We evaluated the role of rituximab during remission induction chemotherapy in relapsed aggressive CD20+ non-Hodgkin lymphoma. Of 239 patients, 225 were evaluable for analysis. Randomized to DHAP (cisplatin-cytarabine-dexamethasone)-VIM (etoposide-ifosfamide-methotrexate)-DHAP (cisplatin-cytarabine-dexamethasone) chemotherapy with rituximab (R; R-DHAP arm) were 119 patients (113 evaluable) and to chemotherapy without rituximab (DHAP arm) 120 patients (112 evaluable). Patients in complete remission (CR) and partial remission (PR) after 2 chemotherapy courses were eligible for autologous stem-cell transplantation. After the second chemotherapy cycle, 75% of the patients in the R-DHAP arm had responsive disease (CR or PR) versus 54% in the DHAP arm (P = .01). With a median follow-up of 24 months, there was a significant difference in failure-free survival (FFS24; 50% vs 24% vs, P < .001), and progression free survival (PFS24; 52% vs 31% P < .002) in favor of the R-DHAP arm. Cox-regression analysis demonstrated a significant effect of rituximab treatment on FFS24 (HR 0.41, 95% confidence interval [CI] 0.29-0.57 versus 0.51, 95% CI 0.37-0.70) and overall-survival (OS24: HR 0.60 [0.41-0.89] vs 0.76 [0.52-1.10]) when adjusted for time since upfront treatment, age, World Health Organization performance status, and secondary age-adjusted international prognostic index. These results demonstrate improved FFS and PFS for relapsed aggressive B-cell NHL if rituximab is added to the re-induction chemotherapy regimen.
Introduction
High-dose chemotherapy with autologous stem-cell transplantation (ASCT) is curative in a proportion of patients with relapsed or refractory aggressive non-Hodgkin lymphoma (NHL).1 In general, the 5-year overall survival is 30% to 50%. Different parameters have been identified that have important impact on the overall survival results. Chemosensitivity, that is, the ability to induce a partial remission (PR) or complete remission (CR) on reinduction chemotherapy before ASCT is especially important.2 Different reinduction regimens have been applied in this setting, including DHAP (cisplatin-cytarabine-dexamethasone), VIM (etoposide-ifosfamide-methotrexate), ICE (ifosfamide-carboplatin-etoposide), or combinations.1,,,,–6 However, so far no distinct differences have been demonstrated in the efficacy of the different chemotherapy regimens although comparative studies have not been performed.
For patients with newly diagnosed aggressive CD20+ B-cell NHL, it has recently been shown that the addition of rituximab to an anthracyclin-based regimen improves the CR rate and overall survival (OS) significantly,7,,–10 with up to 10% to 15% improvement. In patients with relapsed or primary refractory aggressive CD20+ B-cell NHL, no prospective randomized studies have been performed. Kewalramani et al reported the results of 36 relapsed patients treated with rituximab plus ICE (RICE) followed by ASCT, and compared these results with a historical control group.5 They observed a significant improvement in the CR rate after 3 cycles of RICE without a difference in OS so far. Comparable results were described in patients with recurrent B-cell NHL treated with high-dose rituximab in conjunction with ASCT.11 In the present prospective randomized phase 3 study conducted by the Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON), the efficacy of rituximab added to the DHAP-VIM-DHAP regimen followed by ASCT in patients with relapsed or primary refractory aggressive CD20+ B-cell NHL was tested. The results demonstrate that the addition of rituximab to second-line chemotherapy followed by ASCT results in a significant improvement in failure free survival (FFS) and progression free survival (PFS).
Methods
Patients
Patients aged 18 to 65 years who had aggressive CD20+ B-cell NHL, including diffuse large B-cell lymphoma, mediastinal B-cell lymphoma, and follicle center lymphoma grade 3, who relapsed after, or were refractory/progressive on a standard anthracyclin-based (cyclophosphamide, doxorubicin, vincristine, and prednisone [CHOP]-like) regimen were eligible. Before enrollment, all patients were required to have a histologic confirmation of a CD20+ aggressive B-cell NHL. All biopsies were reviewed by hematopathologists of the participating transplantation centers.
Eligible patients had a World Health Organization (WHO) performance status of 0 to 1. Exclusion criteria included central nervous system (CNS) involvement, history of HIV infection, posttransplant lymphoproliferative disorder, or inadequate organ function. Patients were fully evaluated, including computed tomography (CT) scanning of thorax and abdomen, and bone marrow biopsy. All patients gave informed consent for study participation according to the regulations of the Dutch health authorities. The study was performed and evaluated by HOVON in accordance with the Declaration of Helsinki. The participating HOVON institutions and investigators are listed in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Enrollment took place between December 2000 and December 2005.
Study design and treatment
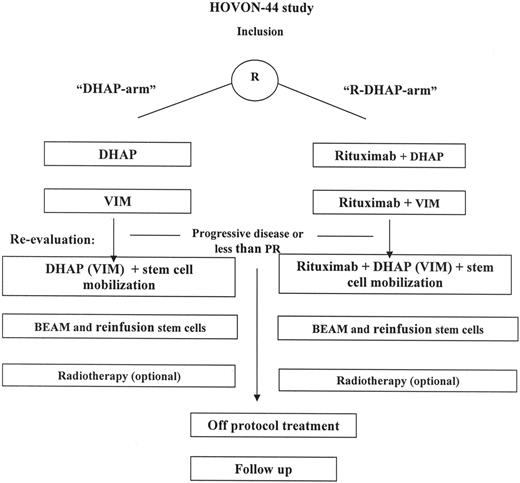
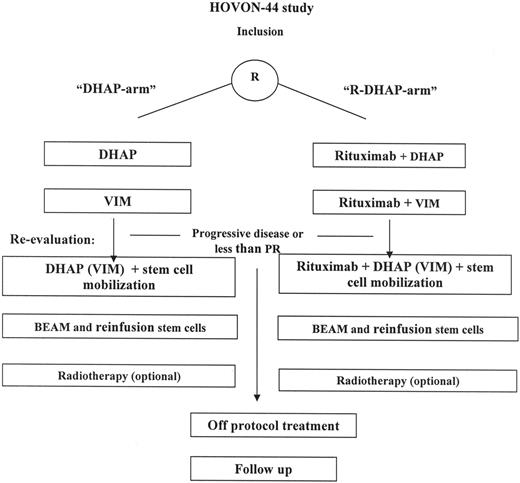
This was a multicenter randomized phase 3 trial. Patients were stratified according to type of response to first-line treatment: response duration more than 3 months versus progression or response duration less than 3 months. The planned treatment consisted of 3 cycles of reinduction chemotherapy6 (Figure 1). Patients received reinduction chemotherapy with DHAP-VIM-DHAP followed by ASCT (DHAP arm) or DHAP-VIM-DHAP in conjunction with rituximab followed by ASCT (R-DHAP arm). The DHAP regimen consisted of cisplatin (100 mg/m2) on day 1 via continuous infusion over 24 hours, followed on day 2 by cytarabine at 2 g/m2 in a 3-hour infusion dose, repeated after 12 hours. Dexamethasone, 40 mg/day given orally or intravenously, was administered for 4 consecutive days. The VIM regimen consisted of etoposide (90 mg/m2) intravenously on days 1, 3, and 5; ifosfamide (1200 mg/m2 intravenously) on day 1 through 5; and methotrexate (30 mg/m2 intravenously) on days 1 and 5. Rituximab (375 mg/m2) was administered on day 5 of the DHAP course or on day 6 of the VIM course. After the second DHAP course, beginning on day 10, granulocyte colony stimulating factor (Filgastrim; Amgen, Thousand Oaks, CA) was administered subcutaneously at a dose of 5 μg/kg each day until the end of leukapheresis. Cycles were given every 4 weeks. In case patients were nonresponsive to (R)-DHAP but responsive to (R)-VIM, it was allowed to repeat the (R)-VIM regimen as the third cycle of reinduction chemotherapy.
Treatment schedule of patients treated according HOVON-44 protocol. Re-evaluation was performed after (R)-DHAP and (R)-VIM. In the case of partial or complete response, patients continued the treatment with (R)-DHAP. In a limited number of patients, nonresponse or toxicity was observed on (R)-DHAP. In this situation the third course consisted of (R)-VIM.
Treatment schedule of patients treated according HOVON-44 protocol. Re-evaluation was performed after (R)-DHAP and (R)-VIM. In the case of partial or complete response, patients continued the treatment with (R)-DHAP. In a limited number of patients, nonresponse or toxicity was observed on (R)-DHAP. In this situation the third course consisted of (R)-VIM.
Peripheral blood stem-cell collection
After the third cycle of chemotherapy, once the white blood cell count recovered from nadir to more than 2 × 109/L, leukapheresis was performed until at least 2 × 106 CD34+ cells/kg had been collected. In case of inadequate peripheral stem cell collection, a bone marrow harvest was allowed as previously described.6
Assessment of response
Response to (R)-DHAP-(R)-VIM was assessed by conventional diagnostic methods, including CT scanning approximately 14 to 21 days after the second chemotherapy course. Bone marrow biopsies were repeated only if samples were abnormal before treatment. Response evaluation was repeated after transplantation or at an earlier time point if clinically indicated. Response was assessed using the International Working Group criteria.12
ASCT
Only those patients who achieved CR or PR after 2 cycles of chemotherapy were considered candidates for ASCT. After the third chemotherapy cycle, these patients received high-dose chemotherapy according to the BEAM (carmustine, etoposide, cytarabine, melphalan) protocol. This included administration of carmustine (300 mg/m2) on day −6, etoposide (200 mg/m2) and cytarabine (200 mg/m2) on day −5 to day −2, and melphalan (140 mg/m2) on day −1. Peripheral blood stem cells were thawed and reinfused on day 0, at least 24 hours after completion of BEAM. Radiotherapy after transplantation of involved areas was allowed.
Supportive care and clinical monitoring
Antibiotic prophylaxis to decontaminate the gastrointestinal tract was applied at a neutrophil count less than 0.5 × 109/L according to local protocols in the various centers. No hematopoietic growth factors were applied after the infusion of stem cells. Therapeutic antibiotic, antiviral, and antimycotic treatment was left to the discretion of the investigator, but was initiated at least at a body temperature more than 38.5°C after 2 readings taken 2 hours apart, and the treatment was discontinued once the patient had remained afebrile for 72 hours. Irradiated platelet transfusions were given if the platelet count was less than 10 × 109/L or in cases of significant bleeding. Irradiated red blood cells were transfused according to the policy of each institution. Complete blood counts and vital signs were monitored daily during hospitalization. Afebrile patients not requiring intravenous treatment were discharged from the hospital at a neutrophil count of more than 0.5 × 109/L.
The sAAIPI
The secondary age adjusted IPI (sAAIPI) was assessed according to absence or presence of 3 risk factors at the start of reinduction treatment in this study: Eastern Cooperative Oncology Group (ECOG) performance score greater than 1, lactate dehydrogenase (LDH) level greater than upper level of normal, and stage III or IV disease.13,14 Patients with 0, 1, 2, or 3 risk factors were considered to have low-, low-intermediate-, high-intermediate-, or high-risk disease, respectively. In the present study, patients were categorized for risk of disease as low (0 risk factors), intermediate (1 risk factor), or high (2-3 risk factors).
Statistics
The data were analyzed as of April 2007. Patient characteristics were compared between the 2 treatment arms using the Pearson χ2 or the Fisher exact test, whichever was appropriate for discrete variables, or the Wilcoxon rank sum test for continuous variables. Study end points were CR and PR rate, FFS, PFS, and OS. FFS was defined as the time from start of treatment to no response after cycle II, progression, relapse, or death as a result of any cause, whichever came first. Patients without progression or relapse who were still alive were censored at the date of last contact. The intention to treat principle was applied. Only patients who turned out not to have been eligible for the study were excluded from analysis, while eligible patients who were not treated according to protocol were analyzed according to treatment arm. Patients who received alternative treatment not according to the protocol were only considered as failure if they did not respond to the treatment at that timepoint. PFS is defined as the time from study entry until disease progression or death as a result of any cause. OS was defined as the time from the start of treatment to death irrespective of cause; patients still alive were censored at the date of last contact.
The chi-square test was used to compare response rates between the 2 arms
The Kaplan-Meier method was used to estimate FFS, PFS, and OS, and 95% confidence intervals (CI)s were calculated. Cox regression analysis was used to calculate the hazard ratio (HR) between the 2 arms with the 95% CI and the P value based on the likelihood ratio test in unadjusted and adjusted analysis. Adjustment was done for several prognostic factors that were identified also by Cox regression analysis. The previous response and previous response duration turned out to be scored inconsistently. Therefore we used a simple factor that is strongly related to the duration of previous response, namely the time since upfront treatment, that is, the interval in months between start of first-line treatment and registration in this study. The logarithm of this variable was used in a Cox regression analysis. A split of this variable into 3 classes with cut-off points at 6 and 12 months, respectively, was used to show the impact of this factor on FFS and OS with survival curves.
All reported P values are 2-sided and a significance level (α = .05) was used.
Results
Patient characteristics
Patients (n = 239) were enrolled in the study; 14 turned out to be not eligible on the basis of incorrect histology (n = 5), second relapse (n = 3), no previous treatment with an antracyclin containing regimen (n = 2), no signs of progression or relapse (n = 2), and incorrect or withdrawal of informed consent (n = 2). Thus, 225 patients were analyzed on the basis of intent to treat, 112 patients in the control arm (DHAP) and 113 patients in the rituximab-containing arm (R-DHAP). Characteristics of the evaluable patients are listed in Table 1. No significant differences between both arms were observed for WHO performance, LDH, and B symptoms. According to the sAAIPI, 25%, 35%, and 40% of the patients in the DHAP-arm belonged to the low-, intermediate-, and high-risk groups, and 15%, 43%, and 42%, respectively, in the R-DHAP arm (not significant). Both arms included mostly patients with diffuse large B-cell NHL (DLBCL), 88% in the DHAP arm and 91% in the R-DHAP arm (Table 2). The majority of the patients (85%) had been treated with a CHOP-like regimen as first-line treatment and only a few had been exposed to rituximab previously (4%). In the DHAP arm, 54% of the patients had received upfront treatment more than 1 year previously versus 43% in the R-DHAP arm (not significant).
Response to chemotherapy and ASCT
Response (CR and PR) after (R)-DHAP and (R)-VIM was attained in 54% of the patients in the DHAP arm and 75% of the patients in the R-DHAP arm (P = .01). These patients were eligible to proceed to ASCT and were treated with a third reinduction course followed by peripheral stem-cell collection. Inadequate stem-cell collection was observed in 1 patient in the DHAP-arm and in 3 patients in the R-DHAP arm. Six patients in the DHAP-arm and 9 patients in the R-DHAP arm received (R)-VIM instead of (R)-DHAP as third reinduction chemotherapy course. Between the second cycle of reinduction chemotherapy and planned ASCT, 6 patients in the DHAP-arm and 7 patients in the R-DHAP arm demonstrated progressive disease and went off protocol. One patient in PR after DHAP and VIM did not proceed and went off protocol. This patient received consolidation chemotherapy, rituximab, and ASCT. In the R-DHAP arm one patient went off protocol in PR after 2 cycles of chemotherapy and received an allogeneic stem-cell transplantation. An additional patient in PR went off protocol without further treatment. For this patient the response improved to complete remission undefined (CRu). Ultimately, ASCT was performed in 52 (46%) patients of the DHAP-arm and in 72 (63%) patients of the R-DHAP arm (P = .01). Radiotherapy after transplantation was given in 3 patients in the DHAP-arm and 9 patients in the R-DHAP arm. The overall response rate in the DHAP-arm was CR, 35% and PR, 15% versus 46% and 27% in the R-DHAP arm, respectively (P = .003). The majority of nonresponding patients were offered third-line chemotherapy, which included rituximab for 31 (28%) nonresponding patients of the DHAP arm and 13 (11%) patients of the R-DHAP arm.
Treatment outcome
The median follow-up was 31 months (range 9-67). In the DHAP arm 60 patients died versus 50 patients in the R-DHAP arm. The main cause of death was progressive disease in 51 (45%) patients in the DHAP-arm and in 37 (33%) patients in the R-DHAP arm.
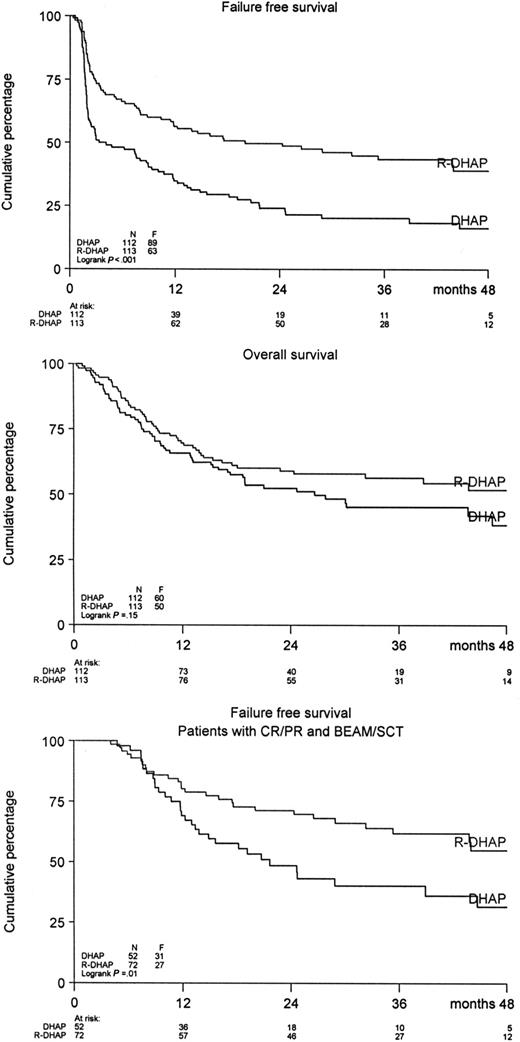
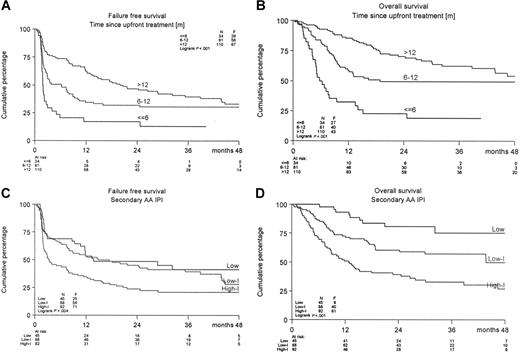
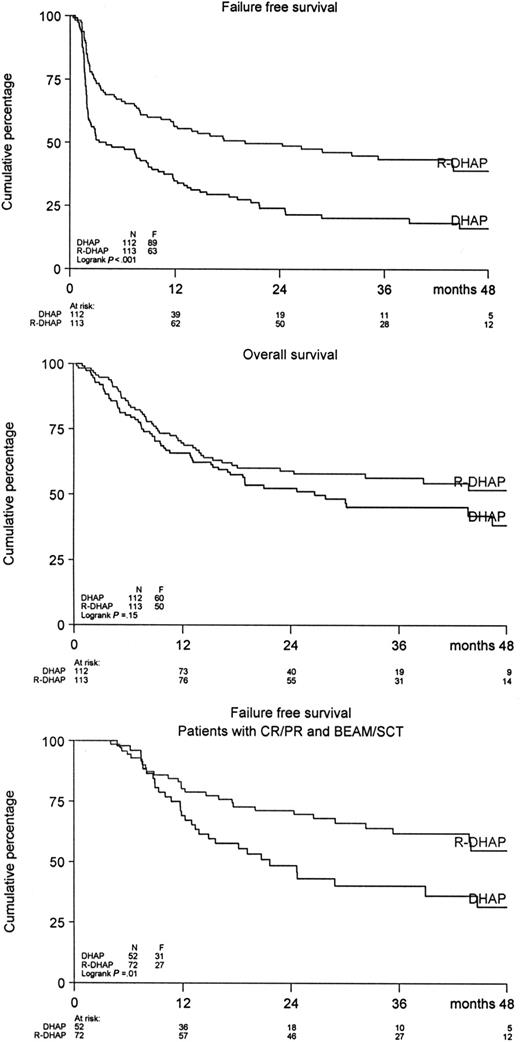
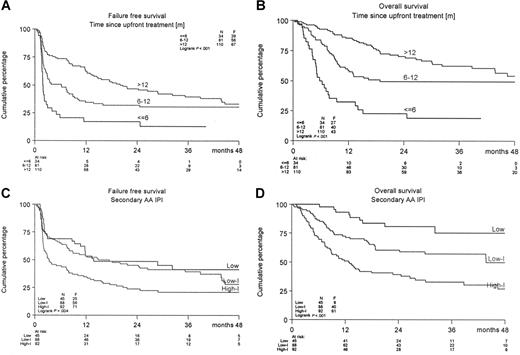
A significant difference was observed in FFS and PFS in favor of the R-DHAP arm at a median follow-up of 24 months (Figure 2). The FFS24 was 24% in the DHAP-arm versus 50% in the R-DHAP arm (P < .001). For the PFS24, these values are 31% versus 52% (P < .002) and for OS24, 52% versus 59% (P = .15), respectively. The effect of rituximab persisted after ASCT. A significantly improved FFS24 was observed for the R-DHAP patients achieving CR/PR after the 2 chemotherapy courses (P = .01, Figure 2). If the patients were stratified according to time since upfront treatment (ie, less than 6 months [n = 34] or 6-12 months [n = 81] or more than 12 months [n = 110], a significant difference was observed in FFS24 and OS24 between the different groups in favor of the patients with the longest time since upfront treatment (Table 3, Figure 3A,B). Several additional prognostic parameters were also tested that might have impact on FFS and OS. As depicted in Table 3, time since upfront treatment, sAAIPI, age, and WHO performance had significant impacts on FFS. The effects on FFS and OS of the different subgroups according to the sAAIPI score are depicted in Figure 3C,D. B-symptoms and LDH above normal were also relevant, but not when adjusted for time since upfront treatment, sAAIPI, age, and WHO performance status. Most of these factors had the same impact on OS. A Cox regression analysis was then performed to demonstrate the effect of rituximab on FFS and OS with and without adjustment for these additional prognostic parameters (ie, time since upfront treatment, sAAIPI, age, and WHO performance). As depicted in Table 4, rituximab treatment had a significant effect on FFS24 (HR 0.41; 95% CI 0.29-0.57) versus 0.51 (95% CI 0.37-0.70) and OS24 (HR 0.60; 95% CI 0.41-0.89) versus 0.76 (95% CI 0.52-1.10) when adjusted for the additional risk factors. The beneficial effect of rituximab on FFS in the R-DHAP treatment arm was similar within subgroups split according to time since upfront treatment (with test of interaction P = .73); less than 6 months, HR 0.45 (95% CI 0.21-0.97), or 6 to 12 months (HR 0.29, 95% CI 0.17-0.51), or more than 12 months (HR 0.45, 95% CI 0.27-0.76). Also with end point OS there was no evidence of a difference in treatment effect in the subgroups split by time since upfront treatment (test for interaction P = .34).
FFS and OS for patients treated with DHAP (n = 112) or R-DHAP (n = 113) and FFS for patients attaining CR/PR after 2 cycles of (R)-DHAP and (R)-VIM, which was followed by a third chemotherapy cycle and ASCT.
FFS and OS for patients treated with DHAP (n = 112) or R-DHAP (n = 113) and FFS for patients attaining CR/PR after 2 cycles of (R)-DHAP and (R)-VIM, which was followed by a third chemotherapy cycle and ASCT.
FFS and OS of treated patients according to time since upfront treatment or according to sAAIPI. (A,B) Time since upfront treatment (< 6, 6-12, > 12 months) or (C,D) sAAIPI.
FFS and OS of treated patients according to time since upfront treatment or according to sAAIPI. (A,B) Time since upfront treatment (< 6, 6-12, > 12 months) or (C,D) sAAIPI.
Discussion
The present study demonstrated that rituximab had a significant impact on the treatment results of second-line chemotherapy in rituximab-naive, relapsed CD20+ aggressive NHL patients. A significant improvement in FFS and PFS was observed in favor of the rituximab treatment. Moreover the beneficial effects of rituximab were observed both in patients with progression on first-line treatment as well in relapsed patients.
So far, different chemotherapy regimens are applied in patients with relapsing aggressive NHL. ICE, DHAP, VIM or combinations are used as reinduction chemotherapy followed by ASCT.1,,,,–6 In general, no significant differences between the different regimens are observed although no direct comparison has been performed. Parameters having the most significant impact on outcome are time since upfront treatment, sAAIPI score at relapse, and response on second-line chemotherapy as determined by fluorine-18-fluorodeoxy-glucose-positron emission tomography (FDG-PET).13,,–16 The prognosis of patients who do not respond on reinduction chemotherapy is poor, as was also shown in the present study, where 76% of the nonresponding patients died versus 33% of the responding patients.
The present study demonstrates that the group of responders on reinduction chemotherapy can be enlarged from 54% to 74% by the addition of rituximab. However it appeared that the impact of the addition of rituximab on OS was smaller than on FFS and PFS and not significant in unadjusted analysis (HR 0.76). When adjusted for important prognostic factors such as time since upfront treatment, sAAIPI, age, and WHO performance status, the effect was stronger (HR 0.60) and statistically significant. This finding can probably be ascribed to the fact that a proportion of the nonresponding and relapsing patients could be treated successfully with a third-line treatment, including rituximab.
In the present study, only 3 infusions with rituximab were given. Compared with ongoing protocols for upfront treatment in DLBCL, the applied dose of rituximab was relatively low. In most upfront studies, 6 to 8 infusions are given in conjunction with an anthracyclin-containing regimen.7,,–10 No convincing data are available demonstrating that infusion of 6 cycles might initiate a better apoptotic response compared with 3 cycles of rituximab. However, there is some clinical data suggesting that more frequent application of rituximab at the start of therapy and higher circulating blood levels might induce a longer and perhaps improved cytotoxic response. Khouri et al investigated this issue by administrating high-dose rituximab in patients with recurrent aggressive NHL who were eligible for ASCT.11 However, the response rate in their study was not distinctly different from the response rate in the R-DHAP arm of our study. Neither was the PFS in responding patients. Future randomized studies will prove whether increasing rituximab dose and or frequency is a feasible and effective approach to enlarge the number of responding patients eligible for ASCT.
At the start of the present study, only a small fraction of the relapsed/progressed patients had a prior exposure to rituximab. This situation has changed significantly. At present rituximab is part of the first-line treatment regimen applied to almost all patients with aggressive CD20+ NHL. Whether, these patients will respond differently at relapse compared with patients in the present study has until now not been studied but seems unlikely for the majority of them. A prerequisite for the effects of rituximab is the presence of CD20 antigen on the cell surface of the malignant lymphoid cells. It is likely that the CD20 antigen will be re-expressed in the majority of patients when rituximab has disappeared from the circulation. This occurs primarily a few weeks after cessation of therapy.17 However, whether patients who are progressive or nonresponding on first-line treatment will have the same beneficial effects of rituximab as observed in the present study is uncertain. On the one hand it is conceivable that the sensitizing effects of rituximab only become significant when it is coadministered with an effective second-line chemotherapy regimen. On the other hand, it has to be considered that the tumor of relapsing patients after first-line treatment with an R-CHOP like regimen might have a more aggressive behavior. The recurrent lymphoid clone is resistant to 5 separate agents with their own cytotoxicity profile compared with 4 agents in the setting of the CHOP-like regimen. Preliminary results from the ongoing international CORAL intergroup study suggest that the treatment results in this group of patients might be less impressive.18
A significant in vivo B-cell depletion will have occurred in patients treated with rituximab. This might have a beneficial effect on the final outcome of the R-DHAP arm. The stem-cell transplant is purged in vivo by rituximab from residual malignant B-cells. On the other hand, the humoral immunity might also be impaired before, during, and after the ASCT, thus increasing the risk for bacterial infections. However, no increased infection rate was observed in the R-DHAP arm (data not shown). Moreover the recovery of granulocytes and platelets was not hampered in the rituximab-treated patients compared with the controls, indicating that this agent can safely be applied in this setting.
In conclusion, this randomized prospective study demonstrates an improved FFS and PFS for patients treated for relapsing aggressive CD20+ NHL if rituximab is added to the reinduction chemotherapy regimen.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.V., W.E.F., and P.C.H. designed the research protocol; E.V., W.J.L.v.P., M.B.v.t.V., J.M.Z., W.E.F., M.H.J.v.O., L.F.V., P.W.W., G.W.v.I., P.J.L., and P.C.H. were involved in performing the clinical research; E.V. and W.L.J.v.P. collected and analyzed the data; E.V., W.J.L.v.P., M.B.v.t.V., J.M.Z., W.E.F., M.H.J.v.O., L.F.V., P.W.W., G.W.v.I., P.J.L., and P.C.H. wrote the paper and critically contributed to the final preparation of the article.
A complete list of the members of the Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON) is provided in Document S1 on the Bloodwebsite.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Edo Vellenga, MD, PhD, University Medical Center Groningen, Department of Hematology, Hanzeplein 1, 9713 GZ Groningen; e-mail: e.vellenga@int.umcg.nl.