Abstract
Granulocyte colony-stimulating factor (G-CSF) induces proliferation of bone marrow–derived cells. G-CSF is neuroprotective after experimental brain injury, but the mechanisms involved remain unclear. Stem cell factor (SCF) is a cytokine important for the survival and differentiation of hematopoietic stem cells. Its receptor (c-kit or CD117) is present in some endothelial cells. We aimed to determine whether the combination of G-CSF/SCF induces angiogenesis in the central nervous system by promoting entry of endothelial precursors into the injured brain and causing them to proliferate there. We induced permanent middle cerebral artery occlusion in female mice that previously underwent sex-mismatched bone marrow transplantation from enhanced green fluorescent protein (EGFP)–expressing mice. G-CSF/SCF treatment reduced infarct volumes by more than 50% and resulted in a 1.5-fold increase in vessel formation in mice with stroke, a large percentage of which contain endothelial cells of bone marrow origin. Most cells entering the brain maintained their bone marrow identity and did not transdifferentiate into neural cells. G-CSF/SCF treatment also led to a 2-fold increase in the number of newborn cells in the ischemic hemisphere. These findings suggest that G-CSF/SCF treatment might help recovery through induction of bone marrow–derived angiogenesis, thus improving neuronal survival and functional outcome.
Introduction
Granulocyte colony-stimulating factor (G-CSF) was identified more than 2 decades ago,1 and was found to mobilize hematopoietic bone marrow cells to the systemic circulation. Although G-CSF has been used in many patients to counter the side effects of chemotherapy as well as to prepare donors for peripheral cell harvesting, some concerns regarding its long-term safety still remain.2-4 G-CSF may have several beneficial effects in animals with stroke.5,6 Thus, it was reported that rats treated with G-CSF following middle cerebral artery occlusion (MCAO) have smaller infarcts and better functional outcome compared with controls.5,7-9 The molecular basis of these neuroprotective effects has not yet been fully determined, but signaling through the JAK-STAT and PI3K-Akt pathways leading to a reduction in proapoptotic factors, and an increase in antiapoptotic factors was suggested to play an important role.10 Furthermore, it was shown that rats that received transplants of G-CSF–mobilized peripheral blood precursor cells (PBPCs) demonstrated a significant improvement in functional recovery following permanent MCAO (PMCAO).11 Moreover, G-CSF reportedly has an important role in inducing neurogenesis in the brain.12 Combinations of G-CSF and stem cell factor (SCF) may further increase the positive effects of G-CSF by augmenting tyrosine kinase–related downstream effects and increasing prosurvival signals.10
Lee and colleagues13 found that G-CSF treatment increased angiogenesis after focal cerebral ischemia. However, the origin of these newly formed blood vessels remains unclear. Thus, they may arise from in situ proliferation of existing endothelial cells in the central nervous system (CNS), or they might originate from bone marrow–derived endothelial precursor cells (BMDECs) that home to the brain. Because G-CSF in combination with SCF mobilizes BMDECs from bone marrow, we used enhanced green fluorescent protein (EGFP) chimeric animals that underwent PMCAO to see if G-CSF/SCF treatment might induce angiogenesis from BMDECs and whether this combination would have beneficial effects on functional outcome.
Methods
Preparation of the mice and surgery
All experiments were approved by the institutional animal care and use committee and were conducted according to National Institutes of Health (NIH) guidelines. Female 4- to 6-week-old C57B mice were subjected to irradiation (2 × 4.5 Gy [450 rad] 6 hours apart) to deplete their own bone marrow (BM), and then were given transplants of BM14 generated from male mice that ubiquitously express GFP (with the exception of erythrocytes) and kept in a sterile environment for 10 days. After recovery, they were subjected to PMCAO as described before.15 This model results in cortical injury limited to the frontal and parietal cortex and spares the subcortical structures. Mice received vehicle or a combination of 200 μg/kg G-CSF and 50 μg/kg SCF per day intraperitoneally, respectively (Peprotech, Rocky Hill, NJ), to increase the number of circulating bone marrow stem cells (BMSCs) for 5 days following the surgery. All mice were given BrdU (50 mg/kg twice daily intraperitoneally; Roche Applied Sciences, Indianapolis, IN) to follow cell proliferation on days 1 to 5 after PMCAO.
Infarct size determination
At 60 days after stroke, serial sections of the forebrains of the mice studied (n = 7 per group) were cut at 200-μm intervals. The sections were stained with toluidine blue and photographed using a DM16000 Leica inverted fluorescence microscope (Wetzlar, Germany), and the hemispheres were traced using Image J software (NIH, Bethesda, MD). Because the cortical stroke tissue had already liquefied and disappeared by the time of death, the infarct volume was calculated as the difference between the sizes of the 2 hemispheres multiplied by the distance between the sections.
Immunohistochemistry
Mice were perfused at different time points (2 to 6 months after surgery) using 4% paraformaldehyde through the ascending aorta. Following perfusion, the brains were taken out and processed using cryoprotection achieved by increasing concentration of sucrose. The brains were then frozen on dry ice, and serial sections were cut at a 10-μm thickness and mounted on positively charged microscope slides. The slides were kept at −80°C until used. Immunohistochemistry was performed to visualize GFP as well as other markers. For photography, all sections were freshly mounted with 0.01 M Tris-HCI buffer (pH 7.8).
The perfused sections were washed in phosphate-buffered saline (PBS) 3 times for 3 minutes, microwaved for antigen retrieval when needed in 10 mM citric acid buffer (pH 6.1) for 5 minutes after the liquid started to boil, and then cooled at room temperature (RT) for 30 minutes. Following pretreatment, the sections were blocked with Universal Blocking Reagent (Biogenex, San Ramon, CA) for 10 minutes. The primary antibody was applied according to Table 1, diluted in 1% bovine serum albumin (BSA) containing 0.25% Triton-X 100 and followed by blocking endogenous peroxidase activity using 3% H2O2 for 15 minutes. In case of a double staining with a second tyramide amplification step, we added 0.5% sodium-azide to the H2O2 solution in order to block the horseradish peroxidase (HRP) still present from the first staining. The secondary antibodies were anti-rabbit HRP polymer conjugate (Zymed-Invitrogen, Carlsbad, CA) applied for 30 minutes, biotinylated donkey anti–chicken IgY or goat Ig-G (Jackson ImmunoResearch, West Grove, PA) at 1:1000 for 1 hour, and HRP-conjugated anti–rat IgG (Jackson ImmunoResearch) at 1:500 overnight. The AlexaFluor or FITC-conjugated tyramides were homemade and used in ratios of 1:2000 or 1:20 000, respectively. When antibodies were derived from the same host, we used a microwave treatment step to eliminate any nonspecific cross reaction.16 Controls were performed with no primary antibodies.
Y chromosome hybridization
To further confirm the origin of the GFP in a few animals, we colocalized Y chromosome in the same cells as GFP as follows: sections were washed in PBS (pH: 7.4) 3 times for 3 minutes, rinsed in distilled water, and incubated in universal blocking reagent for 10 minutes. The sections were then incubated in rabbit anti-GFP antibody (Molecular Probes-Invitrogen, Carlsbad, CA) for 1 hour at room temperature. The endogenous peroxidase activity was blocked with a 3% hydrogen peroxide, and following PBS washes, the secondary antibody—an anti-rabbit HRP polymer conjugate—was applied undiluted for 30 minutes. The staining was then visualized using FITC-conjugated tyramide at 1:10 000 for 10 minutes at RT. To perform Y chromosomal fluorescence in situ hybridization (FISH), the same sections were immersed in 10 mM citric acid (pH 6.1) and microwaved in a kitchen microwave (700 W; GE, Louisville, KY) for 2 × 5 minutes at 50% power after the liquid started to boil. The water that evaporated was replaced with distilled water between and after the microwaving sessions, and the sections were left in the solution to cool for 2 hours at RT. Microwave treatment inactivates any HRP activity that is present in the tissue (ie, endogenous HRP and/or HRP incorporated in reagents used in previous steps).16 The Y chromosomal hybridization was performed as described earlier14 using a 1.5-kb RNA probe (pY3531B) generated against a repeat sequence of the mouse Y chromosome that was labeled with digoxigenin using a labeling kit (Roche Applied Sciences). After the hybridization step and several washes in salt sodium citrate (for details, see http://intramural.nimh.nih.gov/lcmr/snge/), the digoxigenin was detected with an antidigoxigenin antibody that was conjugated to HRP (1:600; Roche Applied Sciences) and visualized using the TSA-Plus CY3 System (1:600; Invitrogen, Carlsbad, CA). Determination of colocalization of the Y chromosome with GFP was performed by counting the cells manually on 3 different sections per animal, 5 animals per group, and by 2 independent people using a DMI6000 Leica inverted fluorescence microscope.
Vascular density analysis
To determine the percentage of area surface of vessels, an alkaline phosphatase reaction was performed using BCIP/NBT substrates (Roche Applied Sciences). The stained sections were scanned and photographed using the motorized stage and brightfield illumination. For colocalization studies between GFP, CD31, the Y chromosome, and BRDU, the immunostained sections were evaluated with a DMI6000 Leica inverted fluorescence microscope or a Leica TCS SP2 with AOBS and 2-Photon on an upright Leica DM RE-7 confocal microscope. Conventional fluorescent images were captured using the Volocity 4.01 software (Improvision; PerkinElmer, Waltham, MA). Z-stacks were collected at 0.5-μm intervals using a motorized stage. After image capturing, iterative restoration was performed at a 95% confidence level. The images were evaluated using the NIH Image J software. The data were analyzed using analysis of variance (ANOVA) and the Prism software (GraphPad, San Diego, CA).
In vitro endothelial cell proliferation assay
Mouse brain endothelial cells were acquired from ATCC (Manassas, VA) and maintained in Dulbecco modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS) at 37°C, 5% CO2.17 Cells were plated in 96-well cell-culture plates, 3000 cells/well, in 150 μL medium without FBS or growth factors. After 24 hours, different concentrations of G-CSF and SCF were added to the medium together with BrdU (1:1000 dilution of BrdU; final dilution, 1:4000). The plates were then placed into a normal (37°C, 5% CO2) or hypoxic (37°C, 5% CO2, 1% O2) incubator for 24 hours. The cell proliferation assay was performed according to the manufacturer's instructions (catalog no. 11647229001; Roche Applied Sciences). Control experiments included cells cultured without the growth factors. The experiment was done twice with triplicate samples.
Results
Infarct volumes
Injury size was measured 60 days after PMCAO. Lesion size was significantly smaller (P = .012) in animals treated with G-CSF/SCF (11.3 + 2.5 mm3) as compared with controls (25.1 + 3.7 mm3; Figure 1).
Infarct volumes in vehicle and G-CSF/SCF–treated mice. Columnar graph showing the infarct volume in millimeters cubed in the brains of the saline-treated versus GSCF-treated mice. Values are means plus SEM. **P < .01.
Infarct volumes in vehicle and G-CSF/SCF–treated mice. Columnar graph showing the infarct volume in millimeters cubed in the brains of the saline-treated versus GSCF-treated mice. Values are means plus SEM. **P < .01.
G-CSF/SCF increases influx of GFP+ cells into the injured brain
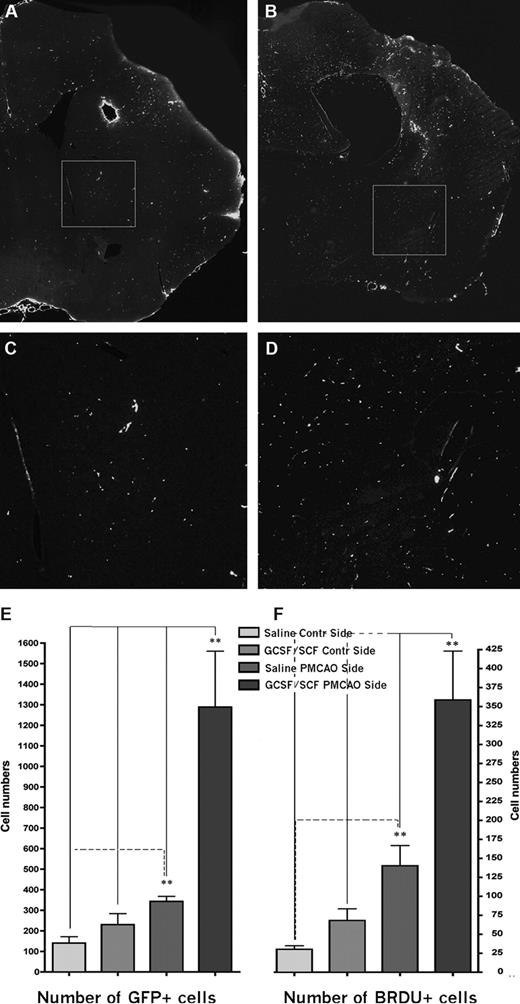
In animals that underwent PMCAO but did not receive G-CSF/SCF that were killed at 2 months, we observed a 2.4 fold increase in the number of GFP+ cells (Figure 2A,C,E) in the ischemic hemisphere compared with the nonischemic hemisphere (343 ± 24.7 vs 140 ± 31; P < .05, n = 4). Most of these cells expressed the microglial marker Iba1 (data not shown). Stimulation of peripheral stem cells using a combination of G-CSF and SCF after PMCAO resulted in a significant, 3.75-fold increase in the number of GFP+ cells in the ischemic hemisphere as compared with the controls (Figure 2B,D,E). In the G-CSF/SCF–stimulated mice, the number of GFP+ cells in the ischemic side were 1288 plus or minus 272 versus 229.8 plus or minus 54.2 in the nonischemic side (P < .01, n = 4; Figure 2E). To ensure that cells expressing GFP originate from donor BM, we used FISH to determine the presence of a Y chromosome in the GFP+ cells (Figure 3 and Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).18 The present findings confirmed our previously published results18 that about 10% of cells expressing the Y chromosome were not positive for GFP (even when signal amplification was used), suggesting that when using only GFP as a marker we significantly underestimate the percentage of brain cells originating from the donor BM. This may be due to silencing of the GFP expression during neural differentiation. At 2 months after the injury, many of the GFP+ cells coexpressed Iba1, suggesting that the early influx of BM-derived cells consists of mainly inflammatory cells. At this time point we could identify occasional GFP cells that colocalized with neuronal markers such as NeuN and rare cells that coexpressed GFP and the glial marker GFAP (not shown).
GFP expression and proliferation at 2 months following PMCAO. (A) Coronal section of a vehicle-treated brain at 2 months after PMCAO at the level of the lesion. GFP-immunopositive cells were visualized using amplified immunostaining. (B) Similar level in the brain like in panel A from a mouse that received daily injection of G-CSF/SCF for 5 consecutive days following the stroke. (C,D) Magnifications of the boxed areas in panels A and B, respectively. Note the significantly higher number of green fluorescent cells in the G-CSF/SCF–treated animal's brain. Horizontal side of box equals 500 μm (A,B) and 125 μm (C,D). Panels A,B: objective, 10×; numeric aperture, 0.3, Plan Fluo. (E) Columnar graph showing the mean number of GFP- and (F) BRDU-immunopositive cells in the sections of the saline-treated versus G-CSF–treated mice in both hemispheres.
GFP expression and proliferation at 2 months following PMCAO. (A) Coronal section of a vehicle-treated brain at 2 months after PMCAO at the level of the lesion. GFP-immunopositive cells were visualized using amplified immunostaining. (B) Similar level in the brain like in panel A from a mouse that received daily injection of G-CSF/SCF for 5 consecutive days following the stroke. (C,D) Magnifications of the boxed areas in panels A and B, respectively. Note the significantly higher number of green fluorescent cells in the G-CSF/SCF–treated animal's brain. Horizontal side of box equals 500 μm (A,B) and 125 μm (C,D). Panels A,B: objective, 10×; numeric aperture, 0.3, Plan Fluo. (E) Columnar graph showing the mean number of GFP- and (F) BRDU-immunopositive cells in the sections of the saline-treated versus G-CSF–treated mice in both hemispheres.
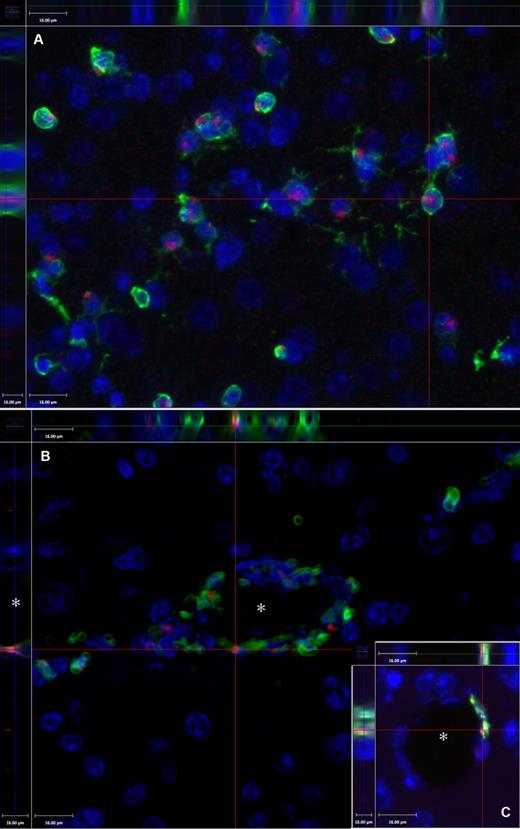
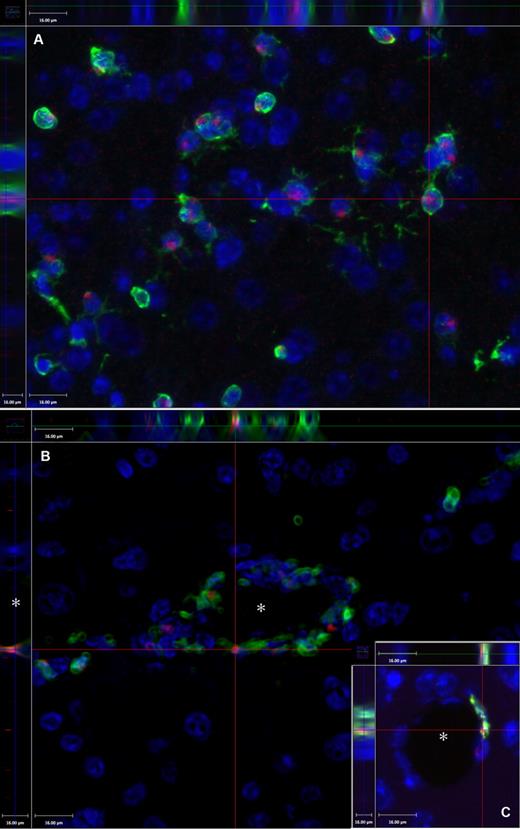
Demonstration of the technique of colocalization of the GFP and the Y chromosome in a female brain at 2 months after PMCAO. Y chromosome hybridization was performed in sex-mismatched GFP BM-transplanted mice brains. (A) Presence of the Y chromosomes in red (Alexa-594); green fluorescence indicates the presence of the GFP, and blue fluorescence labels cell nuclei based on DAPI, a chromosomal stain. The section is one level of a Z-series demonstrating that the Y chromosome is localized in the same cells that are also GFP+ (side panels). (B) Cross-section of a capillary (*) of a Z-series depicting several luminally localized GFP cells that are also positive for the Y chromosome (red dots). The inset (C) shows another example, where the side panel of the confocal image illustrates that the Y chromosome and the GFP colocalize in a cell that borders the vascular lumen (*). Objective, 40×; numeric aperture, 0.6 (A); and objective, 20×; numeric aperture, 0.7 (B). Scale bar equals 16 μm.
Demonstration of the technique of colocalization of the GFP and the Y chromosome in a female brain at 2 months after PMCAO. Y chromosome hybridization was performed in sex-mismatched GFP BM-transplanted mice brains. (A) Presence of the Y chromosomes in red (Alexa-594); green fluorescence indicates the presence of the GFP, and blue fluorescence labels cell nuclei based on DAPI, a chromosomal stain. The section is one level of a Z-series demonstrating that the Y chromosome is localized in the same cells that are also GFP+ (side panels). (B) Cross-section of a capillary (*) of a Z-series depicting several luminally localized GFP cells that are also positive for the Y chromosome (red dots). The inset (C) shows another example, where the side panel of the confocal image illustrates that the Y chromosome and the GFP colocalize in a cell that borders the vascular lumen (*). Objective, 40×; numeric aperture, 0.6 (A); and objective, 20×; numeric aperture, 0.7 (B). Scale bar equals 16 μm.
G-CSF/SCF increases cell proliferation in the ischemic brain
Cell proliferation was studied with BrdU immunostaining at 60 days after PMCAO (n = 4 in each group). Following PMCAO, BrdU+ cells were significantly more abundant in the ischemic hemisphere (140 ± 27 vs 30 ± 5; P < .002; Figure 2F). The number of proliferating cells significantly increased following G-CSF/SCF treatment in both hemispheres, but a much larger increase was noted on the ischemic side (358 ± 64 vs 68 ± 15.5; P < .001). Overall, G-CSF/SCF treatment resulted in a 2.5-fold increase in the absolute number of BrdU+ cells in the ischemic side. When we tried to colocalize GFP with BrdU, we found less than 1% of GFP+ cells that were double labeled; most of these were endothelial cells based on their location, nuclear morphology, and their elongated endothelial-like shape. Specifically, at this time point we could not identify any cells that coexpressed GFP, BrdU, and neuronal markers, and only very rare cells that coexpressed GFP, BrdU, and GFAP. Most of the BRDU+ cells were astrocytes or microglial or endothelial cells. These results suggest that most BM-derived cells failed to proliferate in the brain for long periods of time and that most of those that did proliferate maintained a BM fate. However, because G-CSF/SCF treatment did increase the number of BrdU-expressing cells, it presumably did increase endogenous neurogenesis in the brain.
G-CSF/SCF increases angiogenesis in the injured brain
Alkaline phosphatase staining of vascular endothelium was performed on all brains at the level of the bregma plus 0.5 mm (n = 4 in the G-CSF/SCF and n = 3 in the control groups, respectively). Blood vessel density was also counted in naive mice and in mice that did not undergo PMCAO but received G-CSF/SCF. Ischemic and nonischemic hemispheres were compared in PMCAO mice that received G-CSF/SCF or vehicle. As shown in Figure 4A-C, we found that vessels occupy 3% plus 0.1% of the surface area in naive mice. In control animals that did not suffer from stroke, but received G-CSF/SCF, the surface area occupied by vascular endothelium increases slightly (3.9% ± 0.2%). In ischemic animals in the ischemic hemisphere, the area occupied by blood vessels increased significantly (4.8% ± 0.3%) and there was a further, statistically significant (P < .001) increase in PMCAO mice that also received G-CSF/SCF (6.7% ± 0.45%; Figure 4). Overall, we observed a 2.23-fold increase in the number of blood vessels in ischemic animals treated with G-CSF/SCF compared with naive animals. Interestingly, G-CSF/SCF also significantly increased blood vessel density in the nonischemic hemisphere.
G-CSF/SCF treatment increases angiogenesis and many of the new endothelial cells are of donor BM origin. Vascular density is demonstrated in untreated (A) and G-CSF/SCF–treated (B) mice after stroke (→). The insets are high-magnification representative images of coronal sections from similar rostro-caudal levels of the brains. (C) Columnar graph showing the percentage of the surface area occupied by vessels based on alkaline phosphatase staining in the sections of the saline-treated versus G-CSF/SCF–treated mice in both hemispheres. Vascular density is demonstrated in untreated (D) and G-CSF/SCF–treated (E) mice after stroke. The vascular endothelial cells are immunostained for CD31 (red fluorescence due to Alexa-594), and the GFP cells are immunostained in green following signal amplification using FITC-tyramide. For panels A,B,D,E: objective, 10×; numeric aperture, 0.3, Plan Fluo. Note the increased density of vessels as well as more green vascular structures in the G-CSF/SCF–treated brain. (F) The columnar graph demonstrates the percentage of GFP+ (ie, BM-derived; green) cell surfaces compared with all (red) endothelial (CD31-stained) surfaces in the saline-treated versus G-CS/SCF–treated mice brains in both hemispheres (n = 4). Values are means plus SEM. **P < .01. CC indicates corpus callosum; v, lateral ventricle.
G-CSF/SCF treatment increases angiogenesis and many of the new endothelial cells are of donor BM origin. Vascular density is demonstrated in untreated (A) and G-CSF/SCF–treated (B) mice after stroke (→). The insets are high-magnification representative images of coronal sections from similar rostro-caudal levels of the brains. (C) Columnar graph showing the percentage of the surface area occupied by vessels based on alkaline phosphatase staining in the sections of the saline-treated versus G-CSF/SCF–treated mice in both hemispheres. Vascular density is demonstrated in untreated (D) and G-CSF/SCF–treated (E) mice after stroke. The vascular endothelial cells are immunostained for CD31 (red fluorescence due to Alexa-594), and the GFP cells are immunostained in green following signal amplification using FITC-tyramide. For panels A,B,D,E: objective, 10×; numeric aperture, 0.3, Plan Fluo. Note the increased density of vessels as well as more green vascular structures in the G-CSF/SCF–treated brain. (F) The columnar graph demonstrates the percentage of GFP+ (ie, BM-derived; green) cell surfaces compared with all (red) endothelial (CD31-stained) surfaces in the saline-treated versus G-CS/SCF–treated mice brains in both hemispheres (n = 4). Values are means plus SEM. **P < .01. CC indicates corpus callosum; v, lateral ventricle.
Newly formed blood vessels at the infarct border originate from BM endothelial precursors
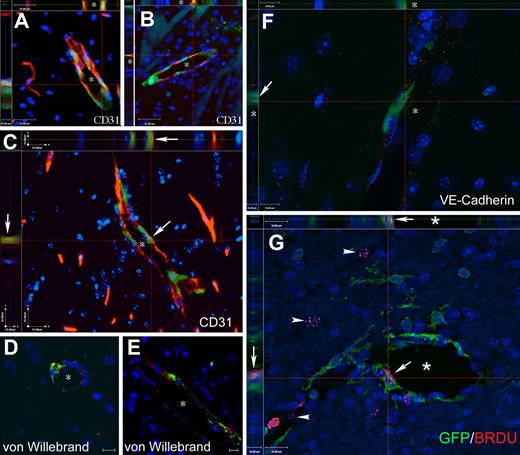
We used GFP- and endothelium-specific immunostaining in the same sections to see if endothelial cells and precursors in newly formed blood vessels derive from BM. We chose 3 markers that are generally accepted to be specific for vascular endothelium: von Willebrand factor, CD31 (PECAM1), and VE-cadherin. GFP immunostaining was amplified to ensure visualization of cells that express the marker protein at very low levels. We found that many endothelial cells in the immediate infarct vicinity expressed GFP. In the untreated ischemic animals GFP+ endothelial cells were identified in both hemispheres with a frequency of 0.1% and 0.08% of all endothelial surface for the nonischemic versus ischemic hemisphere, respectively. In G-CSF/SCF–treated mice there was a robust increase of new GFP+ endothelial cells compared with the saline-treated cells that was more significant on the side of the stroke than in the control side (0.35% ± 0.07% vs 0.22% ± 0.05%; P = .008; Figures 4D-F; S3). The colocalization of CD31 (Figures 5A-C; S2), von Willebrand factor (Figure 5D,E), and VE-cadherin (Figure 5F) and BRDU (Figures S4; 5G) with GFP was demonstrated using confocal Z-stacks (at 0.5-μm intervals) and iterative restoration. Except for occasional rare cells, we could not detect GFP+ endothelial cells in control chimeric animals that did not experience stroke with or without G-CSF/SCF treatment.
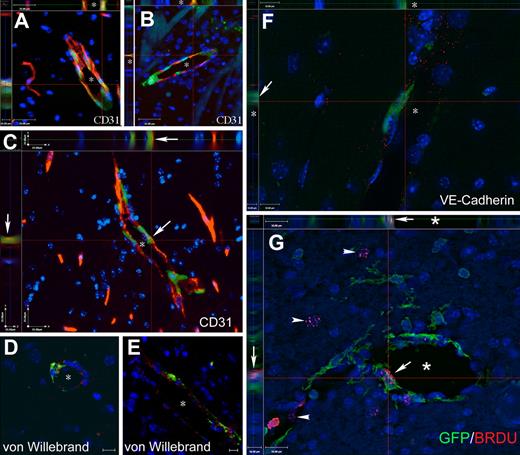
Cells of BM origin populate the vascular endothelium in mice with PMCAO treated with G-CSF/SCF. (A-C) Images from Z-stacks, where sections from G-CSF/SCF–treated PMCAO mice were immunostained with CD31, a specific marker of vascular endothelium, and GFP to track the bone marrow origin. The side panels demonstrate the colocalization of the 2 markers (endothelial marker and GFP) in several cells of the vascular wall. (D,E) demonstrates colocalization of GFP with the von Willebrand factor in vascular endothelium shown in red. (F) One level of a Z-series with side panels demonstrating that a GFP+ cell lining the lumen of a capillary (*) is also labeled with red dots representing the antibody staining for VE-cadherin, another specific endothelial cell marker. (G) Colocalization of BRDU (red dots over labeled nuclei) with GFP in a luminal cell lining a capillary (*). Arrow points to the colocalization of the red and green in the main panel as well as in the side panels. Arrows point to BRDU+ nuclei that do not belong to GFP+ cells. The images are a single level taken out of a Z-series, where the section was optically sliced into 0.5-μm–thin sections and iterative restoration was perfomed using Volocity 4.0 software. Objective, 40×; numeric aperture, 0.6 (A-E); and objective, 63×; numeric aperture, 0.7 (F). Scale bar equals 25 μm (A-C), 10 μm (D-F), and 16 μm (G).
Cells of BM origin populate the vascular endothelium in mice with PMCAO treated with G-CSF/SCF. (A-C) Images from Z-stacks, where sections from G-CSF/SCF–treated PMCAO mice were immunostained with CD31, a specific marker of vascular endothelium, and GFP to track the bone marrow origin. The side panels demonstrate the colocalization of the 2 markers (endothelial marker and GFP) in several cells of the vascular wall. (D,E) demonstrates colocalization of GFP with the von Willebrand factor in vascular endothelium shown in red. (F) One level of a Z-series with side panels demonstrating that a GFP+ cell lining the lumen of a capillary (*) is also labeled with red dots representing the antibody staining for VE-cadherin, another specific endothelial cell marker. (G) Colocalization of BRDU (red dots over labeled nuclei) with GFP in a luminal cell lining a capillary (*). Arrow points to the colocalization of the red and green in the main panel as well as in the side panels. Arrows point to BRDU+ nuclei that do not belong to GFP+ cells. The images are a single level taken out of a Z-series, where the section was optically sliced into 0.5-μm–thin sections and iterative restoration was perfomed using Volocity 4.0 software. Objective, 40×; numeric aperture, 0.6 (A-E); and objective, 63×; numeric aperture, 0.7 (F). Scale bar equals 25 μm (A-C), 10 μm (D-F), and 16 μm (G).
SCF induces proliferation of cerebral vascular endothelium in vitro
Our in vitro data show that SCF induces endothelial proliferation at concentrations between 5 and 100 ng/mL, while G-CSF seems to have no direct effect on proliferation (Figure 6). When the cells are grown in a hypoxic environment, the proliferative effect of SCF is significantly enhanced. The combination of SCF/G-CSF has the same effect as SCF alone.
Proliferative effect of G-CSF, SCF, and its combination on murine cerebral endothelial cells in vitro. A mouse brain endothelial cell line (b.end3) was used in the experiment. Following a 24-hour incubation of the cells with the factors at different concentrations (values are mean nanograms per milliliter media) and BRDU, the proliferation rate was calculated based on optical density (OD) measurements.  represents proliferation at normoxic conditions; ■ shows proliferation rate in hypoxic conditions. Controls received no factors but BRDU. G-CSF seems to be ineffective in the concentrations used, while SCF has a dose-dependent, significant proliferative effect, starting at 5 ng/mL. Combining the 2 factors results in proliferation rates similar to that of SCF alone. Hypoxic conditions seem to further increase the effectiveness of SCF. Values are means plus SD.
represents proliferation at normoxic conditions; ■ shows proliferation rate in hypoxic conditions. Controls received no factors but BRDU. G-CSF seems to be ineffective in the concentrations used, while SCF has a dose-dependent, significant proliferative effect, starting at 5 ng/mL. Combining the 2 factors results in proliferation rates similar to that of SCF alone. Hypoxic conditions seem to further increase the effectiveness of SCF. Values are means plus SD.
Proliferative effect of G-CSF, SCF, and its combination on murine cerebral endothelial cells in vitro. A mouse brain endothelial cell line (b.end3) was used in the experiment. Following a 24-hour incubation of the cells with the factors at different concentrations (values are mean nanograms per milliliter media) and BRDU, the proliferation rate was calculated based on optical density (OD) measurements.  represents proliferation at normoxic conditions; ■ shows proliferation rate in hypoxic conditions. Controls received no factors but BRDU. G-CSF seems to be ineffective in the concentrations used, while SCF has a dose-dependent, significant proliferative effect, starting at 5 ng/mL. Combining the 2 factors results in proliferation rates similar to that of SCF alone. Hypoxic conditions seem to further increase the effectiveness of SCF. Values are means plus SD.
represents proliferation at normoxic conditions; ■ shows proliferation rate in hypoxic conditions. Controls received no factors but BRDU. G-CSF seems to be ineffective in the concentrations used, while SCF has a dose-dependent, significant proliferative effect, starting at 5 ng/mL. Combining the 2 factors results in proliferation rates similar to that of SCF alone. Hypoxic conditions seem to further increase the effectiveness of SCF. Values are means plus SD.
Discussion
The current study identifies BM-derived cells as a major source of poststroke angiogenesis in the brain. The number of newly formed blood vessels increased 2.2-fold when we applied G-CSF/SCF after PMCAO. To ensure that newly formed blood vessels did originate from BMDECs, we used BM from GFP-expressing male donors that engrafted into the BM of recipient female mice prior to PMCAO. We then identified donor marrow cells as expressing GFP and the Y chromosome. Using this system, we could isolate newly formed blood vessels originating from BMDECs from those derived from the brain's resident endothelial cell population. Our results show that most newly formed blood vessels in the infarct vicinity were composed of GFP+/Y+ cells, indicating their BM origin. Given the fact that not all Y+ cells expressed GFP—probably due to silencing of the transgene related with progressive differentiation—it is highly likely that our results based on GFP expression in newly formed blood vessels are an underestimate of the true contribution of BMDECs to angiogenesis in the injured brain.
Our results corroborate previous reports that found increased recruitment of BM-derived cells to the brain following injury and those that found G-CSF to augment this process.19,20 However, while others focused on the short-term effects of this recruitment we waited for 2 months prior to analysis of the effects. This allowed us to avoid the early phase in which most recruited cells represent inflammatory cells and focus on angiogenesis, which takes a longer time to accomplish. In contrast to work reported earlier, in our studies we treated animals with a combination of G-CSF/SCF. This results in a 2-fold increase in circulating CD34+cells21 versus treatment with G-CSF alone. Both G-CSF and SCF are known to stimulate the survival, proliferation, and differentiation of BM cells, including endothelial cells.21-27 Our results suggest that while G-CSF is important in recruiting cells from the BM into the circulation, SCF might be responsible for the proliferation of vascular endothelial cells, many of which are derived from the donor BM. The in vitro data also suggest that hypoxia, which is found in ischemic tissue, significantly enhances the proliferative effect exhibited by SCF. This may be due to an increased expression of endothelial c-kit receptors under hypoxic conditions. The finding of BRDU and GFP double-positive cells (ie, proliferating endothelial cells of BM origin) among the lining of cerebral vasculature in ischemic areas suggest that our in vitro observations may indeed be important in vivo as well.
Our current set of results argues against transdifferentiation of BM-derived cells into neurons as an important mechanism to functional improvement in stroke.28,29 Thus, despite a major increase in the influx of GFP+ BM-derived cells into the injured brain with G-CSF/SCF, we could only identify isolated astrocytes in the brain that coexpressed GFP and almost no GFP+ neurons. In contrast, most BM-derived GFP+ cells in the brain retained their BM fate and represented either microglia, which were most prevalent in the early poststroke period, or endothelial cells, which were the prevalent GFP+ cell type at 2 months after stroke. However, we cannot rule out a more significant contribution of BM-derived neurogenesis in other circumstances.14,30,31
Importantly, G-CSF reduces infarct volumes in injury models by activation of antiapoptotic and anti-inflammatory mechanisms.19,32 A neuroprotective effect for G-CSF/SCF was also identified in the current experiment, lending further validity to our results. G-CSF treatment in stroke animals also results in greater improvements in neurologic deficits. Our data suggest that G-CSF/SCF–driven angiogenesis is likely another mechanism that could contribute to functional gains after stroke by improving metabolism and function of surviving cells in the peri-infarct area. Furthermore, given the increase in BrdU cells that were not of BM origin following G-CSF/SCF therapy, it is also likely that stimulation of endogenous neurogenesis is another mechanism that could improve functional outcome after stroke.
In conclusion, the combination of G-CSF/SCF may have synergistic beneficial effects both on lesion size and on angiogenesis. G-CSF/SCF–driven angiogenesis results from G-CSF–induced recruitment of BM-derived cells into the ischemic brain, and SCF might facilitate the proliferation and survival of endothelial cells. Most of the BM-derived cells maintain their BM identity and do not appear to significantly transdifferentiate into neural cells. These angiogenic and neuroprotective effects translate into better functional outcome after stroke and should promote future clinical studies in humans to study the efficacy of this therapeutic avenue in stroke victims.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors would like to acknowledge Dr David A. Davis (National Cancer Institute, NIH) for use of the hypoxia chamber.
This research was supported by the Division of Intramural Research, National Institute of Dental and Craniofacial Research, NIH. Z.E.T. is supported by OTKA (Hungarian Scientific Research Fund) T-043169.
National Institutes of Health
Authorship
Contribution: Z.E.T. performed histology, analyzed data, and wrote portions of the paper; R.L.L. performed surgeries and helped write the paper; T.S. designed the experiment, performed surgeries, and analyzed data; A.B. and S.P. participated in surgeries and analyzed data; I.S. and S.K. contributed reagents and helped analyze data; A.P. performed sectioning and immunostaining for VE-cadherin; B.M. and K.N. performed the endothelial cell proliferation studies; and E.M. designed the experiment, performed histochemistry, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Éva Mezey, NIH, NIDCR, CSDB, Bldg 49, Rm 5A-76, 49 Convent Dr, Bethesda, MD 20892, e-mail: mezeye@mail.nih.gov.
References
Author notes
Z.E.T., R.R.L., and T.S. contributed equally to this work.









 represents proliferation at normoxic conditions; ■ shows proliferation rate in hypoxic conditions. Controls received no factors but BRDU. G-CSF seems to be ineffective in the concentrations used, while SCF has a dose-dependent, significant proliferative effect, starting at 5 ng/mL. Combining the 2 factors results in proliferation rates similar to that of SCF alone. Hypoxic conditions seem to further increase the effectiveness of SCF. Values are means plus SD.
represents proliferation at normoxic conditions; ■ shows proliferation rate in hypoxic conditions. Controls received no factors but BRDU. G-CSF seems to be ineffective in the concentrations used, while SCF has a dose-dependent, significant proliferative effect, starting at 5 ng/mL. Combining the 2 factors results in proliferation rates similar to that of SCF alone. Hypoxic conditions seem to further increase the effectiveness of SCF. Values are means plus SD.