endothelial adhesiveness for leukocytes15 (Figure 3).
Weibel-Palade bodies are endothelial-specific organelles that store von Willebrand factor. (Left) Oblique section of a Weibel-Palade body from pulmonary artery endothelium of a rat, showing parallel arrangement of internal tubules (H). Reproduced with permission from Weibel and Palade.11 (Right) Electron micrograph (Wagner et al12 ) of human umbilical vein endothelial cells stained for von Willebrand factor with peroxidase showing a cluster of positively stained Weibel-Palade bodies. Left bar is 0.1 μM, and right bar is 0.5 μM. Reproduced from The Journal of Cell Biology.11,12 Copyright 1964 and 1982 The Rockefeller University Press.
Weibel-Palade bodies are endothelial-specific organelles that store von Willebrand factor. (Left) Oblique section of a Weibel-Palade body from pulmonary artery endothelium of a rat, showing parallel arrangement of internal tubules (H). Reproduced with permission from Weibel and Palade.11 (Right) Electron micrograph (Wagner et al12 ) of human umbilical vein endothelial cells stained for von Willebrand factor with peroxidase showing a cluster of positively stained Weibel-Palade bodies. Left bar is 0.1 μM, and right bar is 0.5 μM. Reproduced from The Journal of Cell Biology.11,12 Copyright 1964 and 1982 The Rockefeller University Press.
Neutrophils adhere to cytokine-stimulated cultured endothelial cells. Phase-contrast photomicrograph of the adhesion of human neutrophils to control (left) and interleukin-1–treated (right) human umbilical vein endothelial monolayers taken at the end of a 10-minute adhesion assay. Reproduced with permission from American Society for Clinical Investigation.15
Neutrophils adhere to cytokine-stimulated cultured endothelial cells. Phase-contrast photomicrograph of the adhesion of human neutrophils to control (left) and interleukin-1–treated (right) human umbilical vein endothelial monolayers taken at the end of a 10-minute adhesion assay. Reproduced with permission from American Society for Clinical Investigation.15
Phagocytes are not the only cells that leave the blood vessels. In the 1950s, Gowans demonstrated that radiolabeled lymphocytes, obtained from the thoracic duct of a P32-labeled rat and transfused into a recipient rat, reappeared rapidly in the lymph and thus could recirculate from blood to lymph.16 Clearance from the bloodstream was subsequently shown to occur at specialized lymphocyte-binding high endothelial venules (HEVs) in lymph nodes.17 Further mechanistic analyses became possible through the development by Stamper and Woodruff in 1976 of an in vitro adhesion assay to assess lymphocyte adhesion to HEVs.18 This allowed Butcher et al to observe a remarkable selectivity in lymphocyte adhesion to HEVs according to their tissue origin, where binding preference to Peyer patch or peripheral lymph node HEVs predicted their differential segregation in vivo in these lymphoid tissues.19
The clinical importance of β2 integrins in leukocyte adhesion was demonstrated by the identification and characterization of the first patients with a syndrome of leukocyte adhesion deficiency (LAD) characterized by recurrent bacterial infections and a defect in phagocytosis.20,21 The application of cloning technologies for large molecules in the 1980s yielded the genetic identification of all major classes of adhesion molecules, and a specific nomenclature was proposed to distinguish each family.22-24 At the same time, the first leukocyte-specific chemotactic factor (IL-8) was purified from conditioned medium of lipopolysaccharide-stimulated monocytes and shown to selectively activate neutrophils.25,26 These chemokines were soon found to regulate leukocyte adhesion by increasing the activity and expression of β2 integrins while shedding other adhesion molecules (eg, L-selectin) from the leukocyte surface.27 These findings brought forth the notion that a stimulus could regulate the inflammatory response by both enhancing and reducing separate proinflammatory adhesion pathways. Seminal studies under physiological flow conditions in vitro and by intravital microscopy have revealed the distinct functions of these surface receptors, allowing the formulation of the multiple, sequential steps mediating leukocyte recruitment whereby leukocytes first tether and roll on selectins, become activated by inflammatory chemokines, and then adhere firmly on activated integrins.28-31 The availability of the first endothelial/leukocyte adhesion molecule–deficient32 and mutant mice33 showed that these molecules not only were important individually for leukocyte extravasation, but also influenced leukocyte homeostasis.
Vessel wall
Owing to its privileged situation at the interface between blood and organs, the endothelium is continuously influenced in its gene expression and function by signals originating from both the luminal and abluminal sides. It interprets changes in blood composition and its mechanical forces, and also receives information from the cellular and extracellular matrix constituents of the vessel wall. Thus, the endothelium cannot be viewed in isolation as its properties reflect not only its arterial or venous origins but also the needs of the organ it serves. Endothelium also changes as a consequence of injury or in pathological situations such as infection and can be affected by disease conditions such as diabetes. All these factors can affect expression of adhesion molecules, and thus modulate blood cell interaction with the vessel wall—the subject of this review.
Besides the endothelial monolayer and its matrix basement membrane, the vessel wall contains other cellular constituents such as smooth muscle cells and pericytes. On the arterial side of the circulation, the vessel wall is thicker to withstand pulsatile flow, while veins usually have larger lumens with thinner and less well-organized walls. In recent years, it has become clear that arterial and venous endothelium are distinct entities. During embryonic development, arterial cells already express Ephrin-B2, while venous cells express its cognate receptor Eph-B4. Expression of these molecules, and likely their interaction, is necessary for proper angiogenesis during development.34 The vascular beds behave differently in inflammation as most vascular leakage and leukocyte rolling and extravasation occur in postcapillary venules, but not in arterioles. The leaked interstitial fluid and inflammatory cells return to the blood via lymphatic vessels. Lymphatics are thin endothelium-lined channels, likely of venous origin, but expressing their own specific markers.35
As noted in the preceding section, leukocyte trafficking is not random but is directed by the endothelium (reviewed in Rao et al36 ). Throughout the body, highly specialized endothelia can either promote or inhibit leukocyte traffic by their surface properties. For example, HEVs in lymph nodes express specific adhesion molecules, ligands for L-selectin, and chemokines that are ideally suited for recruitment of naive T lymphocytes to be presented with antigen within the node. At these sites, large numbers of lymphocytes will leave the blood and transmigrate. Interestingly, a subset of transmigrating CD4+ lymphocytes called lymphoid tissue–inducer cells provide the critical signals inducing lymph node organogenesis.37 In contrast, immune cell transmigration is discouraged in other organs such as the brain and eye.38 Reduced trafficking results from low expression of adhesion molecules, reflected by the absence of rolling leukocytes in vessels with blood-brain barrier properties. In contrast to most endothelia, WPBs in brain endothelial cells do not contain P-selectin,39 and the induction of endothelial selectins in the brain following an inflammatory challenge is an order of magnitude less than that of other organs.40 Presumably, owing to the ability of the blood-brain barrier to prevent leakage and little leukocyte traffic, the brain has not developed draining lymphatic vessels.
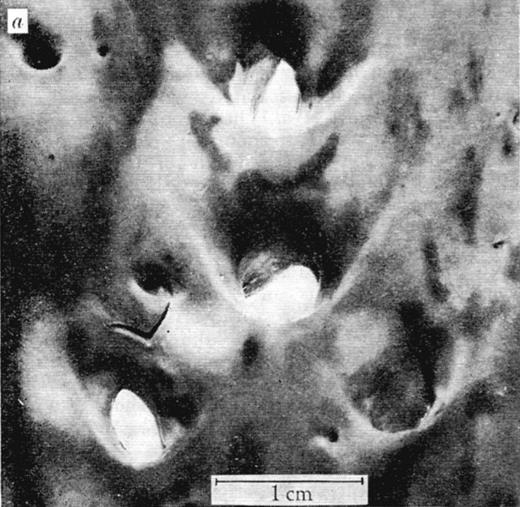
Major decisive influences on the sites of leukocyte transmigration, most notable in arteries, are the local characteristics of blood flow.41 In the 1960s, the arterial distribution of atherosclerotic lesions, formed by transmigrating monocytes accumulating lipids, was found not to be random, and the influence of blood mechanics was considered. Caro et al proposed the hypothesis that “… fluid mechanics has a controlling and inhibiting (or retarding) effect rather than a causative one” on atherogenesis.42 By thorough quantitative evaluation of lesion location within arterial bifurcations (Figure 4) and flow modeling, the authors determined that there were fewer lesions in the areas of arterial bifurcations where shear rates are locally high and laminar and more lesions on the opposite side that experiences lower shear and turbulent flow. Interestingly, the authors proposed that increasing cardiac output through exercise might retard the development of atheroma.42
View of human aorta stained with Sudan III for fat deposits/atheroma (dark stains) from the intimal side. Outer wall branches (experiencing disturbed flow) of the celiac, superior mesenteric, and renal arteries show more extensive staining than the inner walls where shear rates are high and laminar. Reproduced with permission from Macmillan Publishers Ltd.42
View of human aorta stained with Sudan III for fat deposits/atheroma (dark stains) from the intimal side. Outer wall branches (experiencing disturbed flow) of the celiac, superior mesenteric, and renal arteries show more extensive staining than the inner walls where shear rates are high and laminar. Reproduced with permission from Macmillan Publishers Ltd.42
The endothelium senses the flow mechanical forces, and this is reflected in the morphology of endothelial cells in arteries. In laminar flow, endothelial cells assume an elongated shape aligning with the direction of the shear stress, whereas the cells are polygonal in regions of disturbed flow.43 Many early studies brought indications that mechanical forces modified gene expression in endothelium.43,44 In agreement with the previously mentioned hypothesis by Caro et al, Topper et al found that laminar shear stress increases expression of several important atheroprotective genes such as endothelial nitric oxide synthase.45 Laminar shear up-regulates the transcription factor Kruppel-like factor 2 (KLF2), which can act as an activator or repressor of gene expression whose net effect favors protection from the development of atherosclerotic lesions.46 Interestingly, this transcription factor is also down-regulated by TNFα or IL-1β, cytokines that promote inflammatory responses.46,47 KLF2 appears to be an important down-regulator of leukocyte adhesion and thus leukocyte recruitment.48 Unexpectedly, KLF2 expression was recently shown to be increased by statins that were originally designed to decrease cholesterol levels but that show many beneficial vascular side effects.49,50
While effects of shear on gene expression reflect prolonged exposure to the mechanical forces and cytokine exposure can produce adaptive changes in a few hours, leukocyte and platelet recruitment at sites of vascular injury needs to be immediate. Such recruitment is mediated by the release of preformed components of WPBs (Figure 2).51,52 Thus endothelial cells have a regulated pathway of secretion, just as hormone secretory cells do. WPBs are released by secretagogues produced by injury or inflammation. These secretagogues induce signals that either increase intracellular Ca2+, for example after exposure to thrombin53 and histamine,54 or increase cAMP, for example by epinephrine55 and vasopressin.56 WPB secretion occurs both apically and basolaterally. There are few inhibitors of WPB secretion. The best documented is nitric oxide whose primary target is N-ethylmaleimide–sensitive factor (NSF) involved in the WPB membrane fusion process.57
WPBs are responsible for the “first aid-emergency medicine” and therefore are very versatile. They release proteins regulating hemostasis, vascular tone, inflammation and angiogenesis. The major component of WPBs is von Willebrand factor (VWF). VWF biosynthesis and aggregation in the trans-Golgi apparatus drives the formation of this organelle.58 The major component of WPBs is von Willebrand factor (VWF), a multimeric protein held together by disulphide bonds. VWF biosynthesis and aggregation in the trans-Golgi apparatus drives the formation of this organelle.58 WPBs contain the largest VWF multimers, which are the most active in promoting platelet adhesion.59 Several other biologically active components have been reported to be stored in WPBs.52 Among these are the vasoconstrictor endothelin, tissue plasminogen activator, osteoprotegerin active in bone remodeling, and angiopoietin-2 involved in vascular remodeling and inflammation. The receptor P-selectin that mediates both leukocyte and platelet adhesion is found in the organelle membrane, which during secretion fuses with the endothelial plasma membrane.13,14 Interestingly, some components that can be stored in WPBs, such as the chemokine IL-8, are made by endothelial cells only during inflammation and are found stored in the WPBs even after inflammation has subsided. Thus the endothelium retains a memory of the inflammatory process,60,61 perhaps keeping the vessel wall on guard for the next challenge.
Leukocyte rolling, adhesion, and transmigration
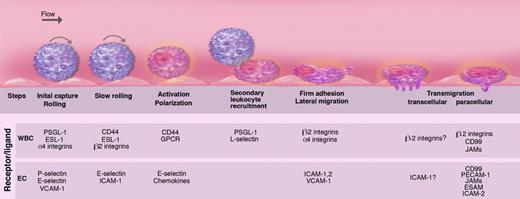
The secretion of the WPBs initiates the multistep process of leukocyte recruitment,28,29 our understanding of which has been refined, but not altered, in major ways over the past decade (Figure 5). In the systemic microvasculature, P-selectin (in acute injury) and E-selectin (in inflammatory conditions) mediate the initial leukocyte capture and rolling along the wall of postcapillary and collecting venules.32,62,63 In specialized endothelia, the initial interactions with endothelial cells can be mediated by other molecules, such as L-selectin in lymph nodes or α4 integrins in Peyer patches or in the bone marrow. These adhesion molecules are concentrated in leukocyte microvilli, fine cellular protrusions allowing the leukocyte to reach down into the endothelial glycocalyx, a meshed network of sulfated glycosaminoglycans (GAGs; reviewed in Weinbaum et al64 ).
Multiple sequential steps mediating leukocyte recruitment during inflammation. Leukocytes are captured and begin to roll on P- and E-selectins and their ligands P-selectin glycoprotein ligand-1 (PSGL-1) and E-selectin ligand-1 (ESL-1). Some leukocytes such as lymphocytes or hematopoietic stem and progenitor cells also roll on α4 integrin and its endothelial receptor vascular cell adhesion molecule-1 (VCAM-1). L-selectin is critical for lymphocyte rolling on HEVs in lymphoid tissues. As inflammation progresses, leukocyte rolling velocity decreases, allowing the integration of activation signals from selectin ligands and G-protein–coupled receptors (GPCRs). These activation signals lead to the polarization of slowly rolling leukocytes and clustering of L-selectin and PSGL-1 to a major pole that allows further leukocyte recruitment through secondary tethers via leukocyte-leukocyte interactions. Leukocyte activation enhances integrin affinity and avidity, leading to firm adhesion on intercellular adhesion molecule-1 (ICAM-1) expressed on endothelial cells. Adherent leukocytes continuously migrate laterally to survey the microvasculature and search for possible sites for transmigration. Leukocytes can transmigrate classically through the junctional (paracellular) pathways via interactions among junctional adhesion molecules (JAMs), CD99 and platelet/endothelial-cell adhesion molecule-1 (PECAM-1), endothelial cell–selective adhesion molecule (ESAM), or alternatively through the endothelial cell (transcellular pathway). Illustration by Marie Dauenheimer.
Multiple sequential steps mediating leukocyte recruitment during inflammation. Leukocytes are captured and begin to roll on P- and E-selectins and their ligands P-selectin glycoprotein ligand-1 (PSGL-1) and E-selectin ligand-1 (ESL-1). Some leukocytes such as lymphocytes or hematopoietic stem and progenitor cells also roll on α4 integrin and its endothelial receptor vascular cell adhesion molecule-1 (VCAM-1). L-selectin is critical for lymphocyte rolling on HEVs in lymphoid tissues. As inflammation progresses, leukocyte rolling velocity decreases, allowing the integration of activation signals from selectin ligands and G-protein–coupled receptors (GPCRs). These activation signals lead to the polarization of slowly rolling leukocytes and clustering of L-selectin and PSGL-1 to a major pole that allows further leukocyte recruitment through secondary tethers via leukocyte-leukocyte interactions. Leukocyte activation enhances integrin affinity and avidity, leading to firm adhesion on intercellular adhesion molecule-1 (ICAM-1) expressed on endothelial cells. Adherent leukocytes continuously migrate laterally to survey the microvasculature and search for possible sites for transmigration. Leukocytes can transmigrate classically through the junctional (paracellular) pathways via interactions among junctional adhesion molecules (JAMs), CD99 and platelet/endothelial-cell adhesion molecule-1 (PECAM-1), endothelial cell–selective adhesion molecule (ESAM), or alternatively through the endothelial cell (transcellular pathway). Illustration by Marie Dauenheimer.
The selectins bind to specialized fucosylated sialoglycoconjugates, such as the tetrasaccharide sialyl Lewis X (sLex), that decorate selected surface glycoproteins. P-selectin glycoprotein ligand-1 (PSGL-1), the most studied ligand, can interact with all 3 selectins under physiological inflammatory situations.65 Expressed as a dimer on most leukocytes, its binding activity is conferred by the few amino acids of the N-terminal region where sulfation of tyrosine residues is critical for binding to P- and L-selectins and by threonine O-linked glycans harboring sLex that binds to all selectins. While several glycosyltransferases have been shown to synthesize selectin ligands, the most dramatic phenotype from genetic deletion studies was reported in mice lacking both fucosyltransferases (Fut4 and Fut7), which exhibit defects of leukocyte recruitment as severe as mice lacking all selectins.66,67 The importance of fucosylation is further illustrated by the human genetic disease LAD type II characterized by recurrent infections, Bombay blood group, mental retardation, and the absence of selectin ligands owing to a mutation in the GDP-fucose transporter gene (reviewed in Etzioni68 ). Regulated glycosylation can also affect ligand function. For example, PSGL-1 is expressed on all CD4+ T helper cells but is functional on Th1, but not Th2, cells, owing to selective Fut7 expression in Th1 cells.69 While several glycoproteins have been suggested to bind to E-selectin, the complete identity of murine E-selectin ligands (ESLs) on neutrophil was only recently elucidated.70 These studies revealed contributions of PSGL-1, ESL-1, and CD44 that reflect their location on the leukocyte surface. PSGL-1, enriched at the very tip of leukocyte microvilli, plays a major role in the initial capture of the leukocyte to the endothelium, while CD44, found on the leukocyte body, controls leukocyte rolling velocity and transduces signals. Studies using RNA interference have revealed that ESL-1, expressed on the microvilli body but excluded from the tip, was the most powerful and versatile ESL, contributing to leukocyte capture, slow rolling, and arrest.70 There could be important leukocyte subset or species differences in selectin ligands that will require further evaluation. For example, CD43 has been suggested to function as an ESL on activated human T cells,71 and a CD44 isoform has been suggested to represent the most abundant ESLs on human CD34+ cells.72 On murine myeloid cells, intravital microscopy studies have revealed that ESL-1 was critical for the transition to slow rolling since its knockdown was sufficient to produce a skipping behavior and higher leukocyte rolling velocities.
Slow rolling allows leukocytes to sample chemokines presented by GAGs on the endothelium. Chemokines and their receptors provide cell- and tissue-specific activation signals that selectively regulate recruitment. T cells, for example, use CCR7 for directed migration toward CCL21 and CCL1973 ; monocytes classically respond to CCL2 through its receptor CCR2 but other chemokine receptors control the recruitment of monocyte subsets in atherosclerotic plaques.74 Recruitment specificity to certain tissues may be enhanced by unique combinations of adhesion receptors and chemokines. For example, CXCL12 and its receptor are broadly expressed, but specific homing of hematopoietic stem cells to bone marrow is enhanced by the collaboration of E-selectin ligands and α4 integrins and their endothelial counterreceptors E-selectin and VCAM-1, which are constitutively expressed in the bone marrow.71 All chemokine receptors signal through heterotrimeric G-protein–coupled receptors whose downstream effectors differ depending on the type of the G-protein alpha-subunit involved, and include phosphatidylinositol 3-kinase (PI3K), phospholipase C (PLC), dedicator of cytokinesis 2 (DOCK2), and the small GTPase RhoA.75 The Ras-related GTPase, Rap1, has also emerged as a key regulator of integrin function.76-78 This notion was further substantiated, notably through the demonstration of a genetic defect, LAD type III, in which chemokine-triggered integrin activation in platelets and leukocytes was impaired due to defective Rap1 activation by the guanine nucleotide exchange factor CalDAG-GEFI.79,80 Slow rolling on E-selectin also transduces signals via CD44 that induce the polarization of rolling leukocytes with segregation of PSGL-1 and L-selectin to a major cluster, even on leukocytes that have not arrested.70 The redistribution of PSGL-1 and L-selectin to high-density areas presumably allows other leukocytes to be recruited through secondary tethers.81 Intravital microscopy studies have revealed that flowing red blood cells can also tether with adherent leukocytes, thereby mediating vaso-occlusion in sickle cell disease.82,83 E-selectin– and P-selectin–induced signals may also collaborate in vivo with chemokine receptor signaling in integrin activation and leukocyte arrest.84-86
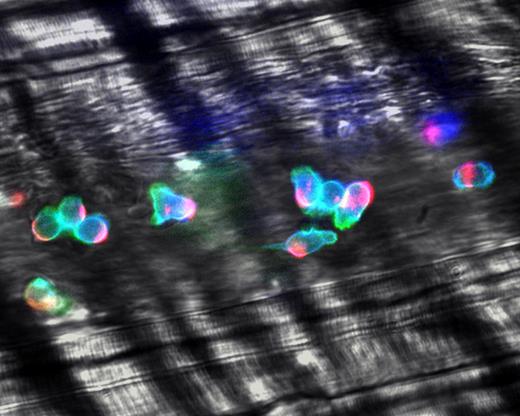
Integrins are heterodimers, formed by an α and β chain, that are normally found in a closed low-affinity conformation on most leukocytes.23 The X-ray crystal structure and nuclear magnetic resonance analyses have revealed at least 3 conformations where the integrin may rest in a bent conformation with its headpiece near the plasma membrane, and activation signals lead to the separation of the α and β subunit cytoplasmic tails, converting the bent conformation into fully extended high-affinity structures in a switchblade-like movement.87,88 A variety of intermediate conformations, with matched affinity states, likely exists. These may contribute to the slow leukocyte rolling prior to firm leukocyte adhesion. In addition to affinity regulation, the overall strength of adhesiveness (ie, avidity) is further controlled by the clustering of integrin molecules. Displacements of surface receptors affects the entire cell surface, leading to cell polarization and segregation of adhesion molecules, chemokine receptors, and associated cytoplasmic constituents to a leading or trailing edge.89 High-speed intravital analyses have revealed that virtually all adherent leukocytes are polarized in inflamed venules (Figure 6), and that the majority of leukocytes actively migrate laterally on the venular surface, searching for appropriate conditions for extravasation.90
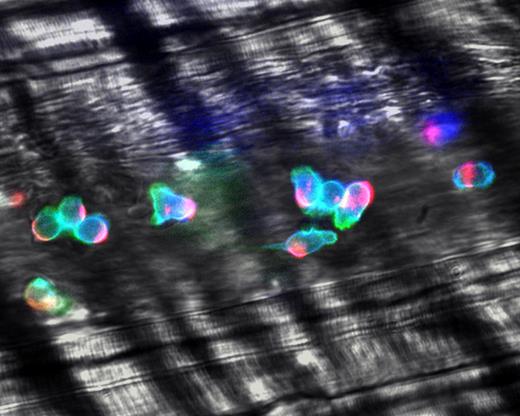
Polarization of adherent leukocytes. In vivo imaging using high-speed high-resolution multichannel fluorescence intravital videomicroscopy of αLβ2 integrin (blue), PSGL-1 (red), and the granulocyte marker Gr-1 (green) expressed on the leukocyte surface in tumor necrosis factor-α (TNF-α) inflamed venules. The majority of leukocytes migrates laterally in inflamed venules. These migrating leukocytes exhibit marked clustering of PSGL-1 at the trailing edge. The large PSGL-1 cluster may contribute to leukocyte recruitment through leukocyte-leukocyte secondary tethers. In contrast, the expression of αLβ2 is relatively homogenous on the leukocyte surface. Reproduced with permission from Nature Publishing Group.90
Polarization of adherent leukocytes. In vivo imaging using high-speed high-resolution multichannel fluorescence intravital videomicroscopy of αLβ2 integrin (blue), PSGL-1 (red), and the granulocyte marker Gr-1 (green) expressed on the leukocyte surface in tumor necrosis factor-α (TNF-α) inflamed venules. The majority of leukocytes migrates laterally in inflamed venules. These migrating leukocytes exhibit marked clustering of PSGL-1 at the trailing edge. The large PSGL-1 cluster may contribute to leukocyte recruitment through leukocyte-leukocyte secondary tethers. In contrast, the expression of αLβ2 is relatively homogenous on the leukocyte surface. Reproduced with permission from Nature Publishing Group.90
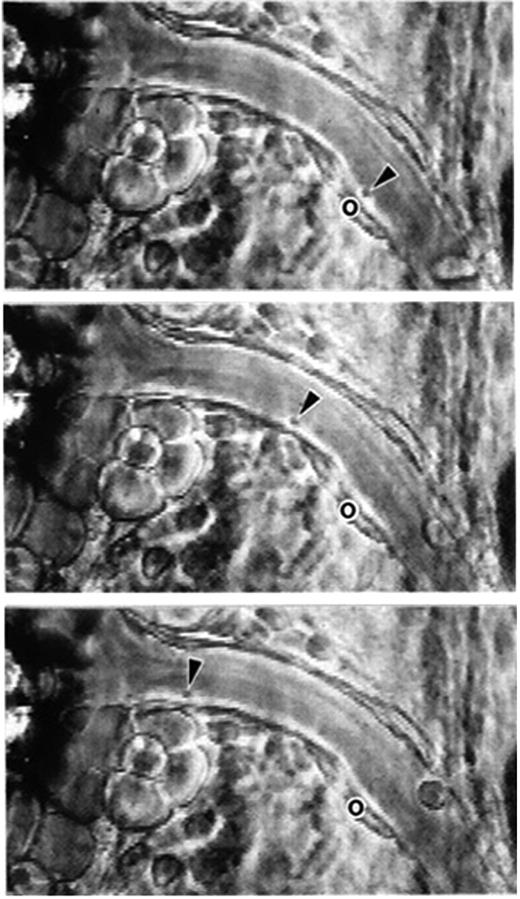
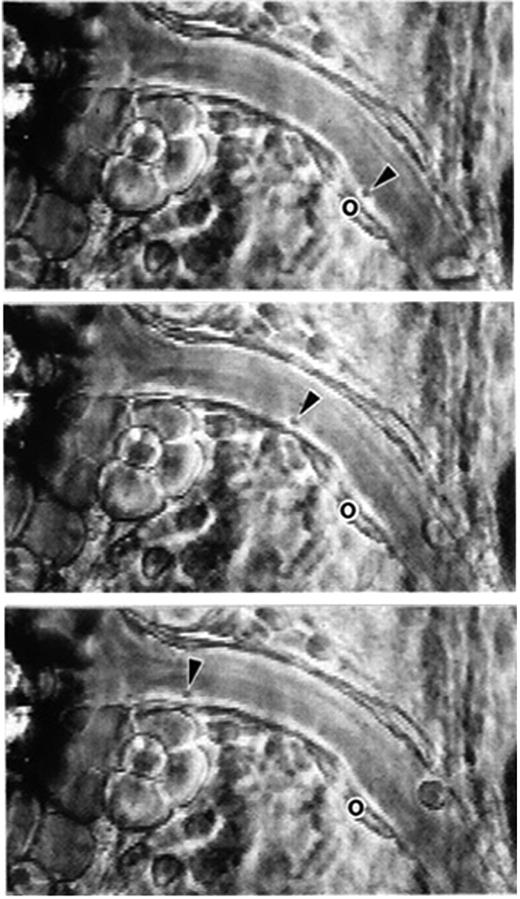
Phase-contrast intravital microscopy showing a platelet rolling on a mesenteric venule after stimulation by the calcium ionophore A23187. “o” indicates the location of the platelet at time 0. Arrowheads point toward the rolling platelet at 0 seconds (top), 1.58 seconds (middle), and 2.96 seconds (bottom). A single much larger and more slowly rolling leukocyte is also seen. Bar represents 30 μm. Reproduced with permission from PNAS.7
Phase-contrast intravital microscopy showing a platelet rolling on a mesenteric venule after stimulation by the calcium ionophore A23187. “o” indicates the location of the platelet at time 0. Arrowheads point toward the rolling platelet at 0 seconds (top), 1.58 seconds (middle), and 2.96 seconds (bottom). A single much larger and more slowly rolling leukocyte is also seen. Bar represents 30 μm. Reproduced with permission from PNAS.7
Until recently, the high dynamism of adherent leukocytes and the receiving endothelial cells had not been appreciated. In vitro studies have uncovered raised structures, enriched in the integrin counterreceptors VCAM-1 and ICAM-1 on the endothelial surface, that surround laterally migrating leukocytes.91,92 The actual anatomic site of leukocyte extravasation has been debated ever since the phenomenon was suggested a century ago.93 Electron microscopy analyses and multiple in vitro studies using human umbilical vein endothelial cells have supported the view that leukocytes extravasate between endothelial cells (paracellular route). The molecular determinants that mediate paracellular transmigration have been characterized and shown to involve adhesion molecules that concentrate at the intercellular junctions, such as PECAM-1, JAMs, or CD99.94 On the other hand, migration directly through endothelial cells (transcellular route) was also suggested decades ago.93 Careful electron microscopy analyses of transmigrating neutrophils in vivo have indicated that transcellular migration occurs in certain physiological settings.95,96 Indeed recent studies have supported the possibility that the 2 pathways may coexist. In vitro studies have revealed that ICAM-1 translocates to F-actin– and caveolin-1–rich regions close to endothelial cell-cell borders, and that adherent T lymphoblasts extend pseudopodia down to the basal endothelial membrane, establishing a transcellular pathway through caveolin- and F-actin–enriched channels.97 This phenomenon may be facilitated by intermediate filament networks.98 The ventral lymphocyte protrusions exhibit characteristic features of podosomes, including an F-actin core structure surrounded by the integrin signaling machinery.99 In hematopoietic cells, podosomes have been described in osteoclasts where they are thought to participate in translocation on bone surfaces. Leukocyte podosomes, however, appear to act as a sensing organ, probing for a suitable path for transcellular migration.99 Although there is in vitro evidence supporting both pathways, it is important to point out that the paracellular pathway appears to dominate in each system tested to date. Further studies are needed to determine the relevance of each pathway in vivo and to define how leukocyte subsets, the type of inflammatory stimulus, or endothelia and the quality of their junctions influence transmigration.
Platelet–vessel wall interactions
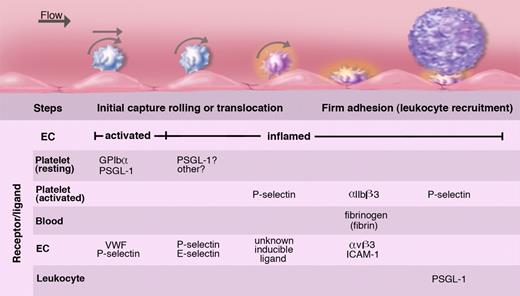
Intravital microscopy analyses of leukocyte-endothelial interactions have revealed that much smaller blood corpuscles, the platelets, also roll on the vessel wall (Figure 7).7 Similarly to leukocyte rolling, platelet rolling increases upon endothelial activation and is observed mainly in veins. The release of WPB components is crucial in mediating this interaction. Interestingly, the identity of the predominant adhesion molecule from the WPB that mediates platelet rolling depends on shear. In small venules where shear is higher, P-selectin supports a true rolling motion of the platelets. The counterreceptor on the platelets is PSGL-1 or GPIbα100,101 (Figure 8). Platelet interactions with stimulated veins of lower shear are much more prominent and are primarily mediated by VWF.102 A carpet of platelets forms within seconds after WPB secretion. These translocate slowly downstream in a manner similar to observations of in vitro translocation of platelet GPIbα on VWF.103 In the absence of additional stimulation, platelets disengage from the vessel wall in a few minutes and return to circulation. Their release is mediated by cleavage of the VWF by the plasma metalloprotease ADAMTS13 (a disintegrin-like and metalloprotease with thrombospondin type I repeats 13),104 the enzyme that is lacking in thrombotic thrombocytopenic purpura (reviewed in Tsai105 ). In the absence of this enzyme, the VWF polymers associate in long strings spanning many endothelial cells to which platelets adhere like beads on a necklace.104,106 Such strings can coalesce and form thrombi in small vessels, a process normally prevented by ADAMTS13.107
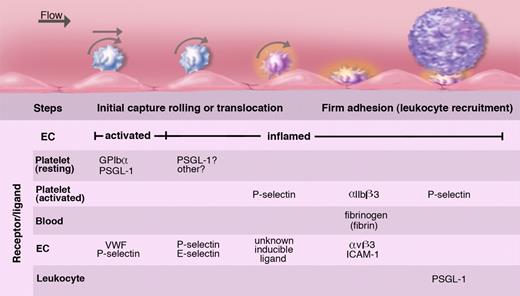
Platelets roll/translocate on stimulated endothelium of veins; this can lead to their firm adhesion. Endothelium either activated to release WPBs or inflamed by cytokines captures resting or activated platelets. These roll on the selectins or move laterally on VWF. In inflammation, fibrinogen or fibrin oligomers can promote firm adhesion of activated platelets by cross-linking the major platelet integrin to endothelial receptors. These adherent platelets expressing P-selectin may recruit leukocytes but may also initiate pathological thrombosis such as occurs in deep veins. The various known ligands and receptors participating in these platelet adhesion events are listed. Illustration by Marie Dauenheimer.
Platelets roll/translocate on stimulated endothelium of veins; this can lead to their firm adhesion. Endothelium either activated to release WPBs or inflamed by cytokines captures resting or activated platelets. These roll on the selectins or move laterally on VWF. In inflammation, fibrinogen or fibrin oligomers can promote firm adhesion of activated platelets by cross-linking the major platelet integrin to endothelial receptors. These adherent platelets expressing P-selectin may recruit leukocytes but may also initiate pathological thrombosis such as occurs in deep veins. The various known ligands and receptors participating in these platelet adhesion events are listed. Illustration by Marie Dauenheimer.
There are many molecular and behavioral parallels between platelet and leukocyte interactions with the vessel wall. Recently, this became even more evident when VWF, the molecule traditionally recognized for platelet adhesion, became implicated in promoting leukocyte rolling and adhesion both in vitro108 and in vivo (A. Chauhan and D.D.W., unpublished data, July 2005). Thus ADAMTS13, which clears the released VWF from the vessel wall, likely down-regulates both thrombosis and inflammation.
In inflamed vessels, in addition to VWF and P-selectin,109 E-selectin, whose expression is induced by cytokines, contributes to resting platelet rolling.110 Platelets, like leukocytes, were also shown to adhere firmly to inflamed endothelium in vivo by the major platelet integrin αIIβ3. αIIβ3 binds to fibrin/fibrinogen anchoring to endothelial cells' αVβ3 or ICAM-1111 (Figure 8). There are other receptors that were shown to mediate firm platelet adhesion to inflamed endothelium in vitro, and their importance awaits in vivo evaluation.112 Since the density of P-selectin on platelets after its release from platelet α-granules is much higher than on endothelium, leukocytes are easily recruited to the adherent activated platelets. They roll on the platelets and after activation transmigrate.113,114 Thus platelets may facilitate leukocyte recruitment to inflamed or injured vessel wall.
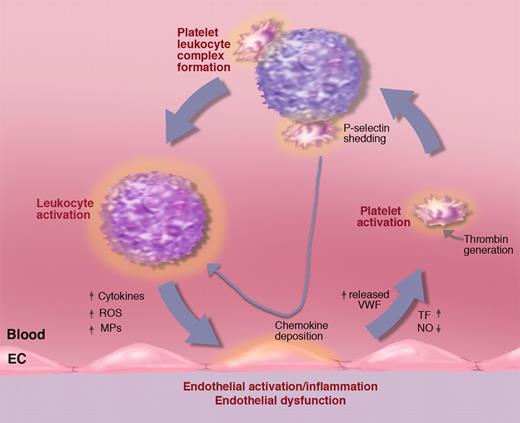
Activated platelets in circulation bind avidly to leukocytes, forming platelet-leukocyte complexes. This is mediated by platelet P-selectin and by PSGL-1 on the leukocyte.115,116 Whenever there is platelet activation, platelet-leukocyte complexes form, and these can also be seen rolling on endothelium.7 The platelet P-selectin signals through PSGL-1 inducing leukocyte integrin activation.117 The integrins can stabilize the interaction with the platelets by binding to ICAM-2 or GPIbα.112 Importantly, P-selectin/PSGL-1 signaling stimulates inflammatory cytokine secretion by monocytes (eg, TNF-α, MCP-1, and IL-8).118 These rolling platelet-leukocyte complexes likely feed the cytokines directly to the vessel wall thus sustaining, or even enhancing, the inflammatory process (Figure 9). Activated platelets also deposit chemokines on endothelium, such as RANTES,119 that promote shear-resistant monocyte adhesion (important in arteries). Experimental infusion of activated platelets into mice leads to systemic WPB secretion and a transient increase in the numbers of rolling leukocytes.120 This may have a pathological impact since repeated infusions of activated platelets promote atherosclerosis.121 Chemokine deposition by platelets and monocyte recruitment in atherosclerosis is P-selectin dependent.122 Clearly activated platelets and their P-selectin importantly feed into a vicious circle of inflammation (Figure 9), showing that thrombosis and inflammation are intimately linked. The pivotal role of P-selectin is emphasized by the observations that even soluble P-selectin, shed from the activated platelets and endothelium, stimulates leukocytes to produce tissue factor123 and to adhere.124 Mice overexpressing soluble P-selectin show many aspects of prothrombotic state125 and chronic inflammation (J. Kisucka and D.D.W., unpublished observations).
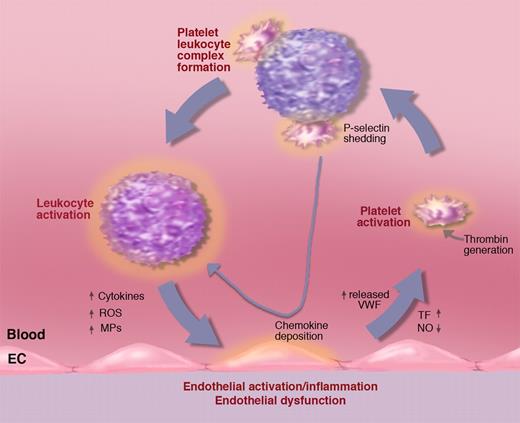
Activated platelets propel the vicious circle of inflammation. A procoagulant state or inflammatory process generating tissue factor (TF) may lead to platelet activation. Activated platelets bind to leukocytes promoting, in turn, leukocyte activation. Platelet leukocyte complexes produce chemokines that, when deposited on the vessel wall, facilitate leukocyte recruitment. Binding to leukocytes causes platelet P-selectin shedding. Elevated soluble P-selectin activates additional leukocytes. Activated leukocytes can produce leukocyte-derived microparticles (MPs) that further promote endothelial activation as well as cytokines and reactive oxygen species (ROSs) advancing inflammation and endothelial dysfunction. Endothelial dysfunction reduces NO and prostaglandin I2 production leading to increased release of WPBs promoting leukocyte and platelet rolling and facilitating platelet activation. Illustration by Marie Dauenheimer.
Activated platelets propel the vicious circle of inflammation. A procoagulant state or inflammatory process generating tissue factor (TF) may lead to platelet activation. Activated platelets bind to leukocytes promoting, in turn, leukocyte activation. Platelet leukocyte complexes produce chemokines that, when deposited on the vessel wall, facilitate leukocyte recruitment. Binding to leukocytes causes platelet P-selectin shedding. Elevated soluble P-selectin activates additional leukocytes. Activated leukocytes can produce leukocyte-derived microparticles (MPs) that further promote endothelial activation as well as cytokines and reactive oxygen species (ROSs) advancing inflammation and endothelial dysfunction. Endothelial dysfunction reduces NO and prostaglandin I2 production leading to increased release of WPBs promoting leukocyte and platelet rolling and facilitating platelet activation. Illustration by Marie Dauenheimer.
There is a silver lining to the platelet cloud during inflammation. Platelets provide important beneficial nurturing effects to the inflamed endothelium and the vessel wall, diminishing the injury produced by the transmigrating leukocytes. Thrombocytopenic mice subjected to inflammatory stimuli in skin, lung, or brain bleed significantly from the inflamed venules, whereas there is no hemorrhage outside the inflamed organ.126 This shows that platelets, through a yet unknown mechanism, support the integrity of the microcirculation during inflammation, perhaps generating the “restorative power of blood” noted by Waller.2
Questions for the future
Although we can be proud of our progress in understanding the molecular and cellular mechanisms mediating leukocyte and platelet interactions with the vessel wall (Figure 5,8), there are still many challenges and open questions ahead. Pathological inflammation and thrombosis are now the biggest killers in the Western world, and we have to seek effective ways to stop the “vicious circle of inflammation.” Activated platelets are present in numerous common diseases such as coronary syndromes, ischemic cerebrovascular disease, peripheral arterial or venous disease, diabetes mellitus, Alzheimer disease, sickle cell disease, rheumatoid arthritis, and even asthma.112 Finding ways to better inhibit platelet activation or platelet-leukocyte complex formation and the resulting signaling would represent a major step forward. In addition, we do not know what molecules or microparticles these platelet-leukocyte complexes generate that cause WPB secretion,120 and activation of the vessel wall, contributing to inflammation and heart disease.127
Laminar shear on the vessel wall has a calming effect on the endothelium. If we knew the cellular receptors and pathways that sense the shear in endothelial cells, perhaps these could be stimulated to enhance the beneficial signaling effects. Vascular dysfunction, such as seen under turbulent flow, is clearly a major promoter of inflammation and thrombosis, in particular in aging. In heart disease, oxidized lipid deposits induce such dysfunction starting by endothelial activation. Such “injury to endothelium” as the basis for atherosclerosis was already proposed in 1976 by Ross and Glomset.128 Could other deposits such as beta amyloid in the brain vasculature have similar effects promoting endothelial injury/inflammation at the level of the blood-brain barrier? Vascular dysfunction in the brain might progress to neurodegeneration as blood vessels and nerves appear linked by astrocyte signaling.129 Learning more about various specialized endothelia, and their interactions with blood cells, will be key to address organ-specific inflammatory diseases.
In addition to leukocytes and platelets, the endothelium can interact with erythrocytes under pathological situations. The best studied example occurs in sickle cell disease where sickle erythrocyte adhesion in venules has been suggested to contribute either directly to vaso-occlusion, and/or indirectly by activating endothelial cells (reviewed in Frenette130 ). Blood cell-cell interactions, such as erythrocyte-leukocyte interactions in small venules, can cause vascular occlusion in sickle cell mice, but the molecular mechanisms are not yet defined. Interestingly, these heterocellular interactions have been documented in normal mice under inflammation (A. Hidalgo and P.S.F., unpublished data), suggesting potentially broader pathological functions. Elucidation of the mechanisms of vascular occlusion in sickle cell disease, where inflammation is severe, may teach us important principles applicable to other more common ischemic vasculopathies.
While the initial steps mediating leukocyte recruitment are relatively well characterized, the mechanisms of transmigration remain less defined. Emerging in vitro studies have revealed a remarkable dynamism of the endothelial membrane and receptor expression in which they actively embrace crawling leukocytes prior to migration.91,92 This emphasizes the active, continuous cross-talk between the endothelial cell and the leukocyte. The actual anatomic site of transendothelial migration, whether transcellular or paracellular, needs to be characterized in vivo using novel high-speed imaging techniques. It is possible that these pathways differ mechanistically, offering new therapeutic opportunities. For example, one could envision the possibility of inhibiting selectively the metastasis of tumor cells, which may preferentially be taking one of these routes.
The recent progress in stem cell biology offers enormous promise for regeneration and the potential cure of several diseases. In addition to providing a conduit for trafficking, the vasculature can provide a niche that houses stem cells. Such a vascular stem cell niche has been described in organs such as the brain,131 testis,132 and bone marrow.133 The vasculature may also nurture cancer stem cells.134 However, much work remains to dissect the constituents, both at the cellular and molecular levels, providing the critical signals that govern survival, retention, quiescence, self-renewal, and differentiation. Some signals may not originate from the niche itself but be delivered from afar, such as the recently described role of the sympathetic nervous system in regulating circadian release of hematopoietic stem cells from the bone marrow.135
We look forward to the years ahead when all the basic knowledge accumulated over the past decades will be harvested to stop disease progression and improve the quality of our lives.
Acknowledgments
We regret that many important papers could not be cited or discussed in detail, due to space limitations. The help with library searches by Stephen Cifuni and paper preparation by Lesley Cowan is appreciated. We are grateful for stimulating discussions with Michael Gimbrone, Richard Hynes, Sergio Lira, Timothy Springer, and Ulrich von Andrian.
This work was supported by the National Institutes of Health (for D.D.W.: R37 HL041002, P01 HL056949, and P01 HL066105; and for P.S.F.: R01 grants DK056638, HL69438, and AI069402). P.S.F. has also received support from the Department of Defense (Idea Development Award PC060271) and an Established Investigator Award from the American Heart Association.
National Institutes of Health
Authorship
Contribution: D.D.W. and P.S.F. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Denisa D. Wagner, Immune Disease Institute and Department of Pathology, Harvard Medical School, Boston, MA 02115; e-mail: wagner@idi.harvard.edu; or Paul S. Frenette, Department of Medicine, Black Family Stem Cell Institute and Immunology Institute, Mount Sinai Medical School, New York, NY 10029; e-mail: paul.frenette@mssm.edu.