Abstract
Severe congenital neutropenia (SCN) was first described just over 50 years ago. The progress in elucidating the clinical features and molecular pathophysiology of SCN closely parallels the progressive growth in our understanding of myelopoiesis. In this historical review, I have delineated this parallel progression in our understanding of the processes of granulocyte differentiation and the pathogenesis of congenital neutropenia. SCN is a heterogeneous disease that can serve as a model for the failure of myelopoiesis, and dissection of its pathogenesis has yielded important insights into the normal process of myeloid development.
Introduction
It has been just over 50 years since Rolf Kostmann, a student at the Karolinska Institute in Stockholm, Sweden, described “infantile genetic agranulocytosis” in his doctoral thesis,1,2 the first description of severe congenital neutropenia (SCN), also termed Kostmann Syndrome. The evolving understanding of this complex and heterogeneous disorder parallels the explosion of new discoveries in the last 50 years that have elucidated the process of myeloid cell proliferation and differentiation. SCN is a model for failure of myelopoiesis, and dissection of its pathogenesis has yielded important insights into the normal process of myeloid development.
Myelopoiesis has evolved to respond to a highly demanding system: neutrophils are constitutively produced in large numbers, survive for only a short time, and must be rapidly up-regulated in response to infectious or inflammatory stresses. The regulation of neutrophil number, the induction of normal maturation, and the continued balance of cell production with apoptosis are crucial to the normal homeostasis of the innate immune response. Granulocytes are produced in the bone marrow under the direction of cytokines, most important of which is granulocyte colony stimulating factor (G-CSF). G-CSF induces a transcriptional program that governs the full spectrum of proliferation, differentiation, functional activation, and apoptosis of hematopoietic progenitors directed along the myeloid lineage.
The original description of SCN preceded the recognition of the existence of a myeloid-specific cytokine by about 20 years, and the cloning and characterization of granulocyte colony stimulating factor (G-CSF) and its receptor (G-CSFR) by about 30 years. Once G-CSF was isolated, it was logical to hypothesize that severe congenital neutropenia arose from a genetic disorder leading to abnormal expression of or failure of response to G-CSF; however, no constitutional mutations in G-CSF or G-CSFR were identified as the source of the disorder. The clinical benefit derived from G-CSF therapy, however, allowed longer survival of SCN patients, and provided a wider understanding of the natural history of the disease, revealing its predilection for malignant transformation as well as facilitating further investigation of its molecular pathogenesis. The identification of the genetic defects underlying SCN did not occur until the era of gene mapping techniques, and discovery of the pathogenic mutations yielded puzzling results that could only be understood in the context of a more fully developed understanding of myelopoiesis, based on 50 years of discoveries elucidating the features of cytokine-mediated regulation of proliferation, differentiation, and apoptosis.
The 1950s: the kinetics of granulocyte production
At the time Kostmann described congenital neutropenia, very little was known about the process of myeloid cell proliferation and differentiation. Early studies of myelopoiesis focused on the characterization of white cell mass, distribution, and turnover. Investigators used a variety of physiologic techniques in an attempt to define the life span of leukocytes and the mechanisms that balanced granulocyte production and destruction. Experiments from the 1930s observing neutrophil counts following marrow suppressing agents resulted in estimates of granulocyte lifespan ranging from 4 days to 2 to 3 weeks (reviewed in3 ). Twenty years later, white cell survival was estimated by several techniques. Kline and Cliffton gave a dose of P32 to hospitalized patients with normal neutrophil counts and estimated the granulocyte lifespan by following release of radiolabeled neutrophils into the peripheral blood.4 Hollingsworth followed leukocyte counts in parabiotic rats, in which short-term cross-circulation was established between an irradiated recipient and a healthy donor to estimate neutrophil survival.5,6 Craddock and colleagues used leukopheresis to induce leukopenia and followed the pattern of recovery of white cell counts.7 In later studies, Cronkite and colleagues administered tritiated thymidine to patients to determine the dynamics of neutrophil senescence and removal from the circulation.8 Not surprisingly, such studies gave rise to a wide range of estimates of the lifespan of the neutrophil, ranging from 30 minutes to 2 weeks.9 Certain defined principles became accepted, however. All studies supported several concepts that have become accepted principles defining neutrophil homeostasis: (1) postmitotic progenitors mature to neutrophils in the bone marrow over several days and constitute the granulocyte reserves; (2) the marrow reserve exceeds the circulating population of neutrophils 5- to 10-fold; (3) neutrophils released from the marrow reserve are the primary contributors to acute leukocytosis; (4) marginated leukocytes exchange freely with circulating neutrophils in the blood; and (5) granulocytes circulate for a variable period of time, after which they enter tissues, from which they do not recirculate in the blood.10 Estimates of daily white cell production, based on the appropriate presumption that production must balance white cell loss, was in the range of 1011 to 1012.10
The concurrent view of neutropenia was that it was an acquired disease, often attributable to the spleen. Cyclic neutropenia was first described in 1910,11 but was not described as a familial disorder, and was thought to be immune mediated, responsive to splenectomy or to adrenocorticotropic hormone (ACTH; reviewed in Reimann and DeBerardinis12 ). Kostmann was the first to document a heritable neutropenia, describing its autosomal recessive inheritance and attributing it to a “primary insufficiency” of the bone marrow.2 The next phase of investigation into the origins of the disorder would come in the context of the identification and study of myeloid cytokines.
Identification, purification, and characterization of G-CSF and its receptor
The importance of humoral factors in hematopoiesis was established in the 1960s. Pioneering techniques developed independently by Metcalf and Sachs resulted in the successful in vitro culture of hematopoietic progenitors in semisolid medium.13-16 These studies established that hematopoietic colony formation was completely dependent on the presence of factors from a variety of sources, including spleen cells, embryonic cells, or primed serum. These were termed “colony stimulating factors” because they were assayed functionally by their ability to stimulate in vitro colony formation.15 Evidence supported the emerging theory that these cytokines influenced all aspects of the hematopoietic differentiation program: proliferation, lineage commitment, and terminal maturation.17 Four major cytokines were described that were responsible for supporting the growth of granulocytes and macrophages: granulocyte-macrophage (GM)-CSF, G-CSF, IL3, and M-CSF. These cytokines had both overlapping and unique effects on myeloid differentiation, and the combinatorial effects helped regulate the balance of granulocyte and monocyte production, the response to increased demand for one cell type over another, and the regulation of proliferation versus maturation.18 Of the myeloid cytokines, the one most critical to neutrophil production is G-CSF. Mice with homozygous inactivating mutations in either G-CSF19 or G-CSFR20 were neutropenic, although their neutrophils were reduced only about 80%, confirming the ability of other cytokines to contribute to neutrophil production. This is in contrast to the GM-CSF knockout mouse, which has normal granulopoiesis.21
The cDNA encoding G-CSF was isolated independently by 2 laboratories in 1986,22,23 and the G-CSF receptor was cloned in 1992.24 Subsequent studies elucidated the many roles of G-CSF in neutrophil differentiation and function. The effects of G-CSF are mediated through binding to its receptor, which within the myeloid lineage is expressed on hematopoietic progenitors, myeloid precursors, and mature neutrophils. Signaling through the G-CSFR involves activation of the Jak-Stat pathway and the ras/MAPK pathway, and the cytoplasmic tail of the receptor has several defined regions that mediate its diverse effects (reviewed in Avalos25 ). The membrane proximal region mediates mitogenic effects, while more distal regions mediate maturation signals. G-CSF also increases functional activation of mature neutrophils, leading to increases in adherence, phagocytosis, respiratory burst, and granule secretion (reviewed in26 ). In addition, G-CSF transduces an antiapoptotic signal through activation of the Akt pathway.27 As described below, naturally occurring mutations in the G-CSF mutation in association with SCN provided important clues in the delineation of those portions of the receptor that are responsible for proliferation and maturation.
The cloning of G-CSF and the G-CSF receptor was a critical step in defining the signaling pathways involved in myelopoiesis. It also led the way to the development of recombinant G-CSF as a critically important agent for the treatment of neutropenia.28 Approved in 1991 for clinical use in patients with chemotherapy-induced neutropenia, it is now widely used for the treatment of neutropenia in nearly every setting. It was shown to have demonstrated efficacy for the treatment of severe congenital neutropenia and cyclic neutropenia, and has become the mainstay of therapy for those diseases.29-31
Role of G-CSF and G-CSFR in SCN and myelodysplastic syndrome/acute myelogenous leukemia
From the time that the role of cytokines first became appreciated, it seemed logical to presume that SCN would be related to a defect in the production of, or the response to, G-CSF. Indeed, in a follow-up to his original report 20 years after he described SCN, Kostmann reported on an additional 9 patients and proposed that the maturation defect was due to a “deficiency of a serum factor.”32 However, studies of G-CSF receptor expression and function suggested that this pathway was in fact intact in SCN patients, and sequence analysis of the receptor was also normal.33 This was further corroborated by the successful treatment of SCN and CN with G-CSF. Although rare patients have been described with G-CSF resistance attributable to a mutation in the external domain of the G-CSFR,34-36 in general the G-SCF receptor signaling pathway appears normal in these patients.
However, subsequent studies identified an acquired point mutation in SCN patients that appeared to be associated with the development of myelodysplastic syndrome/acute myelogenous leukemia (MDS/AML). This mutation created a nonsense mutation resulting in a truncation of the C-terminal cytoplasmic domain of the receptor (Figure 1).37 The role of this mutation in the development of MDS/AML in patients with SCN remains controversial. There are several unanswered questions: Is the mutation pathogenic for the development of MDS/AML? Is there a subset of SCN patients that is more predisposed to developing the mutation and/or MDS/AML? Is it a result of G-CSF therapy?
Schematic diagram of the cytoplasmic domain of the granulocyte colony stimulating factor receptor. Depicted are the regions that mediate signals for proliferation and maturation as well as the 4 tyrosine residues that are phosphorylated on ligand binding. The critical region containing the mutations in severe congenital neutropenia is bracketed; the asterisk denotes the site for the alternative splicing event that generates the class IV isoform.
Schematic diagram of the cytoplasmic domain of the granulocyte colony stimulating factor receptor. Depicted are the regions that mediate signals for proliferation and maturation as well as the 4 tyrosine residues that are phosphorylated on ligand binding. The critical region containing the mutations in severe congenital neutropenia is bracketed; the asterisk denotes the site for the alternative splicing event that generates the class IV isoform.
The point mutation in SCN patients has a physiologic counterpart in normal myeloid development. The G-CSFR mRNA is alternatively spliced to give rise to 7 naturally occurring isoforms. Two of these isoforms are expressed in normal myeloid cells. The class I isoform is the full-length “normal” G-CSF receptor that is expressed at high levels in myeloid progenitors. The class IV isoform, which is expressed at very low levels, splices out the carboxy-terminal end of the full-length receptor and replaces it with an alternative sequence. The consequence is loss of the same portion of the normal G-CSFR as occurs by mutation in patients with SCN, although in the naturally occurring isoform, this is replaced with alternative sequences rather than truncating the receptor altogether.38 Intriguingly, the class IV G-CSFR isoform expression is almost uniformly expressed at markedly increased levels in acute myelogenous leukemia (AML).39
Study of both the class IV G-CSFR isoforms and the SCN-related mutant receptor in model systems have suggested that the truncation of the cytoplasmic domain of the receptor serves to separate the proliferation and maturation signals transduced through the receptor. Overxpression of both the class IV isoform and the SCN-related mutant receptor in murine hematopoietic cells inhibits maturation without decreasing proliferation.37,40 However, this remains controversial, because mice carrying a targeted mutation that recapitulates the SCN mutant receptor have normal resting granulopoiesis, and although they have an increased proliferative response to G-CSF, they have no evidence of impaired myeloid differentiation.41 Cells bearing the mutant receptor also have enhanced cell survival and resistance to apoptosis.27
Is the mutation pathogenic for MDS/AML? Although the aberrant signaling through the truncated G-CSFR may be an appealing candidate factor in the pathogenesis of MDS/AML, its clinical significance is not established. Not all patients with SCN who develop MDS/AML have the mutation, and not all patients who acquire the mutation go on to develop MDS/AML.42,43 It seems clear from a range of studies that the mutation increases the proliferation response of the cells carrying the mutant receptor. Whether this leads to leukemia, allows selective proliferation of a clone that has undergone another leukemic event, or is merely a bystander phenomenon remains uncertain.
Is there a subset of SCN patients that is more predisposed to developing the mutation and/or MDS/AML? This risk is becoming better defined. Patients with severe disease, as evidenced by a higher demand for G-CSF and a correspondingly poor response to G-CSF represent a high risk population, as do patients with G-CSFR mutations. The correlation between G-CSF requirements and risk of MDS/AML gives rise to the obvious question: Is MDS/AML the result of G-CSF therapy? This remains unclear. Patients with severe disease may have an inherent predilection for the development of MDS/AML that is independent of G-CSF. It seems likely that G-CSF allows selective proliferation of preleukemic or leukemic cells, especially in those patients carrying the mutant G-CSFR, although G-CSF itself may not actually induce the leukemic changes. This is supported by recent studies by Sloand et al examining the relationship of G-CSF therapy to the development of MDS/AML associated with monosomy 7 in patients with aplastic anemia. In those patients, monosomy 7 cells were demonstrated to proliferate selectively in vitro in the presence of G-CSF with a steep dose-response curve, while monosomy 7 cells could not be amplified out of normal marrow. However, fluorescence in situ hybridization (FISH) of stored patient specimens before treatment with G-CSF demonstrated rare cells bearing monosomy 7, suggesting that G-CSF was amplifying a preexisting clone. Interestingly, these cells showed overexpression of the class IV isoform of the G-CSFR. What the relationship is between monosomy 7 and selective expression of the class IV isoform of the receptor is unknown.44
In summary, the study of the G-CSFR in SCN patients did not reveal the etiology of their neutropenia, but provided important insights into the natural history of their disease. Further, it served to highlight the complexity of signals arising from the G-CSFR in influencing proliferation and maturation as separable components of the cytokine-induced differentiation program.
SCN and disruption of myelopoiesis
In the past 8 years, investigators have identified several genes that are implicated in the pathogenesis of SCN, details of which are outlined below. At least 2 surprising findings emerge from these studies. First, whereas a mutation in the genes encoding G-CSF or G-CSFR would have seemed a self-evident explanation for the SCN phenotype, none of the identified mutations revealed an obvious basis for the development of neutropenia. Second, the pathogenic genes appeared unrelated biologically: 4 variants of the disease are caused by a granule protein, a mitochondrial protein, a cytoplasmic protein and a transcription factor. Dissection of the molecular pathways leading from these proteins to neutropenia has again underscored the complexity of the myeloid maturation program and the interactions between pathways of cellular proliferation and programmed cell death.
Autosomal dominant SCN and neutrophil elastase
In 1999, Horwitz and colleagues used gene mapping techniques to determine the gene responsible for cyclic neutropenia (CN). They presented convincing genetic evidence that mutations in neutrophil elastase (Ela2) were responsible for nearly all cases of that disorder,45 and subsequent studies revealed that mutations in Ela2 were also responsible for the majority of cases of autosomal dominant SCN.46 Neutrophil elastase is a serine protease that is synthesized at the promyelocyte stage of differentiation and stored in the primary granules of neutrophils.47 More than 50 Ela2 mutations have been described in patients with CN and SCN, and they result in proteins with a wide range of enzyme activity and substrate specificity.48-50 No obvious connection could be made linking the abnormalities of the mutant enzymes and neutropenia, nor was there an obvious explanation why some mutations should cause SCN and others the more benign CN syndrome. Any explanation needed to account for the observations that both SCN and CN are associated with increased intramedullary apoptosis and the finding that SCN associated with Ela2 mutations has an autosomal dominant pattern of inheritance.
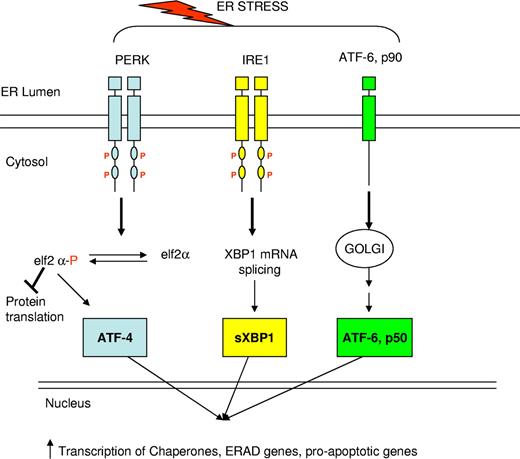
In the past year, elegant studies reported independently by Kollner et al and Grenda et al have delineated the role of the unfolded protein response in SCN.51,52 The unfolded protein response (UPR) is activated in response to the presence of misfolded proteins in the endoplasmic reticulum (ER) (reviewed in Ron and Walter53 ). In response to unfolded or misfolded proteins within the ER, transmembrane proteins sense the presence of ER stress and trigger the UPR (Figure 2). This induces (1) a general decrease in protein synthesis through inhibition of translation initiation; (2) an increase in the expression of chaperone proteins; and (3) activation of ER-associated protein degradation (ERAD). If these responses are inadequate to compensate for the quantity of misfolded protein, the same transmembrane proteins induce apoptosis through changes in the expression of bcl family proteins and through increased caspase expression. Biochemical markers of the UPR include increased expression of the chaperone BiP and increased splicing of XBP1 (X-box binding protein-1), as well as increased expression of the negative transcriptional regulator CHOP and increased expression of caspase 12.53 Expression of mutant elastase derived from patients with SCN or CN in hematopoietic U937 cells and in primary human granulocytic precursors resulted in activation of the UPR, as evidenced by increased expression of BiP and CHOP and increased splicing of XBP1. It also increased surface expression of annexin V, an early marker of apoptosis.51,52 Furthermore, the investigators introduced a mutation into the SCN elastase cDNA to destroy the proteolytic activity of the enzyme, and this change had no impact on the induction of apoptosis, supporting the notion that the pathogenesis of neutropenia induced by mutant Ela2 is independent of the intrinsic activity of the enzyme.
Schematic diagram of the unfolded protein response. In response to endoplasmic reticulum (ER) stress, signals are transmitted from the ER to the cytoplasm via 3 transmembrane proteins in the ER membrane. Signaling from these 3 proteins leads to many downstream responses: Protein kinase RNA (PKR)-like ER kinase (PERK) phosphorylates elF2, which causes a global decrease in protein synthesis. IRE1 activation leads to splicing of XBP1; sXBP1 enters the nucleus and increases transcription of chaperone proteins, ER-associated protein degradation proteins, and proapoptotic proteins. ATF6 is cleaved and the cleaved portion enters the nucleus where it stimulates transcription of XBP1 and other unfolded protein response target genes.
Schematic diagram of the unfolded protein response. In response to endoplasmic reticulum (ER) stress, signals are transmitted from the ER to the cytoplasm via 3 transmembrane proteins in the ER membrane. Signaling from these 3 proteins leads to many downstream responses: Protein kinase RNA (PKR)-like ER kinase (PERK) phosphorylates elF2, which causes a global decrease in protein synthesis. IRE1 activation leads to splicing of XBP1; sXBP1 enters the nucleus and increases transcription of chaperone proteins, ER-associated protein degradation proteins, and proapoptotic proteins. ATF6 is cleaved and the cleaved portion enters the nucleus where it stimulates transcription of XBP1 and other unfolded protein response target genes.
The hypothesis that SCN and CN arise from activation of the UPR is attractive in that it can provide an explanation for many aspects of Ela2-related disease. First, it explains the autosomal dominant pattern of inheritance of the syndromes. Second, it provides a genotype-phenotype link that may account for the observation that mutations in the same enzyme can give rise to 2 distinctly different clinical disorders. In the study by Grenda et al, preliminary examination suggested that the degree of activation of the UPR was correlated with the severity of the phenotype, although further studies are required to confirm this observation.52 The corollary to that observation is that in the rare mutations that give rise to both SCN and CN in different families, there must be gene-modifying effects that lie outside the Ela2 locus.
Kostmann Syndrome and HAX1
The original pedigree described by Kostmann displayed autosomal recessive inheritance of SCN, and these patients did not have mutations in the Ela2 gene. Genome-wide linkage studies reported within the last year by Klein et al have confirmed that the gene for autosomal recessive SCN is HAX1.54 HAX1 is a ubiquitously expressed mitochondrial protein with weak homology to bcl-2 that represses apoptosis. It is hypothesized to have a role in maintaining the mitochondrial membrane potential, loss of which leads to the release of cytochrome c and other proapoptotic proteins into the cytoplasm. Klein et al reported that neutrophils from HAX-1 deficient patients did indeed demonstrate dissipation of mitochondrial membrane potential.54 Unlike Ela2-related SCN, HAX1 represents a loss-of-function mutation, thereby explaining its autosomal recessive pattern of inheritance. Thus, although both Ela2 and HAX1 related neutropenia share an increase in apoptosis as a final common pathway to their pathogenesis, the induction of increased apoptosis occurs by completely distinct mechanisms.
Other rare causes of SCN
Rare cases of SCN have been attributed to mutations in other neutrophil-specific genes. X-linked SCN has been reported in a few patients with novel mutations in the Wiskott-Aldrich syndrome protein (WASp).55,56 WASp is found only in hematopoietic cells, where it regulates actin polymerization involved in a spectrum of activities, including cell-cell interactions, cell movement, and cell signaling. Mutations in WASp commonly associated with Wiskott Aldrich syndrome (immunodeficiency, thrombocytopenia, and eczema) are inactivating mutations that lead to reduced or absent WASp activity. In contrast, mutations giving rise to SCN are activating mutations that cause constitutive WASp activity. Expression of these mutant WASp proteins by gene transfer into a hematopoietic cell line suggested that the mutant protein caused unregulated actin polymerization, interfering with mitosis and cytokinesis. Primary progenitors from these patients showed decreased proliferation as well as increased apoptosis.57
As noted, 3 patients have been described with germ line mutations in the extracellular domain of the G-CSFR as the cause of G-CSF resistant SCN. These mutations are thought to inhibit receptor trafficking and dimerization in response to G-CSF.34-36
The transcription factor Gfi-1 has also been implicated in a small number of cases of SCN.58 Gfi-1 is a zinc-finger protein that has been characterized as a transcriptional repressor.59 In myeloid cells, it has been shown to down-regulate expression of C/EBPϵ and neutrophil elastase. Zhuang et al have suggested that Gfi-1 mutant proteins associated with SCN cause apoptosis through up-regulation of C/EBPϵ expression,60 although it is tempting to postulate that up-regulation of neutrophil elastase by loss of Gfi-1 activates the UPR and induces neutropenia by that mechanism.52
Despite its rarity, SCN is a very heterogeneous disease. The delineation of the defects involved in its pathogenesis has served to highlight the complexity of the myeloid maturation program. It has also served to underscore the prominent role of apoptosis in the regulation of neutrophil mass. Whereas investigators first hypothesized that this disease likely arose from a defect in G-CSF-induced proliferation, the final common pathway for this disparate set of gene defects appears to be a mechanism leading to an increase in apoptotic cell death. Indeed, the clinical efficacy of G-CSF in treating SCN probably reflects its activity as an antiapoptotic protein rather than its role in inducing stem cell proliferation and maturation.
Conclusion
Fifty years after the first description of severe congenital neutropenia, the causative mutations underlying the disease and the links between those mutations and altered neutrophil mass are becoming clear. G-CSF has prolonged survival from this once uniformly fatal disease, though revealing the unfortunate tendency for leukemic transformation. The elucidation of the pathophysiology of this rare disease and its late complications has provided critical insights into the basic biology of myelopoiesis, illuminating the intricate processes involved in proliferation, maturation, function, and apoptosis of the hematopoietic stem cell.