The platelet von Willebrand factor (vWF) receptor, glycoprotein Ib-IX (GPIb-IX), mediates platelet adhesion and induces signaling leading to integrin activation. Phosphoinositol 3-kinase (PI3K) is important in GPIb-IX–mediated signaling. PI3K–dependent signaling mechanisms, however, are unclear. We show that GPIb-IX–induced platelet aggregation and stable adhesion under flow were impaired in mouse platelets deficient in PI3K effectors, Akt1 and Akt2, and in human platelets treated with an Akt inhibitor, SH-6. Akt1 and Akt2 play important roles in early GPIb-IX signaling independent of Syk, adenosine diphosphate (ADP), or thromboxane A2 (TXA2), in addition to their recognized roles in ADP- and TXA2–dependent secondary amplification pathways. Knockout of Akt1 or Akt2 diminished platelet spreading on vWF but not on immobilized fibrinogen. Thus, Akt1 and Akt2 are both required only in the GPIb-IX–mediated integrin activation (inside-out signaling). In contrast, PI3K inhibitors abolished platelet spreading on both vWF and fibrinogen, indicating a role for PI3K in integrin outside-in signaling distinct from that in GPIb-IX–mediated inside-out signaling. Furthermore, Akt1- or Akt2-deficiency diminished vWF–induced cGMP elevation, and their inhibitory effects on GPIb-IX–dependent platelet adhesion were reversed by exogenous cGMP. Thus, Akt1 and Akt2 mediate GPIb-IX signaling via the cGMP–dependent signaling pathway.
Introduction
In the event of vascular injury, circulating platelets adhere to the exposed subendothelial matrix. Under high shear-rate flow conditions such as in arteries, the interaction between matrix-bound von Willebrand factor (vWF) and its platelet receptor, glycoprotein Ib-IX (GPIb-IX), is required for initial platelet adhesion.1,2 vWF binding to GPIb-IX also induces intracellular signals leading to integrin activation and, thus, integrin–dependent stable platelet adhesion, spreading, aggregation, and thrombus formation.3,,–6 Normally, soluble vWF does not bind to GPIb-IX in circulation. The interaction between vWF and GPIb-IX can be induced by a vWF “conformational” change caused by its binding to collagen, its immobilization, and/or high shear stress.7,8 Under experimental conditions, botrocetin and ristocetin are used to mimic the collagen–induced changes in vWF and initiate vWF binding to GPIb-IX and, thus, GPIb-IX–mediated signaling.2,9,10
The GPIb-IX–mediated integrin activation signal (inside-out signaling) has been shown to involve many intracellular signaling molecules and pathways, including activation of protein kinases, lipid kinases, elevation of calcium, activation of the immunoreceptor tyrosine-based activation motif (ITAM) pathway (which is also important in collagen–induced platelet activation pathway), synthesis of thromboxane A2 (TXA2), and release of granule content adenosine diphosphate (ADP).6,11,,,,,,–18 However, the molecular pathways of GPIb-IX signaling, particularly the early signaling pathways, remain poorly understood. We have recently reported that sequential activation of cGMP–dependent protein kinase I, p38, and extracellular stimuli responsive kinase (ERK) is important in GPIb-IX–mediated signaling leading to integrin activation.15,19,20 In addition, vWF binding to GPIb-IX has been shown to induce NO synthesis and cGMP elevation.15,21 Thus, it is important to understand the upstream signaling mechanisms that activate the NO-cGMP-p38-ERK pathway and the role of this pathway in early GPIb-IX signaling.
It was reported that the Src family protein kinases (SFK), c-Src and Lyn, form complexes with PI3K and GPIb-IX after vWF stimulation.22 PI3K has also been reported to interact with GPIb-IX via 14-3-3.23 Previous studies using pharmacologic inhibitors suggested that PI3K is important in GPIb-IX–mediated, integrin–dependent platelet adhesion, spreading, and aggregation.16,17,24 PI3K has also been shown to play roles in integrin outside-in signaling.25,26 It is unclear, however, how PI3K plays a role in mediating GPIb-IX-initiated inside-out signaling leading to integrin activation and integrin outside-in signaling.
One of the well-established downstream pathways of PI3K is to induce the recruitment of the phosphoinositide–dependent protein kinase (PDK) and Akt family of protein kinases to the membrane and consequent phosphorylation and activation of Akt.27,28 There are 3 known isoforms of Akt: Akt1, Akt2, and Akt3.28 Akt1 and Akt2 have been reported to express in both human and mouse platelets.29,–31 Recent studies showed that both Akt1 and Akt2 play roles in the amplification of platelet aggregation induced by G protein–coupled receptors and collagen receptors.30,–32 In this study, we have examined the role of Akt in GPIb-IX signaling using both knockout mouse models and pharmacologic inhibitors. We present data showing that both Akt1 and Akt2 play important roles in GPIb-IX–mediated integrin–dependent adhesion, spreading, and aggregation. We also show that PI3K mediates GPIb-IX early signaling, leading to integrin activation via the Akt1- and Akt2-dependent pathway, which is distinct from the role of PI3K in integrin outside-in signaling. Furthermore, we show that both Akt1 and Akt2 are important to GPIb-IX–mediated elevation of platelet cGMP and that exogenously added cGMP rescues the defect of GPIb-IX–mediated platelet activation in Akt1 and Akt2 knockout platelets. Thus, our study indicates that Akt1 and Akt2 play important roles in early GPIb-IX signaling leading to platelet activation, and a major downstream mechanism for this role of Akt is mediated via the NO-cGMP-PKG signaling pathway.
Methods
Preparation of platelets
For studies using human platelets, fresh blood was drawn by venipuncture from healthy volunteers (performed in the General Clinical Research Center at the University of Illinois Medical Center). Institutional Review Board approval was obtained from the University of Illinois at Chicago, and informed consent from volunteers was obtained in accordance with the Declaration of Helsinki. To prepare washed human platelets, one-seventh volume of ACD was used as anticoagulant.33 Washed platelets were isolated from platelet-rich plasma by differential centrifugation of whole blood with 0.1 μg/mL prostaglandin E1 and 1 U/mL apyrase (Sigma-Aldrich, St Louis, MO). For studies using mouse platelets, fresh blood from mouse inferior vena cava was anticoagulated with ACD as previously described.34 Platelets were isolated by differential centrifugation and washed twice with CGS buffer (sodium chloride 0.12 M, trisodium citrate 0.0129 M, D-glucose 0.03 M, pH 6.5), and resuspended in modified Tyrode buffer as previously described.33 In some experiments, platelets were washed and resuspended according to Liu et al.35
Platelet aggregation
Platelet aggregation was measured in a turbidometric platelet aggregometer (Chronolog, Havertown, PA) at 37°C with stirring (1000 rpm). Washed platelets (3 × 108/mL) were resuspended in modified Tyrode buffer. vWF and ristocetin (Sigma-Aldrich) or botrocetin (kindly provided by Dr Michael C. Berndt, Monash University, Melbourne, Australia)36 were added to washed platelets to induce platelet aggregation. To test the effects of Akt inhibitor SH-6 (Calbiochem, La Jolla, CA), SH-6 or dimethylsulfoxide (DMSO) was preincubated with platelets at 37°C for 2 minutes before addition of agonists.
Platelet spreading on immobilized vWF or fibrinogen
Chamber slides with microtiter wells (Nalgen Nunc, Rochester, NY) were coated with 30 μg/mL vWF or fibrinogen in 0.1 M NaHCO3 (pH 8.3) at 4°C overnight. Washed platelets (2 × 107/mL) preincubated with or without inhibitors were allowed to adhere and spread on vWF– or fibrinogen–coated wells at 37°C for 90 minutes. Botrocetin (1 μg/mL) was also added in vWF–coated wells. After 3 washes with phosphate-buffered saline (PBS; 0.01 M NaH2PO4, 0.15 M NaCl, pH 7.4), the cells were fixed, permeabilized, and stained with fluorescein-labeled phalloidin (Molecular Probes, Eugene, OR) as previously described.37 Adherent platelets were viewed with a Leica RMI RB inverted fluorescence microscope (Leica Microsystems, Wetzlar, Germany) using a HCX PL FLUOTAR lens at 100×/1.30 NA oil objective. Images were acquired using a CoolSNAP HQ CCD camera (Photometrics, Tucson, AZ) and processed with RS Image version 1.4 software (Photometrics, Tucson, AZ). The spreading area of single platelets was measured using ImageJ software (National Institutes of Health, Bethesda, MD) with pixel numbers as unit of size. Three randomly selected fields from different tests were used for statistical analysis. Bars represent the average surface area (± SD) of individual platelets. Statistical significance of the difference between groups was analyzed using Student t test.
Platelet adhesion under shear stress
Glass slides were coated with vWF (30 μg/mL) overnight as described in “Platelet spreading on immobilized vWF or fibrinogen.” Washed platelets (3 × 108/mL) were pretreated with different inhibitors, SH-6, wortmannin, piceatannol (Calbiochem), A3P5P, 2MeSAMP, or aspirin (Sigma-Aldrich) for 5 minutes, and then loaded onto the slides. A cone-plate rheometer (Rheostress 1, Thermo-HAAKE, Paramus, NY) was used to introduce shear stress to the platelets. For the cGMP reversal experiment, 10 μM 8-bromo-guanosine 3′, 5′-cyclic monophosphate (8-bromo-cGMP; Calbiochem) was added after inhibitor treatment. A fluorescence dye mepacrine (10 μM; Sigma-Aldrich) was added to the platelets before the application of constant shear rate (800 s−1) to the platelets for 5 minutes. Slides were then quickly rinsed in a container with approximately 200 mL PBS 3 times to wash out platelets that are not stably adherent. Slides were viewed with a Leica DMI RB fluorescence microscope (Leica Microsystems) using an N PLAN L lens at 40×/0.55 NA objective with 1.5× magnification. Stable adherent platelets were counted in 10 randomly selected microscope fields. Bars indicate the average numbers (± SD) of adherent platelets per field. Statistical significance of the difference between groups was analyzed using Student t test.
Platelet rolling under shear in a flow chamber
Analysis of platelet rolling on vWF under shear stress was performed essentially as previously described38 with modifications as follows. Glass slides (22 × 40 mm, Warner Instrument, Hamden, CT) were coated with vWF (30 μg/mL) in 2 mM ammonium acetate (pH 7.0) at 37°C for 2 hours. After blocking with 5% bovine serum albumin (BSA) in PBS at 22°C for 2 hours, the coated slide together with a rubber gasket (100-μm height) and cover slide (Harvard Apparatus, Holliston, MA) were assembled into a flow chamber as described by the manufacturer (RC-30, Harvard Apparatus, Holliston, MA). Washed platelets (3 × 108/mL) and red blood cells (2.5 × 109/mL) from Akt1−/−, Akt2−/−, or wild-type mouse were reconstituted in Tyrode buffer containing 1% BSA, 5 mM EDTA, and 10 μM mepacrine. Platelets were perfused through the chamber under 800 s−1 shear rate using a syringe pump (Harvard Apparatus), visualized with a Leica DMI RB fluorescence microscope (Leica Microsystems) using an N PLAN L lens at 40×/0.55 objective with 1.5× magnification (equivalent to 60× magnification). Images were acquired using a CoolSNAP HQ CCD camera (Photometrics) and an HDD recorder, and were processed with ISEE imaging version XII software (Inovision, Raleigh, NC). Rolling platelets were counted in 10 randomly selected fields of 0.01 mm2 within a 30-second time period from 3 different experiments for the statistics. Bars indicate the average numbers (± SD) of rolling platelets per 1 mm2.
vWF binding to platelets
Washed platelets (3 × 108/mL) were resuspended in PBS containing 1% BSA and an integrin antagonist arginine-glycine-aspartate-serine (RGDS; 1 mM). Platelets were incubated with vWF (10 μg/mL) alone as control or vWF plus botrocetin (2 μg/mL) at 22°C for 30 minutes. After washing with PBS once, platelets were incubated with 10 μg/mL fluorescein isothiocyanate-labeled anti-vWF antibody SZ-29 (kindly provided by Dr Changeng Ruan, Jiangsu Institute of Hematology, Suzhou, China) at 22°C for 30 minutes. In some samples, platelets were preincubated with SH-6 at 22°C for 5 minutes before addition of agonists. The vWF binding was analyzed by flow cytometry.39
Immunoblot detection of Akt phosphorylation
Washed platelets (3 × 108/mL) were stirred (1000 rpm) in a platelet aggregometer for 1 minute after adding ristocetin (0.5 mg/mL), or ristocetin and vWF (25 μg/mL). Platelets were also preincubated with DMSO, wortmannin (100 nM), or LY294002 (20 μM). The reaction was stopped by addition of equal volume of 2× sodium dodecyl sulfate (SDS) sample buffer, containing 0.2 mM E64, 2 mM PMSF and 34 μg/mL aprotinin (Sigma-Aldrich). Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene difluoride membranes and immunoblotted with the polyclonal antibody specifically recognizing Akt phosphorylated at serine-473 or with a polyclonal anti-Akt antibody (Cell Signaling Technology, Beverly, MA) to indicate loading levels.
vWF-induced platelet cGMP elevation
Washed platelets (human 5 × 108/mL, mouse 3 × 108/mL) 400 μL were incubated with or without vWF (15 μg/mL) and botrocetin (1.5 μg/mL) in a platelet aggregometer with stirring (1000 rpm) at 37°C for 8 minutes. The reaction was stopped by adding the equal volume of ice-cold 12% (w/v) trichloroacetic acid (400 μL). Samples were mixed and centrifuged at 2000g for 15 minutes at 4°C, and the supernatant was extracted 4 times with 5 volumes of water-saturated diethyl ether followed by lyophilization. The cGMP enzyme immunoassay kit (Amersham-Pharmacia Biotech, Piscataway, NJ) was used to measure the cGMP concentrations. Bars represent the average (± SD) cGMP concentration derived from 3 experiments.
Results
Akt1 and Akt2 are important in GPIb-IX–mediated and integrin-dependent platelet aggregation
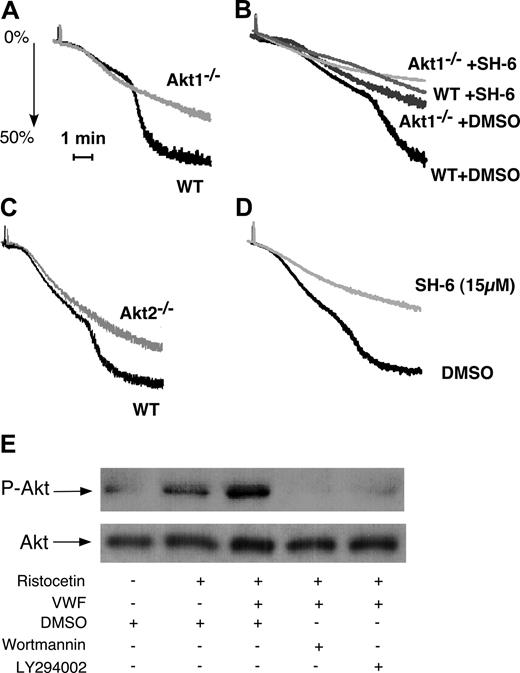
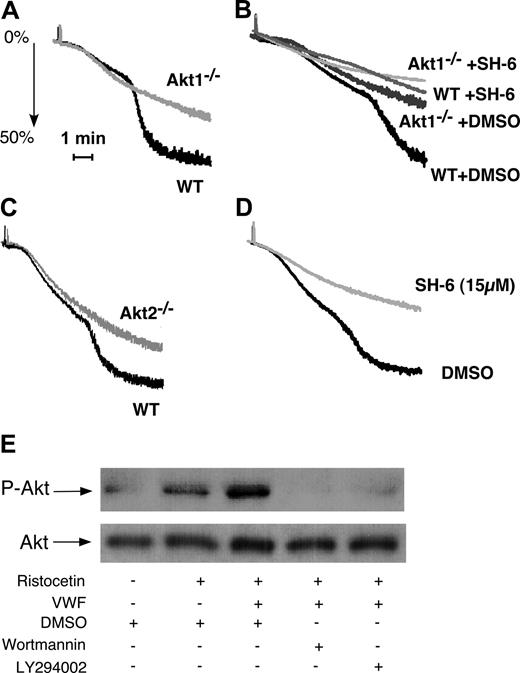
To determine whether Akt1 is important in GPIb-IX signaling, washed platelets from Akt1-knockout mice were stimulated with vWF in the presence of botrocetin. Wild-type mouse platelets undergo the typical 2-wave aggregation on exposure to botrocetin and vWF (Figure 1A). The first wave represents mainly the platelet “agglutination” induced by vWF binding to GPIb-IX but also contains a proportion of integrin-dependent platelet aggregation.35 The second wave is the secretion- and integrin-dependent platelet aggregation and thus reflects the GPIb-IX–mediated integrin activation. Akt1-null platelets exhibited impaired second-wave aggregation in response to botrocetin and vWF (Figure 1A,B), indicating that Akt1 plays an important role in GPIb-IX–induced, integrin-dependent platelet aggregation. Interestingly, an inhibitor of Akt family of kinases, SH-6, caused slightly more potent inhibition of botrocetin-induced integrin-dependent mouse platelet aggregation than Akt1 knockout at a concentration (15 μM) that is relatively specific for Akt, suggesting the possible involvement of other Akt isoforms in GPIb-IX–mediated platelet aggregation (Fig 1B). Indeed, Akt2 knockout platelets also showed impaired platelet aggregation in response to botrocetin and vWF (Figure 1C). Thus, both isoforms of Akt in platelets, Akt1 and Akt2, are important in mediating GPIb-IX–dependent platelet activation.
The role of Akt in GPIb-IX–mediated platelet aggregation and the PI3K-dependent Akt activation induced by vWF. (A) Washed wild-type (WT) and Akt1−/− mouse platelets were stimulated with botrocetin (2 μg/mL) and vWF (7.5 μg/mL). Platelet aggregation was monitored using a turbidometric aggregometer at 37°C and 1000 rpm stirring speed. (B) WT and Akt1−/− platelets were treated with DMSO or SH-6 (15 μM) for 2 minutes and then stimulated with botrocetin (1.2 μg/mL) and vWF (10 μg/mL). (C) WT and Akt2−/− platelets were stimulated with botrocetin and vWF as in panel B. (D) Washed human platelets were preincubated with Akt inhibitor SH-6 (15 μM) or the vehicle (DMSO) for 2 minutes, and then stimulated with botrocetin and vWF as in panel B. (E) Washed human platelets (5 × 108/mL) were preincubated with DMSO, PI3K inhibitors, wortmannin (100 nM), or LY294002 (20 μM), then stimulated with ristocetin (0.5 mg/mL) in the absence or presence of 25 μg/mL vWF for 1 minute in a platelet aggregometer. Platelets were solubilized and immunoblotted with a rabbit antibody specifically recognizing Akt phosphorylated at serine-473 (p-Akt) or with a rabbit anti-Akt antibody to indicate loading levels (Akt).
The role of Akt in GPIb-IX–mediated platelet aggregation and the PI3K-dependent Akt activation induced by vWF. (A) Washed wild-type (WT) and Akt1−/− mouse platelets were stimulated with botrocetin (2 μg/mL) and vWF (7.5 μg/mL). Platelet aggregation was monitored using a turbidometric aggregometer at 37°C and 1000 rpm stirring speed. (B) WT and Akt1−/− platelets were treated with DMSO or SH-6 (15 μM) for 2 minutes and then stimulated with botrocetin (1.2 μg/mL) and vWF (10 μg/mL). (C) WT and Akt2−/− platelets were stimulated with botrocetin and vWF as in panel B. (D) Washed human platelets were preincubated with Akt inhibitor SH-6 (15 μM) or the vehicle (DMSO) for 2 minutes, and then stimulated with botrocetin and vWF as in panel B. (E) Washed human platelets (5 × 108/mL) were preincubated with DMSO, PI3K inhibitors, wortmannin (100 nM), or LY294002 (20 μM), then stimulated with ristocetin (0.5 mg/mL) in the absence or presence of 25 μg/mL vWF for 1 minute in a platelet aggregometer. Platelets were solubilized and immunoblotted with a rabbit antibody specifically recognizing Akt phosphorylated at serine-473 (p-Akt) or with a rabbit anti-Akt antibody to indicate loading levels (Akt).
In human platelets, botrocetin (or ristocetin) is also known to induce similar 2-wave platelet aggregation.19 The second wave and part of the first wave of platelet aggregation induced by botrocetin (or ristocetin, not shown) were abolished by 15 μM SH-6 (Figure 1D), suggesting that Akt in human platelets, similarly, is important in GPIb-IX–induced integrin-dependent platelet aggregation. To determine whether vWF binding to GPIb-IX activates Akt, ristocetin and vWF were added to platelets for 1 minute in the platelet aggregometer, and the platelets were then solubilized and immunoblotted for Akt phosphorylated at Ser473 (Figure 1E). In the presence of ristocetin, vWF induced a significant increase in Akt phosphorylation compared with ristocetin alone (Figure 1E). Akt phosphorylation was abolished by PI3K inhibitors, indicating that vWF induces PI3K-dependent activation of Akt.
The role of Akt1 and Akt2 in platelet spreading on vWF and fibrinogen
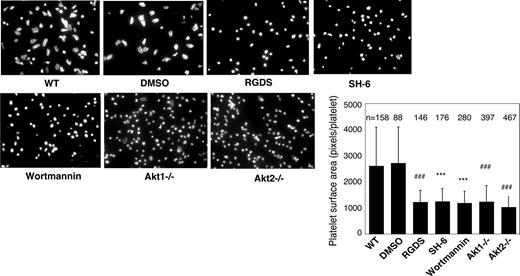
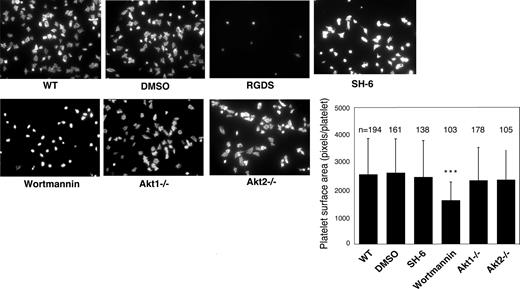
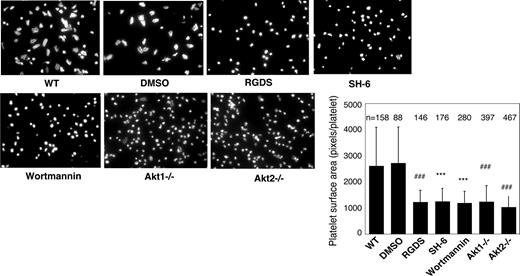
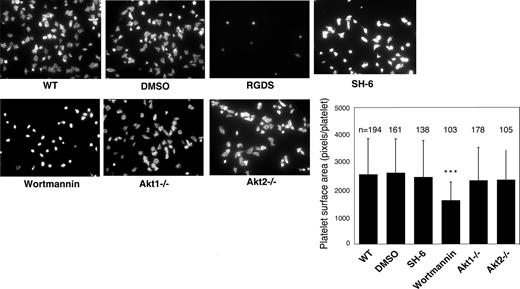
Previous studies showed that platelet spreading on purified vWF requires GPIb-IX–mediated initial platelet adhesion, GPIb-IX–mediated integrin activation (inside-out signaling) that allows integrin-vWF interaction,37,40 and integrin outside-in signals. Integrin binding to immobilized fibrinogen, however, does not require inside-out signaling; thus, platelet spreading on fibrinogen only requires outside-in signaling induced by integrin-fibrinogen interaction. To determine whether Akt plays a role in the integrin inside-out signaling induced by GPIb-IX or in the integrin outside-in signaling, wild-type, Akt1 knockout, Akt2 knockout, or 15 μM SH-6–treated mouse platelets were allowed to spread on vWF or fibrinogen. Wild-type platelets spread on vWF (Figure 2) and fibrinogen (Figure 3). RGDS peptide, an integrin antagonist, inhibited platelet spreading on vWF and abolished platelet adhesion and spreading on fibrinogen, indicating their dependence on integrin-ligand interaction. Both Akt1-deficient and Akt2-deficient platelets showed diminished platelet spreading on vWF compared with wild-type mouse platelets (Figure 2). Similarly, the SH-6–treated platelets also exhibited impaired spreading on vWF-coated surface compared with the vehicle DMSO (Figure 2). Interestingly, neither Akt1-deficiency nor Akt2-deficiency affected platelet spreading on fibrinogen (Figure 3). In addition, SH-6, at the same concentration (15 μM) that inhibited platelet spreading on vWF, did not significantly affect platelet spreading on fibrinogen. These data indicate that both Akt1 and Akt2 play important roles in GPIb-IX–mediated integrin activation (inside-out signaling), but Akt1 and Akt2 are not both required for outside-in integrin signaling. In contrast, PI3K inhibitors significantly reduced platelet spreading on both vWF and fibrinogen (Figures 2 and 3), indicating that PI3K mediates integrin outside-in signaling via a pathway that is distinct from GPIb-IX–mediated, Akt1- and Akt2-dependent inside-out signaling. It is important to note that a higher concentration of SH-6 (30 μM) also inhibited platelet spreading on both vWF and fibrinogen (not shown). However, because SH-6 as a phosphoinositide competitor may also affect Akt-independent PI3K activity at high concentrations41,42 and Akt1/2 double knockout is lethal, we have not been able to conclusively differentiate whether the PI3K-dependent outside in signaling is Akt-independent or requires the presence of any one of the 2 Akt isoforms.
Effects of PI3K and Akt inhibitors and Akt knockout on platelet spreading on immobilized vWF. Microtiter chamber slides were coated with 30 μg/mL vWF. Washed WT mouse platelets (2 × 107/mL) were preincubated with or without vehicle (DMSO), SH-6 (15 μM), or wortmannin (100 nM) for 5 minutes. WT, Akt1−/−, or Akt2−/− platelets were allowed to adhere and spread on vWF for 90 minutes in the presence of botrocetin (1 μg/mL). Adherent platelets were stained with fluorescein-labeled phalloidin and photographed under a fluorescence microscope as described in “Platelet spreading on immobilized vWF and fibrinogen.” Platelets were also treated with the integrin inhibitor, RGDS (1 mM), to indicate the integrin–dependent platelet spreading. Shown in the figure are representative pictures from one of 3 experiments with similar results and a bar graph of the quantitative analysis of platelet surface area as an indicator of spreading. The data are from 3 randomly selected fields from 3 experiments. The bars in the graph represent the average surface area (± SD) of individual platelets. ###P < .001 versus WT platelets. ***P < .001 versus DMSO–treated WT platelets. Numbers of platelets analyzed for each group are indicated above the bars.
Effects of PI3K and Akt inhibitors and Akt knockout on platelet spreading on immobilized vWF. Microtiter chamber slides were coated with 30 μg/mL vWF. Washed WT mouse platelets (2 × 107/mL) were preincubated with or without vehicle (DMSO), SH-6 (15 μM), or wortmannin (100 nM) for 5 minutes. WT, Akt1−/−, or Akt2−/− platelets were allowed to adhere and spread on vWF for 90 minutes in the presence of botrocetin (1 μg/mL). Adherent platelets were stained with fluorescein-labeled phalloidin and photographed under a fluorescence microscope as described in “Platelet spreading on immobilized vWF and fibrinogen.” Platelets were also treated with the integrin inhibitor, RGDS (1 mM), to indicate the integrin–dependent platelet spreading. Shown in the figure are representative pictures from one of 3 experiments with similar results and a bar graph of the quantitative analysis of platelet surface area as an indicator of spreading. The data are from 3 randomly selected fields from 3 experiments. The bars in the graph represent the average surface area (± SD) of individual platelets. ###P < .001 versus WT platelets. ***P < .001 versus DMSO–treated WT platelets. Numbers of platelets analyzed for each group are indicated above the bars.
Effect of PI3K and Akt inhibitors and Akt knockout on platelet spreading on immobilized fibrinogen. Microtiter chamber slides were coated with 30 μg/mL fibrinogen. Washed WT mouse platelets (2 × 107/mL) were preincubated with vehicle (DMSO), SH-6 (15 μM), or wortmannin (100 nM) for 5 minutes. WT, Akt1−/−, or Akt2−/− platelets were allowed to adhere and spread on immobilized fibrinogen for 90 minutes. Platelets were also treated with the integrin inhibitor, RGDS (1 mM), to indicate the integrin-dependent platelet adhesion and spreading. Adherent platelets were stained with fluorescein-labeled phalloidin and photographed under a fluorescence microscope as described in “Platelet spreading on immobilized vWF and fibrinogen.” Shown in the figure are the representative pictures from one of 3 experiments with similar results and a bar graph of the quantitative analysis of platelet surface area as an indicator of spreading. The data are from 3 randomly selected fields from 3 experiments. The bars in the graph represent the average surface area (± SD) of individual platelets. ***P < .001 versus DMSO–treated WT platelet. Numbers of platelets analyzed for each group are indicated above the bars.
Effect of PI3K and Akt inhibitors and Akt knockout on platelet spreading on immobilized fibrinogen. Microtiter chamber slides were coated with 30 μg/mL fibrinogen. Washed WT mouse platelets (2 × 107/mL) were preincubated with vehicle (DMSO), SH-6 (15 μM), or wortmannin (100 nM) for 5 minutes. WT, Akt1−/−, or Akt2−/− platelets were allowed to adhere and spread on immobilized fibrinogen for 90 minutes. Platelets were also treated with the integrin inhibitor, RGDS (1 mM), to indicate the integrin-dependent platelet adhesion and spreading. Adherent platelets were stained with fluorescein-labeled phalloidin and photographed under a fluorescence microscope as described in “Platelet spreading on immobilized vWF and fibrinogen.” Shown in the figure are the representative pictures from one of 3 experiments with similar results and a bar graph of the quantitative analysis of platelet surface area as an indicator of spreading. The data are from 3 randomly selected fields from 3 experiments. The bars in the graph represent the average surface area (± SD) of individual platelets. ***P < .001 versus DMSO–treated WT platelet. Numbers of platelets analyzed for each group are indicated above the bars.
The role of Akt1 and Akt2 in integrin-dependent stable platelet adhesion to vWF under shear stress
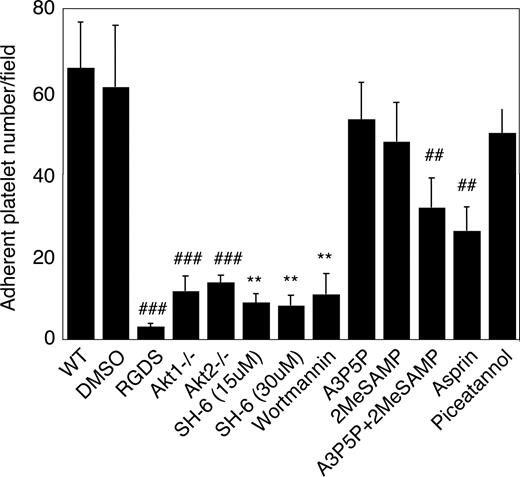
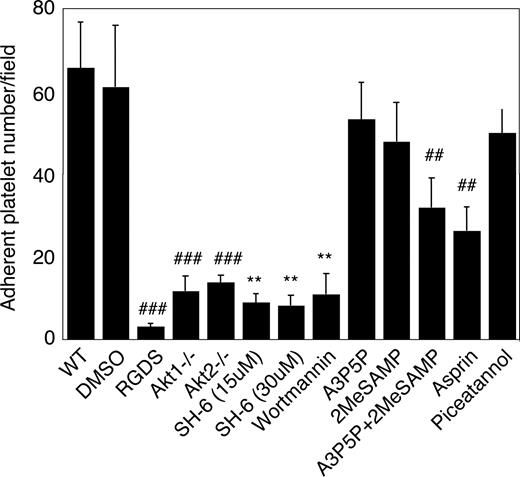
To understand the role of Akt in platelet adhesion and activation under shear stress, a new platelet adhesion assay was developed to investigate stable platelet adhesion to immobilized vWF under uniform and constant shear stress introduced by a cone and plate rheometer. A shear rate of 800 s−1 (≈8 dyne/cm2) was chosen to mimic the physiologic artery or arteriole shear rates. Normal human and mouse platelets showed stable adhesion under these conditions. Stable platelet adhesion was inhibited by integrin inhibitor RGDS, indicating that it is dependent on integrin αIIbβ3, and thus reflects GPIb-IX–mediated integrin activation (Figure 4). Stable platelet adhesion to vWF under these conditions was almost totally abolished in Akt1-knockout or Akt2-knockout mouse platelets and in human platelets treated with 15 μM SH-6, indicating important roles for both Akt1 and Akt2 in GPIb-IX–mediated, integrin-dependent stable platelet adhesion under flow. PI3K inhibitor wortmannin similarly inhibited stable platelet adhesion. It is known that PI3K and Akt play important roles in G-protein coupled receptor signaling induced by agonists such as ADP and TXA2,30,–32,34 both of which are released and important in GPIb-IX–mediated platelet activation. To determine the roles of ADP and TXA2 in the activation of PI3K and Akt pathway, we examined the effects of ADP receptor antagonists, and a cyclooxygenase inhibitor, aspirin, on stable platelet adhesion under flow. Preincubation of platelets with a P2Y1 antagonist, A3P5P, or a P2Y12 antagonist, 2MeSAMP, only slightly affected platelet adhesion under flow. Preincubation with both A3P5P and 2MeSAMP together significantly but partially inhibited platelet adhesion. Similarly, aspirin also partially inhibited platelet adhesion to vWF. The partial inhibitory effect of both ADP antagonists and aspirin are contrasted by the nearly complete inhibition by Akt1 knockout, Akt2 knockout, or 15 μM SH-6, indicating that the role of Akt is at least partially independent of ADP or TXA2 in stimulating GPIb-IX–mediated stable platelet adhesion, and that ADP and TXA2 are important amplifiers that promote full scale platelet adhesion and aggregation. Stable platelet adhesion under these conditions was not significantly inhibited by the Syk inhibitor, piceatannol, suggesting that integrin–dependent stable platelet adhesion to vWF under flow is independent of the GPIb-IX-ITAM-Syk signaling pathway. These data indicate that the PI3K-Akt pathway plays an important role in the early GPIb-IX-signaling pathway leading to integrin activation (Figure 4).
The role of Akt in stable platelet adhesion to vWF under shear stress. Washed mouse platelets were preincubated with DMSO, RGDS (1 mM), SH-6 (15 and 30 μM), wortmannin (100 nM), the P2Y1 antagonist A3P5P (0.5 mM), the P2Y12 antagonist 2MeSAMP (10 μM), A3P5P and 2MeSAMP, aspirin (1 mM), or the Syk inhibitor piceatannol (10 μM) for 2 minutes, loaded onto the vWF-coated glass slides with mepacrine (10 μM), and then subjected to constant shear rate (800 s−1) for 5 minutes. After washing, adherent platelets were photographed under the fluorescent microscope as described in “Platelet adhesion under shear stress.” Numbers of adherent platelets/field were quantitated. Data (mean ± SD) were obtained from 10 randomly selected fields from each of 3 experiments. ##P < .01 vs WT platelet. **P < .01 vs DMSO-treated platelet. P < .01 for the difference between ADP receptor antagonists and SH-6 or between aspirin and SH-6. P < .05 for difference between ADP receptor antagonists and Akt knockout or between aspirin and Akt knockout.
The role of Akt in stable platelet adhesion to vWF under shear stress. Washed mouse platelets were preincubated with DMSO, RGDS (1 mM), SH-6 (15 and 30 μM), wortmannin (100 nM), the P2Y1 antagonist A3P5P (0.5 mM), the P2Y12 antagonist 2MeSAMP (10 μM), A3P5P and 2MeSAMP, aspirin (1 mM), or the Syk inhibitor piceatannol (10 μM) for 2 minutes, loaded onto the vWF-coated glass slides with mepacrine (10 μM), and then subjected to constant shear rate (800 s−1) for 5 minutes. After washing, adherent platelets were photographed under the fluorescent microscope as described in “Platelet adhesion under shear stress.” Numbers of adherent platelets/field were quantitated. Data (mean ± SD) were obtained from 10 randomly selected fields from each of 3 experiments. ##P < .01 vs WT platelet. **P < .01 vs DMSO-treated platelet. P < .01 for the difference between ADP receptor antagonists and SH-6 or between aspirin and SH-6. P < .05 for difference between ADP receptor antagonists and Akt knockout or between aspirin and Akt knockout.
The effect of Akt knockout or Akt inhibition on vWF binding to GPIb-IX
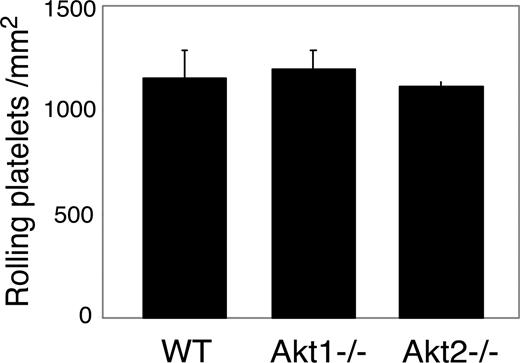
vWF binding function of GPIb-IX can be regulated by intracellular signals.39,43 Thus, it is possible that the role of Akt in GPIb-IX signaling may be the consequence of its up-regulation of the vWF binding function of GPIb-IX. To exclude this possibility, SH-6–treated platelets and Akt null platelets were analyzed for botrocetin-induced vWF binding and for GPIb-IX–mediated transient platelet adhesion to vWF. Botrocetin-induced vWF binding was not affected in SH-6–treated or Akt1−/− platelets compared with wild-type mouse platelets (not shown). Similarly, transient adhesion of platelets to vWF under 800 s−1 shear stress in the presence of integrin inhibitor EDTA was not affected by Akt1 or Akt2 knockout (Figure 5). These data indicate that the inhibitory effects of Akt knockout or Akt inhibition on platelet activation are not the result of their effects on vWF binding function of GPIb-IX. Thus, Akt plays an important role in the vWF binding–induced GPIb-IX signaling.
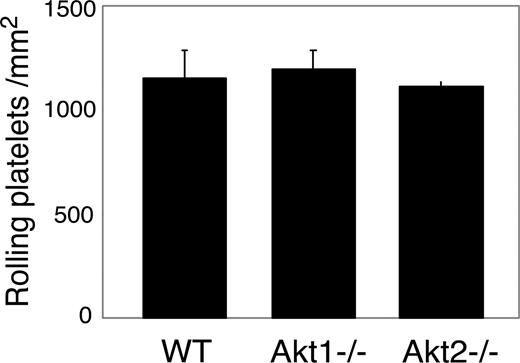
The effect of Akt knockout or Akt inhibition on GPIb-IX-vWF binding–mediated transient platelet adhesion on vWF. Mepacrine-labeled WT or Akt1−/− or Akt2−/− platelets reconstituted in washed red cells in Tyrode solution in the presence of 10 mM EDTA were perfused through a vWF-coated flow chamber at 800 s−1. Transient platelet adhesion to vWF surface were recorded in real time. Number of platelets rolling on vWF (mean ± SD) were counted from 10 randomly selected fields from each of 3 experiments.
The effect of Akt knockout or Akt inhibition on GPIb-IX-vWF binding–mediated transient platelet adhesion on vWF. Mepacrine-labeled WT or Akt1−/− or Akt2−/− platelets reconstituted in washed red cells in Tyrode solution in the presence of 10 mM EDTA were perfused through a vWF-coated flow chamber at 800 s−1. Transient platelet adhesion to vWF surface were recorded in real time. Number of platelets rolling on vWF (mean ± SD) were counted from 10 randomly selected fields from each of 3 experiments.
The cGMP pathway is a downstream mediator of Akt–dependent GPIb-IX signaling
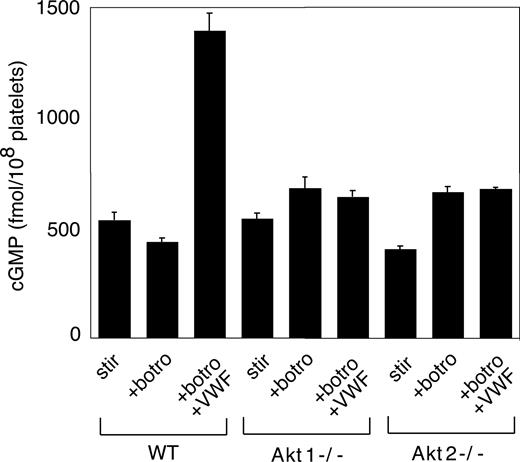
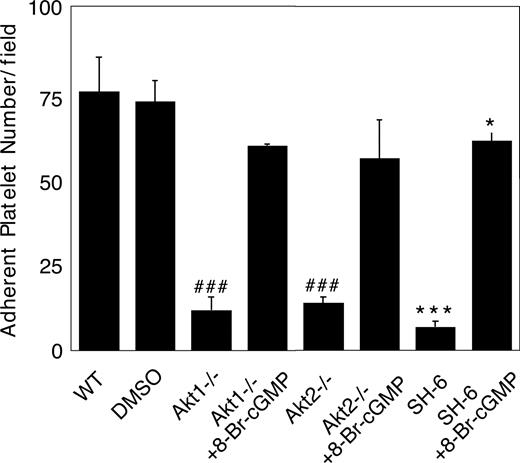
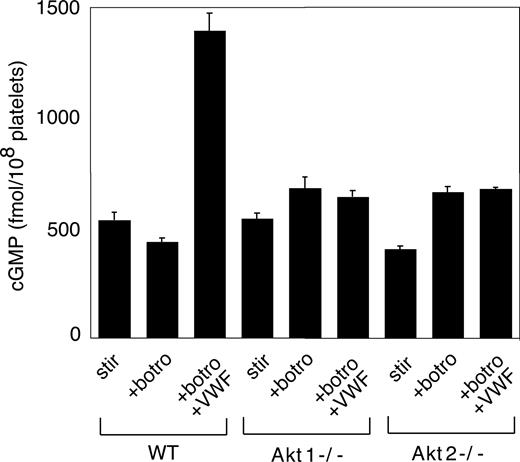
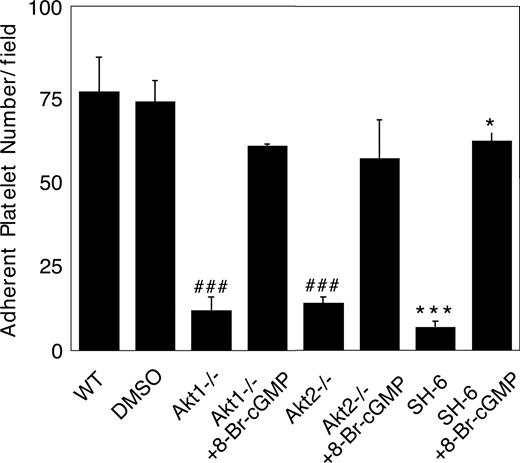
We recently showed that the cGMP–dependent protein kinase-p38-ERK pathway plays a stimulatory role in GPIb-IX–mediated platelet activation.20 vWF binding to GPIb-IX induces increased NO and cGMP levels in platelets.21 Akt has been known to stimulate eNOS and thus NO synthesis.32,44 To investigate whether Akt mediates GPIb-IX–induced signaling by activating the NO-cGMP signaling pathway, we examined whether GPIb-IX–dependent elevation of cGMP levels in response to vWF was affected in platelets deficient in Akt1 or Akt2. As shown in Figure 6, Akt1 knockout or Akt2 knockout abolished vWF-induced cGMP elevation. More important, the inhibitory effect of Akt1 knockout, Akt2 knockout, or SH-6 on stable platelet adhesion to vWF under flow conditions was mostly corrected by adding a low concentration of membrane-permeable cGMP analog, 8-bromo-cGMP (Figure 7). These results indicate that Akt mediates elevation of cGMP induced by vWF binding to GPIb-IX, and the cGMP signaling pathway is a downstream mediator of Akt in GPIb-IX–dependent platelet activation.
The role of Akt in GPIb-IX–dependent elevation of platelet cGMP. Washed WT, Akt1−/−, and Akt2−/− platelets were stimulated with botrocetin alone or botrocetin (1.5 μg/mL) and vWF (10 μg/mL) in the aggregometer for 8 minutes. The cGMP levels (mean ± SD) in platelets were measured as described in “vWF-induced platelet cGMP elevation.” Data were obtained from 3 tests.
The role of Akt in GPIb-IX–dependent elevation of platelet cGMP. Washed WT, Akt1−/−, and Akt2−/− platelets were stimulated with botrocetin alone or botrocetin (1.5 μg/mL) and vWF (10 μg/mL) in the aggregometer for 8 minutes. The cGMP levels (mean ± SD) in platelets were measured as described in “vWF-induced platelet cGMP elevation.” Data were obtained from 3 tests.
Correction of impaired stable platelet adhesion to vWF in Akt–deficient or Akt inhibitor–treated platelets by exogenous cGMP. Washed mouse platelets were preincubated with DMSO or SH-6 (15 μM). WT, Akt1−/−, or Akt2−/− platelets were then treated with or without 10 μM 8-bromo-cGMP and immediately allowed to adhere to vWF under constant shear rate (800 s−1) for 5 minutes. After washing, stably adherent platelets were counted under a fluorescence microscope as described in “Platelet adhesion under shear stress.” Data (mean ± SD) were obtained from 10 randomly selected fields from each of 3 experiments. ###P < .001 versus WT platelets. ***P < .001 versus DMSO–treated WT platelets. *P < .05 versus DMSO-treated WT platelets.
Correction of impaired stable platelet adhesion to vWF in Akt–deficient or Akt inhibitor–treated platelets by exogenous cGMP. Washed mouse platelets were preincubated with DMSO or SH-6 (15 μM). WT, Akt1−/−, or Akt2−/− platelets were then treated with or without 10 μM 8-bromo-cGMP and immediately allowed to adhere to vWF under constant shear rate (800 s−1) for 5 minutes. After washing, stably adherent platelets were counted under a fluorescence microscope as described in “Platelet adhesion under shear stress.” Data (mean ± SD) were obtained from 10 randomly selected fields from each of 3 experiments. ###P < .001 versus WT platelets. ***P < .001 versus DMSO–treated WT platelets. *P < .05 versus DMSO-treated WT platelets.
Discussion
In this study, we have shown that Akt1 and Akt2 both play important roles in GPIb-IX–mediated early signals that lead to integrin activation, and that both Akt isoforms mediate GPIb-IX signaling via the cGMP signaling pathway. We have also provided evidence that PI3K mediates GPIb-IX–induced integrin inside-out signaling via a pathway that is distinct from the role of PI3K in integrin outside-in signaling. The latter does not require the presence of both Akt1 and Akt2.
vWF-induced GPIb-IX signaling involves multiple molecules and pathways, including the Src family of tyrosine kinases (SFK), PI3K, phospholipase C, cytosolic calcium, NO, and cGMP, and also involves activation of ITAM-Syk pathways, synthesis of TXA2, and secretion of granule contents such as ADP.6,11,,,,,–17 The early link between GPIb-IX and these pathways is not totally clear. The cytoplasmic domain of GPIb-IX interacts with several signaling and cytoskeletal proteins, including 14-3-3,45,46 filamin,47 and calmodulin.48 Recently, a complex of PI3K and Src family protein tyrosine kinases (SFK), c-Src and Lyn, has been suggested to interact with the cytoplasmic domain of GPIb-IX.22 Indeed, the SFK inhibitors and knockout of the SFK member, Lyn, abolished GPIb-IX signaling, including the PI3K activation and calcium elevation,16,49 suggesting that SFK is an early link in GPIb-IX signaling. The importance of PI3K in GPIb-IX signaling has also been recognized as PI3K inhibitors impaired platelet spreading and aggregation on a vWF substrate.14 However, the downstream signaling mechanism of PI3K during vWF-induced platelet activation remains unclear. Thus, our data presented in this study provide new evidence that PI3K mediates GPIb-IX signaling via the Akt1 and Akt2 signaling pathways. This observation is consistent with the findings that vWF induces Akt phosphorylation (Figure 1).49 Thus, an early GPIb-IX signaling pathway is revealed that involves GPIb-IX–mediated sequential activation of PI3K and Akt.
GPIb-IX–mediated platelet activation requires not only the early platelet activation pathway, but also several amplification signaling pathways, including the ITAM-Syk signaling pathway,13 TXA2 pathway, and ADP secretion.17,18 PI3K and Akt are known to play roles in these pathways. However, here we show evidence that the PI3K-Akt1/Akt2 pathway plays a role in a GPIb-IX–mediated signaling process that is independent of these amplification pathways. First, we show that GPIb-IX–mediated, integrin-dependent stable adhesion of platelets to immobilized vWF under shear stress is not significantly affected by the Syk inhibitor but is abolished by inhibition of Akt and PI3K, indicating that the ITAM-Syk pathway is not required in this PI3K- and Akt-dependent event. Second, we show that ADP antagonists and TXA2 pathway inhibitors only partially inhibit stable platelet adhesion to vWF under shear stress, which is almost completely abolished by the Akt inhibitor and knockout, indicating that the Akt1/Akt2-mediated integrin-dependent stable platelet adhesion to vWF is at least partially independent of ADP and TXA2 signaling pathways. Thus, the PI3K-Akt1/Akt2 pathway mediates GPIb-IX early signaling, leading to integrin-dependent stable platelet adhesion and aggregation.
It has been shown previously that PI3K is important in the outside-in signaling of integrins,25,50 leading to cell spreading. Thus, PI3K inhibitors inhibit integrin-dependent cell spreading on various integrin ligands. Therefore, although PI3K was recognized to be important in platelet adhesion and spreading on vWF and in vWF-mediated platelet aggregation, it was not clear whether PI3K plays a role in integrin outside-in signaling or in GPIb-IX–mediated inside-out signaling. We show here that the role of PI3K in integrin outside-in signaling as indicated by spreading on fibrinogen is distinct from the role of PI3K in GPIb-IX–mediated platelet adhesion and spreading on vWF because only the latter is dependent on both Akt1 and Akt2. Thus, our study establishes that GPIb-IX–induced PI3K- Akt1/Akt2 signaling pathway mediates integrin inside-out signaling, distinct from the role of PI3K in outside-in signaling.
We previously showed that cGMP-dependent protein kinase plays a stimulatory role in GPIb-IX–mediated platelet activation.51 Indeed, we and others have shown that vWF binding to GPIb-IX induces platelet NO synthesis and elevation of platelet cGMP levels.15,21 We have also shown that the PI3K-Akt-NO-cGMP pathway plays an important role in stimulating platelet granule secretion induced by several platelet agonists.32,51,52 However, it appears that the role of the cGMP pathway in GPIb-IX–mediated integrin activation is unlikely to be the same as the role of this pathway in stimulating platelet secretion, because the cGMP-dependent integrin activation can be shown in the reconstituted integrin activation model in CHO cells that lack the platelet granules and, thus, the platelet granule secretion mechanisms.15,19 In addition, the vWF-induced cGMP levels are much higher than that induced by other platelet agonists,15,32 indicating a different coupling mechanism. However, how vWF induces the activation of the NO-cGMP pathway remains unclear. In this study, we have shown that Akt1 knockout and Akt2 knockout both diminished the vWF induced platelet cGMP elevation, and importantly, that the inhibitory effect of Akt1- or Akt2-knockout or the Akt inhibitor on GPIb-induced, integrin-dependent stable platelet adhesion to vWF can be reversed by supplementing exogenous cGMP. In this respect, Akt1 has been shown to stimulate eNOS and, thus, activate the NO-cGMP pathway in platelets and other cells.32,44 Our data indicate that an important role of Akt in vWF-mediated signaling is to activate the NO-cGMP-PKG pathway, which have been shown to mediate GPIb-IX–dependent integrin activation via the p38 and ERK signaling pathways. Importantly, knockout of either Akt1 or Akt2 caused nearly complete inhibition of cGMP elevation, as well as stable platelet adhesion to vWF, suggesting that Akt1 and Akt2 have distinct roles that cannot be significantly compensated by the presence of the other isoform. Thus, it is possible that Akt1 and Akt2 are involved in activation of the cGMP pathway by different mechanisms.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Heart, Lung, and Blood Institute/National Institutes of Health grants HL062350, HL068819, and HL080264 (X.D.) and the American Heart Association Midwest Affiliate Predoctoral Fellowship Award (H.Y.).
National Institutes of Health
Authorship
Contribution: H.Y. performed a major part of the experiments and wrote the paper; A.S. performed important experiments and was involved in the design of experiments and data analysis; N.H. provided vital reagents and data analysis; and X.D. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xiaoping Du, Department of Pharmacology, University of Illinois College of Medicine, 835 South Wolcott Ave, Chicago, IL 60612; e-mail: xdu@uic.edu.