Abstract
Novel molecular targeted therapies, such as imatinib for chronic myelogenous leukemia (CML), represent the first agents that inhibit cancer cells more than other dividing cells, such as immune cells. We hypothesize that imatinib may create a window in which the immune response is partially restored while apoptotic leukemic cells are present, thus rendering leukemic cells immunogenic as patients enter remission. To detect and quantify antileukemia immune responses in an antigen-unbiased way, we used cryopreserved autologous pretreatment blood samples (representing predominantly leukemic cells) as stimulators to detect antileukemia T-cell responses in CML patients in remission on imatinib. We studied patients over time to address the dynamics of such responses. Our data show that antileukemia T-cell responses develop in the majority of CML patients (9 of 14) in remission and that CD4+ T cells producing tumor necrosis factor-α (median 17.6%) represent the major response over interferon-γ. This confirms the immune system's ability to respond to leukemia under certain conditions. Such responses may be further amplified as a potential therapy that synergizes with imatinib for improved control of CML.
Introduction
Chronic myelogenous leukemia (CML) is driven by deregulation of the abl tyrosine kinase1 because of bcr/abl translocation.2 Imatinib is the first of a new breed of molecular targeted cancer therapies; it inhibits the abl tyrosine kinase3 and has become the first-line therapy for CML. With imatinib, nearly all patients achieve hematologic remission2 and 75% achieve cytogenetic remission.4 Nonetheless, patients relapse on imatinib discontinuation4 and may develop resistance.3 Strategies to enhance imatinib's efficacy are needed. Combining imatinib with immunotherapy represents a promising strategy; however, the role of the immune response remains unclear. Anthracyclins have been shown to induce immunogenic death of cancer cells in mice.5 In vitro, imatinib renders leukemic cells immunogenic.6,7 Indeed, low frequency (generally < 1%) CD8+ T-cell responses to 4 leukemia-associated antigens (LAAs), abl kinase, proteinase 3, telomerase, and Wilm's tumor 1, were detected in CML patients after therapy with interferon-α8,9 or imatinib.8 However, the magnitude and duration of the total antileukemia T-cell response remain unclear because these LAAs represent only a subset of leukemia antigens. Moreover, CD4+ T cells probably play an important role but remain largely unaccounted.10 To circumvent the need to use defined LAAs, we used cryopreserved autologous pretreatment leukemic blood samples (representing a pool of LAAs) as stimulators to detect antileukemia CD4+ and CD8+ T-cell responses in blood samples from CML patients in remission on imatinib. We studied patients over time to gain insights into the dynamics of these responses and their possible role in the control of CML.
Methods
Patients
Whole blood (all patients and time points) and bone marrow (P5 at diagnosis, P11 at 4 months) samples from 14 CML patients treated with imatinib (Novartis, East Hanover, NJ) were collected at diagnosis and at several time points after imatinib treatment. Peripheral blood mononuclear cells (blood samples) were acquired by density gradient centrifugation using Ficoll-Paque (GE Healthcare, Little Chalfont, United Kingdom) and cryopreserved in liquid nitrogen until use. Blood samples were thawed and rested overnight at 37°C, before experiments were performed. All assays were done in triplicates and repeated at least once. Pretreatment blood samples alone and remission blood samples alone served as controls, if not otherwise indicated. Pretreatment blood samples were irradiated at 4000 cGy before use. All patients were examined for HLA-A2 using a fluorescein isothiocyanate conjugated anti-HLA-A2 Ab (kind gift from M. Roederer, National Institutes of Health [NIH], Bethesda, MD). This study has been approved by the Panel on Human Subjects in Medical Research of Stanford University, and conducted in accordance with the principles of the Declaration of Helsinki. Informed consent was obtained from all patients before blood sampling in accordance with the Declaration of Helsinki.
Interferon-γ ELISPOT assay
The assay was performed essentially as described.11 In brief, 105 remission blood samples were incubated for 20 hours with 105 autologous pretreatment samples in interferon-γ (IFN-γ) (anti-h-IFN-γ mAb 1-D1K; Mabtech, Mariemont, OH) precoated plates (MultiScreen HTS IP; Millipore, Bedford, MA) in Iscove modified Dulbecco medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Invitrogen), and 2% human serum (cytotoxic T lymphocyte; Atlanta Biologicals, Lawrenceville, GA). After incubation, biotinylated detection anti-h-IFN-γ mAb 7-B6-1 (Mabtech) was added for 2 hours, followed by streptavidin-alkaline phosphatase (Mabtech) for 1 hour, and, as the final step, the alkaline phosphatase substrate BCIP/NBT (Moss, Pasadena, MD) for 20 minutes. Tap water was added to stop the color reaction. Pretreatment blood samples alone, remission blood samples alone, and 2 serial remission samples incubated together served as negative controls. The plates were imaged and quantified by ZellNet Consulting (Fort Lee, NJ).
Tumor necrosis factor-α and interferon-γ cytokine flow cytometry
The assay was performed essentially as described.12 In brief, 106 remission blood samples were stimulated with autologous pretreatment leukemic cells for 2 hours in a 96-well plate in CTL medium at 37°C; 10 μg/mL of brefeldin A (Sigma-Aldrich, St Louis, MO) was added for an additional 11 hours. Leukemic cells alone as well as remission cells alone served as controls. Cells were then stained with mouse anti–human CD3 Pacific Blue, mouse anti–human CD4 AmCyan, mouse anti–human CD8 allophycocyanin (APC)-Cy7, and mouse anti–human CD56 fluorescein isothiocyanate for 1 hour at room temperature, followed by treatment with FACS Lysing Solution and FACS Permeabilizing Solution 2 (BD Bioscienes, San Jose, CA), according to the manufacturer's directions. This was followed by an hour-long incubation with mouse anti–human IFN-γ phycoerythrin (PE)-Cy7, mouse anti–human tumor necrosis factor-α (TNF-α) APC, and mouse anti–human IL-2 PE (all antibodies BD Biosciences), and analyzed using a BD LSR II flow cytometer (BD Biosciences).
Generation of T-cell clones
CD8+ T-cell clones were generated from stimulated remission blood sample (4 months) from P11 based on the CD107 assay as described.13 CD4+ T-cell clones were generated based on CD4 expression. CD4+ and CD8+ T cells were sterile sorted one cell/well into 96-well plates using a BD Vantage flow cytometer (BD Biosciences), and expanded for at least 4 weeks using irradiated (4000 cGy) peripheral blood mononuclear cells (Stanford University blood bank) and irradiated (12 000 cGy) JY cell line (ATCC, Manassas, VA).14 16 CD4+ and 19 CD8+ T-cell clones were successfully expanded and tested for reactivity against autologous leukemic cells.
Sequencing of T-cell clones
RNA was extracted from the CD4+ and CD8+ T-cell clones using Trizol (Invitrogen). cDNA was synthesized from RNA using the OmniScript RT kit (Qiagen, Valencia, CA) according to the protocol, and the T cell receptor (TCR) Vβ gene expression of each T-cell clone was quantified using real-time polymerase chain reaction with the QuantiTect SYBR Green kit (Qiagen) on a LightCycler instrument. The degenerate forward primer VBUN and reverse primer FPR27 were used15 (ELIM Biopharmaceuticals, Hayward, CA) to amplify the gene expression for the potentially different TCR Vβ from each T-cell clone. The integrity of the amplification products was checked using the Nanochip (Agilent Technologies, Palo Alto, CA), and were sequenced by the Stanford PAN facility.
Beadlyte multiplex cytokine assay
A total of 5 × 104 CD4+ or CD8+ T-cell clones were incubated with autologous pretreatment leukemic cells at a ratio of 1:1 in microcentrifuge tubes (VWR, West Chester, PA) for 13 hours. The supernatants were collected and stored at −80°C, until use in the multiplex cytokine assay using the Beadlyte human multicytokine detection system 1 for IL-2, IL-4, granulocyte-macrophage colony-stimulating factor, TNF-α, and IFN-γ (Upstate Biotechnology, Charlottesville, VA).16 In brief, 50 μL of the supernatant was added to individual wells in a 96-well plate in triplicates, followed by 25 μL of Beadlyte antihuman multicytokine beads 1. The plate was incubated for 2 hours in the dark at room temperature on a plate shaker; 25 μL of Beadlyte antihuman multicytokine 1, biotin was added for 1.5 hours in the dark at room temperature on a plate shaker, followed by 25 μL Beadlyte streptavidin-PE (1:25) for 30 minutes. The reaction was stopped by adding 25 μL Beadlyte stop solution, and the plate was read at the Luminex 100 IS System, version 2.3 (Luminex, Austin, TX). T-cell clones without stimulation or stimulated with autologous remission blood samples served as controls.
Lysate pulsed T-cell clones from P11
Tumor cell lysates were generated from 3 × 105 pretreatment leukemic cells via 4 freeze/thaw cycles in dry ice + ethanol and water bath at 37°C; 1.5 × 105 cells from remission blood sample (6 months) from P11 were pulsed with lysate for 24 hours at 37°C and then added to 3 × 105 CD4+ or CD8+ T-cell clones. Cytokine flow cytometry (CFC) for TNF-α and IFN-γ was performed as described.12 T-cell clones stimulated with remission blood samples pulsed with lysate from the AML cell line THP-1 (ATCC) as well as mitogen phytohemagglutinin (PHA) + ionomycin served as negative controls. Leukemia stimulation of CD8+ T cells was blocked by addition of 1 μg/mL anti-MHC class I antibodies W6/32 (ATCC) and 100 ng anti-CD8 antibodies (BD Biosciences), and CD4+ T cells by addition of 10 μg/mL anti-MHC II antibodies (anti-DR, DP, DQ; BD Biosciences) and 10 μL anti-CD4 antibodies (Invitrogen) for 30 minutes before start of incubation and kept throughout the incubation period.
Peptide pulsed T-cell clones from P11
Remission blood samples from P11 were tested for MHC I (HLA-A0201, HLA-B8) and MHC II (HLA-DRB1, HLA-DRB4, and HLA-DQ2) antigens using specific PE-conjugated antibodies for flow cytometry as well as real-time polymerase chain reaction. IFN-γ release in 5 × 104 CD8+ T-cell clones (HLA-A0201+) was assessed in ELISPOT assay on stimulation with 10 μg/mL of the HLA-A0201 restricted leukemia associated heteroclitic peptides PR-1 169-177 (VLQELNVTV), WT-1 126-135 (RMFPNAPYL), WT-1 187-195 (SLGEQQYSV), bcr 912-920 (FLNVIVSHA), and bcr 1181-1189 (FLLDHLKRV) as well as general tumor associated peptides survivin 95-104 (ELTGEFLKL), p53 264-272 (LLGRNSFEV), p68 168-176 (YLLPAIVHI), and hTERT 540-548 (ILAKFLHWL; Stanford University PAN facility). Stimulation with the melanoma associated peptide gp100 209-217 (IMDQVPSFV) and PHA as well as the melanoma clone 476.139 as effector cells served as controls.
In vitro stimulation of remission blood samples
Remission blood samples from 2 patients (P10 at 28 months and 32 months, P14 at 3 months), which did not show a cytokine response to autologous pretreatment leukemic cells directly ex vivo, were incubated with lysate from autologous leukemic cells or irradiated leukemic cells in vitro at a ratio of 1:1 in the presence of 30 U/mL rhu-IL-2 (NIH) and 25 U/mL rhu-IL-7 (PeproTech, Rocky Hill, NJ). After 2 weeks, cells were analyzed in ELISPOT, CFC, and CD15417 assays.
Statistics
All results were expressed as median (range). Statistical analyses were performed using the 2-tailed Mann-Whitney U test. Results from comparisons were considered significant when P was less than or equal to .05.
Results
Patients
Fourteen patients were included in the study (Table 1). All patients achieved hematologic remission within 1 to 3 months. Ten patients achieved complete cytogenetic remission, and 4 achieved major cytogenetic remission. All patients also achieved at least major molecular responses and sustained molecular as well as cytogenetic responses over time (up to 60 months), except patient 9 (P9), who relapsed after 3 years, and P13, who relapsed after 4 years (after stopping treatment due to imatinib intolerance).
Significant tumor necrosis factor-α and interferon-γ responses in remission under imatinib
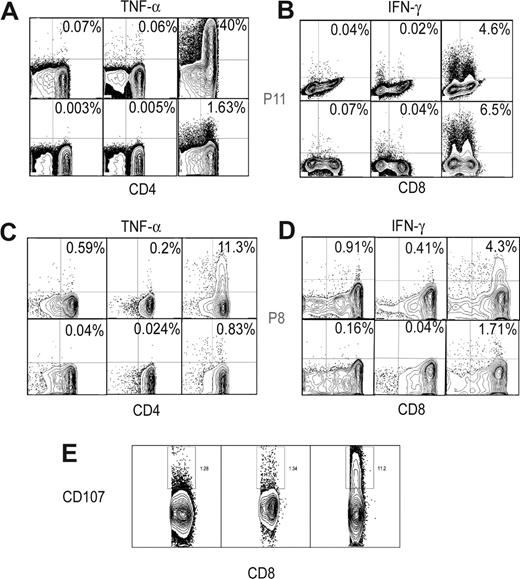
In 9 of the 14 patients, we found evidence for immune responses in at least one remission time point by IFN-γ ELISPOT analysis: pretreatment leukemic plus remission samples produced significantly higher IFN-γ responses (median, 35 spot forming cells [SFCs], range 22-76 SFCs) than pretreatment or remission samples alone, or 2 autologous remission samples together (5, 1-22 SFCs; P < .001; Figures 1A,B, S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). In 6 of these patients, the IFN-γ responses were further analyzed via cytokine flow cytometry (CFC) to delineate the cell type of origin of IFN-γ and to look for production of other cytokines. Intriguingly, TNF-α responses exceeded IFN-γ in most patients and were mainly produced by CD4+ T cells (Figures 1C, 2A-D). In one patient (P11), up to 40% of remission CD4+ T cells produced TNF-α (Figure 2A,B) to pretreatment samples. NK (CD56+) cells did not show a significant response to these cytokines on stimulation by CFC (data not shown). These results suggest that robust anti-leukemia immune responses develop in some patients under imatinib treatment, with CD4+ T cells producing TNF-α as a major response.
Antileukemia T-cell responses develop in remission after imatinib. (A) IFN-γ ELISPOT data (triplicate) depicted from P12: leukemic cells alone (first row), remission sample alone (second row), and leukemic and remission samples (last row). (B) Detection of antileukemia immune responses in 9 of 14 patients via IFN-γ ELISPOT. Leukemic cells alone (□), remission sample alone (■), and leukemic and remission samples ( ). The following stimulated remission samples from the patients are shown and depicted as median and range: P1, 5 months; P2, 12 months; P3, 6 months; P4, 18 months; P6, 14 months; P8, 15 months; P11, 4 months; P12, 5 months; P13, 12 months. All responses were statistically significant (P < .05) compared with leukemic or remission samples alone. (C) TNF-α and IFN-γ production (CFC) by CD4+ T and CD8+ T cells in stimulated remission samples (■) from 6 patients. Leukemic cells alone (□) and remission samples alone (
). The following stimulated remission samples from the patients are shown and depicted as median and range: P1, 5 months; P2, 12 months; P3, 6 months; P4, 18 months; P6, 14 months; P8, 15 months; P11, 4 months; P12, 5 months; P13, 12 months. All responses were statistically significant (P < .05) compared with leukemic or remission samples alone. (C) TNF-α and IFN-γ production (CFC) by CD4+ T and CD8+ T cells in stimulated remission samples (■) from 6 patients. Leukemic cells alone (□) and remission samples alone ( ). The same remission samples shown in panel B were analyzed (*P < .05, statistically significant responses over background).
). The same remission samples shown in panel B were analyzed (*P < .05, statistically significant responses over background).
Antileukemia T-cell responses develop in remission after imatinib. (A) IFN-γ ELISPOT data (triplicate) depicted from P12: leukemic cells alone (first row), remission sample alone (second row), and leukemic and remission samples (last row). (B) Detection of antileukemia immune responses in 9 of 14 patients via IFN-γ ELISPOT. Leukemic cells alone (□), remission sample alone (■), and leukemic and remission samples ( ). The following stimulated remission samples from the patients are shown and depicted as median and range: P1, 5 months; P2, 12 months; P3, 6 months; P4, 18 months; P6, 14 months; P8, 15 months; P11, 4 months; P12, 5 months; P13, 12 months. All responses were statistically significant (P < .05) compared with leukemic or remission samples alone. (C) TNF-α and IFN-γ production (CFC) by CD4+ T and CD8+ T cells in stimulated remission samples (■) from 6 patients. Leukemic cells alone (□) and remission samples alone (
). The following stimulated remission samples from the patients are shown and depicted as median and range: P1, 5 months; P2, 12 months; P3, 6 months; P4, 18 months; P6, 14 months; P8, 15 months; P11, 4 months; P12, 5 months; P13, 12 months. All responses were statistically significant (P < .05) compared with leukemic or remission samples alone. (C) TNF-α and IFN-γ production (CFC) by CD4+ T and CD8+ T cells in stimulated remission samples (■) from 6 patients. Leukemic cells alone (□) and remission samples alone ( ). The same remission samples shown in panel B were analyzed (*P < .05, statistically significant responses over background).
). The same remission samples shown in panel B were analyzed (*P < .05, statistically significant responses over background).
CD4+ and CD8+ T-cell responses via flow cytometry. TNF-α (A,C) and IFN-γ responses (B,D) (first column, leukemic cells alone; second column, remission sample alone; third column, leukemic and remission samples) in CD4+ and CD8+ T cells of P11, 4 months (A,B) and P8, 15 months (C,D). (E) CD8+ CD107+ responses (third column, leukemic and remission samples) in patient P11, 4 months compared with leukemic cells alone (first column) and remission sample alone (second column).
CD4+ and CD8+ T-cell responses via flow cytometry. TNF-α (A,C) and IFN-γ responses (B,D) (first column, leukemic cells alone; second column, remission sample alone; third column, leukemic and remission samples) in CD4+ and CD8+ T cells of P11, 4 months (A,B) and P8, 15 months (C,D). (E) CD8+ CD107+ responses (third column, leukemic and remission samples) in patient P11, 4 months compared with leukemic cells alone (first column) and remission sample alone (second column).
T-cell responses are sustained in patients in remission over time
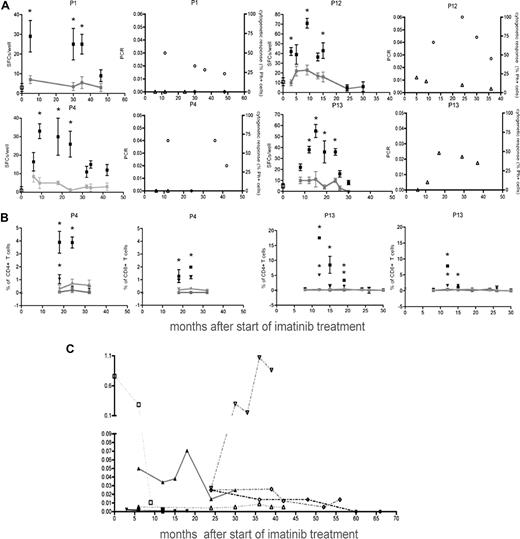
In 4 patients, multiple remission time points were analyzed to address the dynamics of these responses (Figure 3A). Antileukemia immune responses became detectable around the time of cytogenetic remission (3-8 months), peaked at 9 to 16 months, and declined to background levels at 24 to 46 months (Figure 3A). Decline of antileukemia T-cell responses coincided with molecular responses, reflecting major tumor load reduction (Figure 3A). Several time points were also analyzed by CFC to address the TNF-α cytokine responses (Figure 3B). These again showed that CD4+ T-cell responses dominated over time, with higher TNF-α than IFN-γ production. Dynamics of the TNF-α responses detected by CFC correlated with the IFN-γ responses detected in ELISPOT assays (r = 0.50, P = .003). All patients had tumor load reduction under treatment with imatinib (Figure 3C).
Dynamics of antileukemia immune responses. (A) Immune responses in 4 patients via IFN-γ ELISPOT analysis. Stimulated remission samples (■), leukemic cells alone (□), and remission samples alone ( ). Background levels are connected by lines (*P < .05, statistically significant responses over background). Ratio of the tumor load measured by molecular responses (bcr-abl transcript/bcr-abl control gene, left panel, ○) and levels of Ph+ cells in chromosome analysis (right panel, ▵) at the corresponding time points. (B) TNF-α (■) and IFN-γ(▾) responses by CD4+ and CD8+ T cells (CFC) from patients P4 and P13 over time. Leukemic cells alone (TNF-α (■), IFN-γ(▾)) and remission samples alone (TNF-α (
). Background levels are connected by lines (*P < .05, statistically significant responses over background). Ratio of the tumor load measured by molecular responses (bcr-abl transcript/bcr-abl control gene, left panel, ○) and levels of Ph+ cells in chromosome analysis (right panel, ▵) at the corresponding time points. (B) TNF-α (■) and IFN-γ(▾) responses by CD4+ and CD8+ T cells (CFC) from patients P4 and P13 over time. Leukemic cells alone (TNF-α (■), IFN-γ(▾)) and remission samples alone (TNF-α ( ), IFN-γ(
), IFN-γ( ). Background levels are connected by lines (*P < .05, statistically significant responses over background). (C) Tumor load over time from patients P5(▴), P6(▾), P7 (♦), P8 (●), connected by solid lines, and patients P9 (□), P11 (▿), and P14 (○), connected by dotted lines. Error bars indicate ranges of triplicate measurements. Results are representative of 2 or 3 independent experiments.
). Background levels are connected by lines (*P < .05, statistically significant responses over background). (C) Tumor load over time from patients P5(▴), P6(▾), P7 (♦), P8 (●), connected by solid lines, and patients P9 (□), P11 (▿), and P14 (○), connected by dotted lines. Error bars indicate ranges of triplicate measurements. Results are representative of 2 or 3 independent experiments.
Dynamics of antileukemia immune responses. (A) Immune responses in 4 patients via IFN-γ ELISPOT analysis. Stimulated remission samples (■), leukemic cells alone (□), and remission samples alone ( ). Background levels are connected by lines (*P < .05, statistically significant responses over background). Ratio of the tumor load measured by molecular responses (bcr-abl transcript/bcr-abl control gene, left panel, ○) and levels of Ph+ cells in chromosome analysis (right panel, ▵) at the corresponding time points. (B) TNF-α (■) and IFN-γ(▾) responses by CD4+ and CD8+ T cells (CFC) from patients P4 and P13 over time. Leukemic cells alone (TNF-α (■), IFN-γ(▾)) and remission samples alone (TNF-α (
). Background levels are connected by lines (*P < .05, statistically significant responses over background). Ratio of the tumor load measured by molecular responses (bcr-abl transcript/bcr-abl control gene, left panel, ○) and levels of Ph+ cells in chromosome analysis (right panel, ▵) at the corresponding time points. (B) TNF-α (■) and IFN-γ(▾) responses by CD4+ and CD8+ T cells (CFC) from patients P4 and P13 over time. Leukemic cells alone (TNF-α (■), IFN-γ(▾)) and remission samples alone (TNF-α ( ), IFN-γ(
), IFN-γ( ). Background levels are connected by lines (*P < .05, statistically significant responses over background). (C) Tumor load over time from patients P5(▴), P6(▾), P7 (♦), P8 (●), connected by solid lines, and patients P9 (□), P11 (▿), and P14 (○), connected by dotted lines. Error bars indicate ranges of triplicate measurements. Results are representative of 2 or 3 independent experiments.
). Background levels are connected by lines (*P < .05, statistically significant responses over background). (C) Tumor load over time from patients P5(▴), P6(▾), P7 (♦), P8 (●), connected by solid lines, and patients P9 (□), P11 (▿), and P14 (○), connected by dotted lines. Error bars indicate ranges of triplicate measurements. Results are representative of 2 or 3 independent experiments.
Cytokine responses in T-cell clones from P11
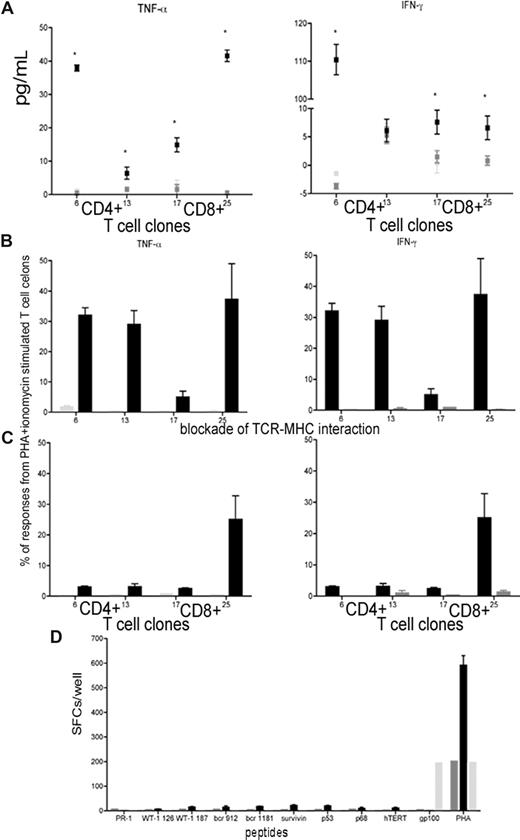
To further validate the antileukemia immune responses, we generated CD8+ T-cell clones from patient P11 (at the peak remission time point) based on CD107 mobilization13 on stimulation with autologous leukemic cells (Figure 2E); CD4+ T-cell clones were also generated. 16 CD4+ and 19 CD8+ T-cell clones were expanded and analyzed for responsiveness to autologous leukemic cells using 2 stimulation modes (whole leukemic cells or lysates) and 2 detection techniques (multiplex cytokine assay or CFC). TNF-α and IFN-γ responses were detected in 2 CD4+ T-cell clones and 2 CD8+ T-cell clones on specific stimulation compared with stimulation with remission samples pulsed with lysate from the AML cell line THP-1 (Figure 4A,B). These responses were suppressed through blockade of TCR-MHC interactions (Figure 4C), demonstrating that these responses were antigen-specific. To identify target antigens of the CD8+ T-cell clones (HLA-A0201+), we stimulated each clone with HLA-A*0201 restricted peptides from known LAAs (PR1, WT1 126, WT1 187, bcr912, bcr1181, survivin, p53, p68, and hTERT). The clones did not respond to these peptides (Figure 4D), suggesting that they recognize currently unidentified LAAs and therefore would have been missed with peptide-based screening. Furthermore, LAAs potentially vary inter-individually and even intra-individually with each patient responding to different LAAs at the same time or to different LAAs sequentially.
Responses of T-cell clones to tumor lysate, but not to TAAs. (A) TNF-α and IFN-γ responses of 2 CD4+ and 2 CD8+ T-cell clones stimulated with autologous leukemic cells (■) via multiplex cytokine array compared with unstimulated T-cell clones ( ) and T-cell clones stimulated with remission sample (
) and T-cell clones stimulated with remission sample ( ). (B,C) Responses in T-cell clones stimulated with remission sample pulsed with lysate from autologous leukemic cells (■) and remission sample pulsed with lysate from the AML cell line THP1 (
). (B,C) Responses in T-cell clones stimulated with remission sample pulsed with lysate from autologous leukemic cells (■) and remission sample pulsed with lysate from the AML cell line THP1 ( ) using CFC for TNF-α and IFN-γ (B) and blocked with anti-CD4 and anti-MHC II antibodies or anti-CD8 and anti-MHC II antibodies, respectively (C). Results were compared with PHA and ionomycin-stimulated T-cell clones. (D) T-cell responses in CD8+ T-cell clones pulsed with HLA-A0201 restricted peptides from LAAs and TAAs using ELISPOT assay compared with PHA stimulated CD8+ T-cell clones, and CD8+ T-cell clones stimulated with a peptide from the melanoma-associated antigen gp100. Results are shown as median and range.
) using CFC for TNF-α and IFN-γ (B) and blocked with anti-CD4 and anti-MHC II antibodies or anti-CD8 and anti-MHC II antibodies, respectively (C). Results were compared with PHA and ionomycin-stimulated T-cell clones. (D) T-cell responses in CD8+ T-cell clones pulsed with HLA-A0201 restricted peptides from LAAs and TAAs using ELISPOT assay compared with PHA stimulated CD8+ T-cell clones, and CD8+ T-cell clones stimulated with a peptide from the melanoma-associated antigen gp100. Results are shown as median and range.
Responses of T-cell clones to tumor lysate, but not to TAAs. (A) TNF-α and IFN-γ responses of 2 CD4+ and 2 CD8+ T-cell clones stimulated with autologous leukemic cells (■) via multiplex cytokine array compared with unstimulated T-cell clones ( ) and T-cell clones stimulated with remission sample (
) and T-cell clones stimulated with remission sample ( ). (B,C) Responses in T-cell clones stimulated with remission sample pulsed with lysate from autologous leukemic cells (■) and remission sample pulsed with lysate from the AML cell line THP1 (
). (B,C) Responses in T-cell clones stimulated with remission sample pulsed with lysate from autologous leukemic cells (■) and remission sample pulsed with lysate from the AML cell line THP1 ( ) using CFC for TNF-α and IFN-γ (B) and blocked with anti-CD4 and anti-MHC II antibodies or anti-CD8 and anti-MHC II antibodies, respectively (C). Results were compared with PHA and ionomycin-stimulated T-cell clones. (D) T-cell responses in CD8+ T-cell clones pulsed with HLA-A0201 restricted peptides from LAAs and TAAs using ELISPOT assay compared with PHA stimulated CD8+ T-cell clones, and CD8+ T-cell clones stimulated with a peptide from the melanoma-associated antigen gp100. Results are shown as median and range.
) using CFC for TNF-α and IFN-γ (B) and blocked with anti-CD4 and anti-MHC II antibodies or anti-CD8 and anti-MHC II antibodies, respectively (C). Results were compared with PHA and ionomycin-stimulated T-cell clones. (D) T-cell responses in CD8+ T-cell clones pulsed with HLA-A0201 restricted peptides from LAAs and TAAs using ELISPOT assay compared with PHA stimulated CD8+ T-cell clones, and CD8+ T-cell clones stimulated with a peptide from the melanoma-associated antigen gp100. Results are shown as median and range.
T-cell responses in prestimulated remission samples in P10
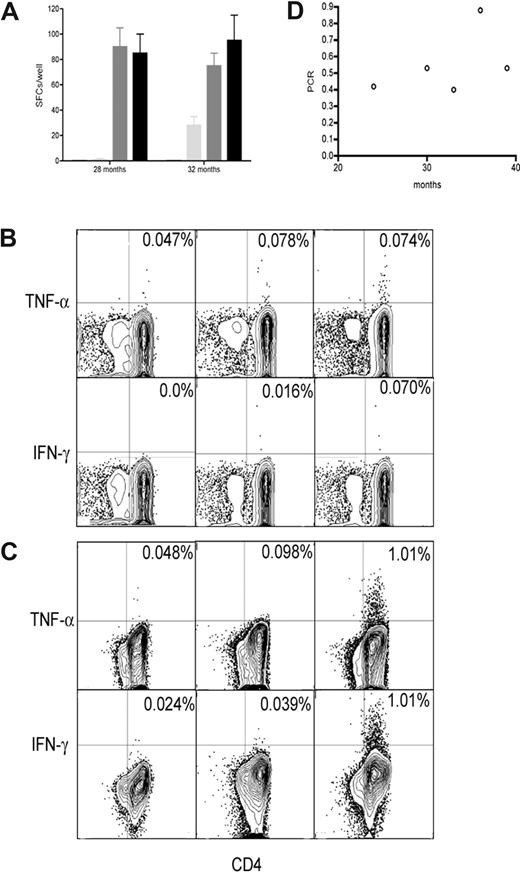
In 2 patients with no detectable immune responses (P10, 28 months and 32 months; and P14, 3 months), remission samples were stimulated with cryopreserved autologous leukemic cells in vitro for 14 days. After stimulation, an antileukemia T-cell response became detectable in P10 in ELISPOT assay (Figure 5A) and CFC (Figure 5B,C), suggesting that a response was present but below detection without pre-stimulation. These responses were also predominantly from CD4+ T cells. These time points showed an association with molecular response (Figure 5D).
Immune responses in remission after imatinib after prestimulation. (A) Immune responses in prestimulated remission samples using IFN-γ ELISPOT. Remission samples from P10 stimulated with autologous leukemic cells (■, third column), with lysate from autologous leukemic cells (■), unstimulated remission samples ( ), and leukemic cells alone (□). Results are shown as median and range. Immune responses in prestimulated remission samples using CFC: (B) 28 months; (C) 32 months. Remission samples from P10 stimulated with autologous leukemic cells (third column), unstimulated remission samples (second column), and leukemic cells alone (first columns). (D) Tumor load from patient P10 over time (○).
), and leukemic cells alone (□). Results are shown as median and range. Immune responses in prestimulated remission samples using CFC: (B) 28 months; (C) 32 months. Remission samples from P10 stimulated with autologous leukemic cells (third column), unstimulated remission samples (second column), and leukemic cells alone (first columns). (D) Tumor load from patient P10 over time (○).
Immune responses in remission after imatinib after prestimulation. (A) Immune responses in prestimulated remission samples using IFN-γ ELISPOT. Remission samples from P10 stimulated with autologous leukemic cells (■, third column), with lysate from autologous leukemic cells (■), unstimulated remission samples ( ), and leukemic cells alone (□). Results are shown as median and range. Immune responses in prestimulated remission samples using CFC: (B) 28 months; (C) 32 months. Remission samples from P10 stimulated with autologous leukemic cells (third column), unstimulated remission samples (second column), and leukemic cells alone (first columns). (D) Tumor load from patient P10 over time (○).
), and leukemic cells alone (□). Results are shown as median and range. Immune responses in prestimulated remission samples using CFC: (B) 28 months; (C) 32 months. Remission samples from P10 stimulated with autologous leukemic cells (third column), unstimulated remission samples (second column), and leukemic cells alone (first columns). (D) Tumor load from patient P10 over time (○).
Discussion
Molecular targeted therapies, such as imatinib, represent an exciting new treatment modality for cancer. Unlike chemotherapy, which is nonspecific and can concurrently inhibit cancer and immune cells, imatinib (and other molecular targeted therapies) can suppress cancer cells more than immune cells. We hypothesized that, as leukemic cells decrease under imatinib therapy, immune function is partially restored while apoptotic leukemic cells are present, thus rendering leukemic cells immunogenic at a critical inflection point. Indeed, using leukemic cells (pretreatment peripheral blood mononuclear cells) as targets, we showed that antileukemia T-cell responses develop in the majority of analyzed CML patients in hematologic and cytogenetic remission under imatinib treatment. Furthermore, such responses are predominantly CD4+ T cells producing TNF-α, and in one patient represent 40% of CD4+ T cells. These responses would have been missed by screening for IFN-γ responses to the limited set of LAAs currently identified. Use of cryopreserved autologous leukemic cells is a useful, antigen-unbiased approach, which allows broader detection of antileukemia T-cell responses, and TNF-α appears to be an important cytokine in such responses.
Results of in vitro studies on the effect of imatinib on T-cell responses are contradictory.18-23 Mechanisms by which imatinib treatment influences antileukemia T-cell responses, and the molecular targets to which these cells are directed, need to be further investigated. The major subset of the antileukemia T-cell responses in this study are CD4+ T cells, which may play a more important role in the immune response against CML24 than previously thought. They may not only help shape25,26 and sustain an antileukemia CD8+ T-cell response27 but may also help shape antileukemia CD4+ T-cell responses via T helper cell–T helper cell cooperation28 (cooperation of CD4+ T cells). Two key molecules are CD40 and OX40 costimulatory molecules, which are known to play an important role in T-cell activation through B cells as APCs. The underlying mechanism of this cooperation is the up-regulation of CD40L as well as CD28 and OX40 on the surface of helper CD4+ T cells, which leads to binding to CD40 on the surface of APCs followed by an up-regulation of OX40L expression on the APCs and a more efficient presentation of LAAs to CD4+28 and CD8+ T cells.29
The cytokines TNF-α and IFN-γ, which were mainly produced by the specific CD4+ T cell in patients in remission, might maintain and sustain the proliferation of antileukemia T cells.28,30,31 Especially TNF-α, which is important for maintaining long-living anti-leukemia memory T cells,32 may be essential in this process. Its significance was also shown in patients with acute graft-versus-host disease who had successfully undergone allogeneic BMT.33 Another mechanism of function is the up-regulation of MHC class I and II molecules on the surface of APCs by TNF-α and IFN-γ28,31,34 for a better presentation of LAAs to T cells. TNF-α-producing T cells also contribute to the elimination of leukemic cells through the interaction of TNF-α with TNF-α receptors, which are expressed at high levels on leukemic cells,31 and the production of other proinflammatory cytokines, such as IL-1, IL-2, IL-6, and IL-8.34,35 Therefore, analysis of IFN-γ production by antileukemia T cells30,36 alone might not reveal the entire immune response. Other cytokines, such as TNF-α and IL-2, are also important for antileukemia T-cell responses and need to be investigated.
The temporal dynamics of these responses were studied in several patients. T-cell responses develop around the time of clinical remission and are sustained for several months. However, these responses ultimately wane in all patients, even though leukemic cells persist at low levels in a minimal residual disease state. It is puzzling why the antileukemia immune response does not lead to complete eradication of leukemic cells. In one subject, an antileukemia immune response could be amplified on stimulation with irradiated leukemic cells (or lysates) at a time point at which such a response was undetectable directly ex vivo. This suggests that low levels of immune reactivity persist that may be amplified via vaccination strategies.
Together, our results show that robust T-cell responses to CML develop in some patients under imatinib treatment. These responses are dominated by CD4+ T cells producing TNF-α. Mechanisms by which imatinib treatment leads to antileukemia T-cell responses, and the molecular targets to which these cells are directed, will be further investigated. This knowledge may be useful for the development of immunotherapeutic strategies against CML, and raises the hope that immunotherapy may synergize with imatinib to eradicate residual leukemic cells for a durable cure of the disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Wesley Witteles, Alexandre Johannsen, and Rhoda Falkow for technical support.
This work was supported by a Research Scholar Award from the American Cancer Society and NIH 5R01CA130817 and partially by a Dean's Postdoctoral Fellowship of the Stanford University Medical School (C.I-U.C.).
National Institutes of Health
Authorship
Contribution: C.I-U.C. and P.P.L. designed all the experiments; C.I-U.C. performed all the experiments; H.T.M. contributed to cytokine flow cytometry; C.I-U.C. and P.P.L. wrote the paper; all authors discussed the results and commented on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter P. Lee, Division of Hematology, Stanford University Medical School, CCSR 1155, 269 Campus Drive, Stanford, CA 94305; e-mail: ppl@stanford.edu.




 ). The following stimulated remission samples from the patients are shown and depicted as median and range: P1, 5 months; P2, 12 months; P3, 6 months; P4, 18 months; P6, 14 months; P8, 15 months; P11, 4 months; P12, 5 months; P13, 12 months. All responses were statistically significant (P < .05) compared with leukemic or remission samples alone. (C) TNF-α and IFN-γ production (CFC) by CD4+ T and CD8+ T cells in stimulated remission samples (■) from 6 patients. Leukemic cells alone (□) and remission samples alone (
). The following stimulated remission samples from the patients are shown and depicted as median and range: P1, 5 months; P2, 12 months; P3, 6 months; P4, 18 months; P6, 14 months; P8, 15 months; P11, 4 months; P12, 5 months; P13, 12 months. All responses were statistically significant (P < .05) compared with leukemic or remission samples alone. (C) TNF-α and IFN-γ production (CFC) by CD4+ T and CD8+ T cells in stimulated remission samples (■) from 6 patients. Leukemic cells alone (□) and remission samples alone (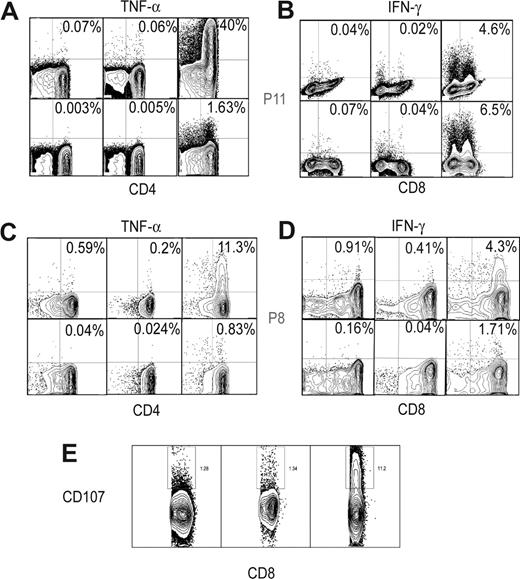

 ), IFN-γ(
), IFN-γ( ). Background levels are connected by lines (*P < .05, statistically significant responses over background). (C) Tumor load over time from patients P5(▴), P6(▾), P7 (♦), P8 (●), connected by solid lines, and patients P9 (□), P11 (▿), and P14 (○), connected by dotted lines. Error bars indicate ranges of triplicate measurements. Results are representative of 2 or 3 independent experiments.
). Background levels are connected by lines (*P < .05, statistically significant responses over background). (C) Tumor load over time from patients P5(▴), P6(▾), P7 (♦), P8 (●), connected by solid lines, and patients P9 (□), P11 (▿), and P14 (○), connected by dotted lines. Error bars indicate ranges of triplicate measurements. Results are representative of 2 or 3 independent experiments.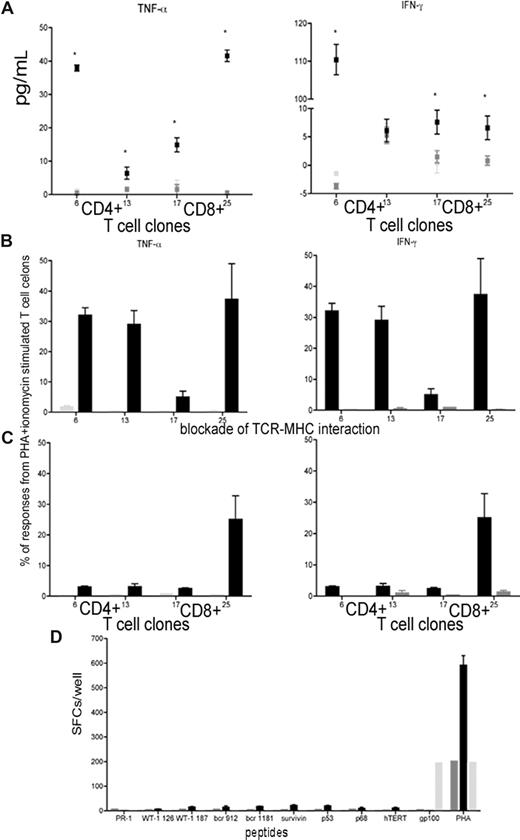
 ) using CFC for TNF-α and IFN-γ (B) and blocked with anti-CD4 and anti-MHC II antibodies or anti-CD8 and anti-MHC II antibodies, respectively (C). Results were compared with PHA and ionomycin-stimulated T-cell clones. (D) T-cell responses in CD8+ T-cell clones pulsed with HLA-A0201 restricted peptides from LAAs and TAAs using ELISPOT assay compared with PHA stimulated CD8+ T-cell clones, and CD8+ T-cell clones stimulated with a peptide from the melanoma-associated antigen gp100. Results are shown as median and range.
) using CFC for TNF-α and IFN-γ (B) and blocked with anti-CD4 and anti-MHC II antibodies or anti-CD8 and anti-MHC II antibodies, respectively (C). Results were compared with PHA and ionomycin-stimulated T-cell clones. (D) T-cell responses in CD8+ T-cell clones pulsed with HLA-A0201 restricted peptides from LAAs and TAAs using ELISPOT assay compared with PHA stimulated CD8+ T-cell clones, and CD8+ T-cell clones stimulated with a peptide from the melanoma-associated antigen gp100. Results are shown as median and range.