Abstract
We recently demonstrated in zebrafish the developmental migration of emerging hematopoietic stem cells (HSCs) that is thought to occur in mammalian embryos, from the aorta-gonad-mesonephros (AGM) area to the successive hematopoietic organs. CD41 is the earliest known molecular marker of nascent HSCs in mammalian development. In this study, we show that in CD41-green fluorescent protein (GFP) transgenic zebrafish embryos, the transgene is expressed by emerging HSCs in the AGM, allowing us for the first time to image their behavior and trace them in real time. We find that the zebrafish AGM contains no intra-aortic cell clusters, so far considered a hallmark of HSC emergence. CD41GFPlow HSCs emerge in the subaortic mesenchyme and enter the circulation not through the dorsal aorta but through the axial vein, the peculiar structure of which facilitates their intravasation. The rise in CD41-gfp expression among c-myb+ HSC precursors is asynchronous and marks their competence to leave the AGM and immediately seed the caudal hematopoietic tissue (which has a hematopoietic function analogous to that of the mammalian fetal liver). Imaging the later migration of CD41-GFP+ precursors to the nascent thymus reveals that although some reach the thymus by extravasating from the nearest vein, most travel for hours through the mesenchyme from surprisingly diverse and remote sites of extravasation.
Introduction
The diversity of hematopoietic cell types that is characteristic of vertebrates is generated from hematopoietic stem cells (HSCs). During ontogeny, the site where hematopoiesis occurs changes (eg, from yolk sac to liver to bone marrow in mammals). During development, cells with long-term HSC potential have first been found in an intraembryonic region called the AGM (aorta-gonad-mesonephros), and it has been assumed that these cells enter the blood circulation to first seed the fetal liver, expand and generate fetal hematopoiesis there, then travel again to seed the bone marrow, the definitive hematopoietic organ.1 The embryologic origin of the HSC precursors in the AGM is still much debated. Because they seem to enter the circulation through “intra-aortic clusters” (IACs) of cells anchored in and interrupting the ventral aortic endothelium, it has been proposed that the HSCs derive from the ventral aortic endothelium, hence its designation “hemogenic endothelium.”2 Other authors have pointed out the earlier expression of early hematopoietic markers in the subaortic mesenchyme and proposed rather that HSCs are born in the mesenchyme and then cross the aortic endothelium to form the IACs and enter circulation.3
The zebrafish appears as an interesting new model to clarify these issues, owing to the immediate accessibility and transparency of the embryo and the quickly increasing number of cellular, molecular, and genetic tools available in this species. In the zebrafish embryo, a cell cord of c-myb+, runx1+ cells appears at the onset of blood circulation in the trunk, between the juxtaposed dorsal aorta (DA) and axial posterior cardinal vein (PCV), in a very thin mesenchyme that we called the DA/PCV joint, or DP joint.4 As the definitive HSCs in mammals, these cells are runx1- and Notch-dependent, unlike the primitive wave of hematopoiesis that occurs earlier in the embryo.5,6 We and others4,7 recently established through cell tracing that the DP joint does contain multipotent blood precursors that first enter the circulation to seed a transient site of hematopoiesis, which we called the caudal hematopoietic tissue (CHT). There they expand and differentiate, later emigrating to seed the thymus and the pronephric kidney, the definitive site of hematopoiesis in fish. In this respect, the CHT has a hematopoietic function similar to the fetal liver in mammals, whereas the DP joint appears homologous to the AGM of amniotes, both anatomically and functionally. The close similarities in early HSC dynamics between fish and mammals disclosed by these studies encouraged us to search for ways to visualize directly, in live zebrafish embryos, the generation and dynamics of HSC precursors in the AGM.
A candidate transgenic zebrafish line to achieve this was the CD41-gfp line.8 In mammals, CD41, originally known as a marker of platelets, was recently found to be the earliest known surface marker of nascent HSCs that distinguishes them from the endothelial lineage during embryogenesis.3,9
Here we show that the definitive blood precursors in the zebrafish AGM express the CD41-gfp transgene at a low but sufficient level to image their behavior. A surprising conclusion is that the onset of CD41-gfp expression among these cells is asynchronous and quickly followed by their entry into the circulation, not through the DA but through the axial vein, the peculiar structure of which, similar to that of a lymphatic vessel, facilitates their intravasation.
The CD41-gfp line also allowed us to image the journey of the first immigrants to the thymus, revealing that most occurs through the mesenchyme, from surprisingly diverse and remote locations relative to the thymus.
Methods
Zebrafish stocks and embryo treatments
Zebrafish CD41-gfp8 transgenic adults and embryos were raised and staged according to Westerfield.10 Anti-runx15 and anti-PU.111 morpholinos were injected in 1- to 4-cell embryos at doses of 1 and 5 ng, respectively. To inhibit Notch signaling, N,[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester (DAPT), (100 μmol/L; Calbiochem, Fontenay-sous-Bois, France) was applied as described previously.5
Light and electron microscopy
Differential interference contrast (DIC) video and wide-field fluorescence microscopy were performed as described previously,4,12 through the 60×/1.00 numeric aperture, water-immersion objective of a Nikon 90i microscope (Nikon-France, Champigny-sur-Marne, France). For electron microscopy, embryos at 26, 36, and 48 hours postfertilization (hpf) were fixed and processed as described previously.13
Time-lapse confocal fluorescence imaging of live zebrafish embryos and larvae
CD41-gfp dechorionated embryos or hatched larvae were incubated for 1 hour with 50 μmol/L Bodipy TR (Invitrogen, Carlsbad, CA),14 rinsed twice in embryo medium, anesthetized with Tricaine methanesulfonate,10 and immobilized in 1% LMP agarose (35-48 hpf) or 3% methylcellulose (3-5 days postfertilization [dpf]) on 35-mm glass-bottomed dishes (Iwaki, Chiba, Japan), covered with Tricaine methanesulfonate containing embryo medium, and imaged on various confocal inverted microscopes as described here. Temperature was maintained at 28°C by placing the dish in a PeCon open chamber (PeCon, Erbach, Germany).
Conventional time-lapse confocal microscopy was performed either on a Zeiss LSM510 (Carl Zeiss, Le Pecq, France) (Figure 1F,J,K; Video S4,S5,S7,S8, available on the Blood website; see the Supplemental Materials link at the top of the online article) or on a Leica SP5 (Leica-France, Rueil-Malmaison, France) (Figure 1B,C and Video S1).
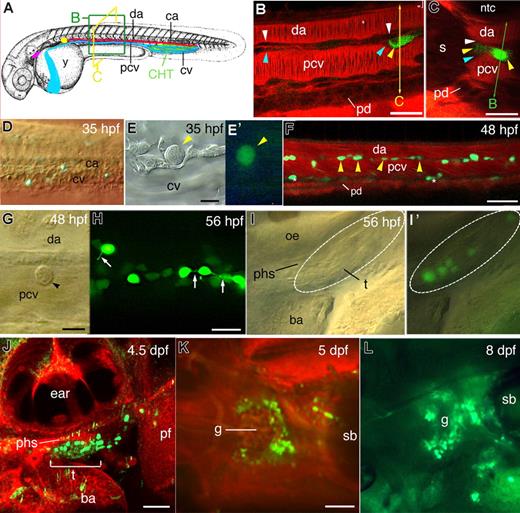
CD41-gfp transgene hematopoietic expression in live zebrafish embryos, from AGM to kidney.(A) Anatomic landmarks relevant to this study in the zebrafish embryo; although represented at 35 hpf,26 it also indicates the future location of the thymus (purple) and pronephric kidney, the site of definitive hematopoiesis (yellow); the optical sectioning planes used in (B) and (C) are represented. (B-L) Live CD41-gfp embryos and larvae; (B,C,F,J,K) Confocal fluorescence images, with Bodipy TR (red) as vital histologic stain; all lateral views, rostral to the left, except (C,K,L). (B,C) Mid-trunk zone at 38 hpf; successive optical longitudinal (B) and cross (C) sections of the same embryo were obtained by laser scanning in the xy and xz mode, respectively. Yellow arrowheads, GFPlow cells in the DP joint, the zebrafish AGM; white arrowheads, DA endothelium; blue arrowheads, PCV endothelium. (D) DIC and fluorescence overlay showing the first GFPlow cells in the CHT area at 35 hpf. (E,E′) DIC (E) and fluorescence (E′) image of a CD41-GFPlow HSC-like cell in the CHT at 35 hpf. (F) GFPlow cells (arrowheads) in the trunk DP joint between DA and PCV at 48 hpf; asterisks, nonhematopoietic, pronephric duct associated GFPlow cells. (G) DIC image of a CD41-GFPlow circulating cell rolling on the PCV dorsal wall at 48 hpf. (H) Fluorescence image of GFPlow (arrowheads) and GFPhigh cells in the CHT at 56 hpf; arrows point at the tethers linking GFPhigh prothrombocytes to each other or to the microenvironment. (I,I′) DIC (I) and DIC+ fluorescence overlay (I′) images of the first CD41-GFPlow immigrants in the left thymus anlage at 56 hpf. (J) Left thymus at 4.5 dpf. (K,L) Pronephros area in dorsal view at 5 dpf (K) and dorso-lateral view at 8 dpf (L). da indicates dorsal aorta; pcv, posterior cardinal vein (axial vein); ca, caudal artery; cv, caudal vein; y, yolk sac; CHT, caudal hematopoietic tissue; s, somite muscle; ntc, notochord; pd, pronephric duct; phs, primary head sinus; ba, branchial arches; oe, otic epithelium; t, thymus; pf, pectoral fin; g, pronephric glomerulus; and sb, swimbladder. Bars, 20 μm in (B,C,H); 10 μm in (E,G); 50 μm in (F,J,K).
CD41-gfp transgene hematopoietic expression in live zebrafish embryos, from AGM to kidney.(A) Anatomic landmarks relevant to this study in the zebrafish embryo; although represented at 35 hpf,26 it also indicates the future location of the thymus (purple) and pronephric kidney, the site of definitive hematopoiesis (yellow); the optical sectioning planes used in (B) and (C) are represented. (B-L) Live CD41-gfp embryos and larvae; (B,C,F,J,K) Confocal fluorescence images, with Bodipy TR (red) as vital histologic stain; all lateral views, rostral to the left, except (C,K,L). (B,C) Mid-trunk zone at 38 hpf; successive optical longitudinal (B) and cross (C) sections of the same embryo were obtained by laser scanning in the xy and xz mode, respectively. Yellow arrowheads, GFPlow cells in the DP joint, the zebrafish AGM; white arrowheads, DA endothelium; blue arrowheads, PCV endothelium. (D) DIC and fluorescence overlay showing the first GFPlow cells in the CHT area at 35 hpf. (E,E′) DIC (E) and fluorescence (E′) image of a CD41-GFPlow HSC-like cell in the CHT at 35 hpf. (F) GFPlow cells (arrowheads) in the trunk DP joint between DA and PCV at 48 hpf; asterisks, nonhematopoietic, pronephric duct associated GFPlow cells. (G) DIC image of a CD41-GFPlow circulating cell rolling on the PCV dorsal wall at 48 hpf. (H) Fluorescence image of GFPlow (arrowheads) and GFPhigh cells in the CHT at 56 hpf; arrows point at the tethers linking GFPhigh prothrombocytes to each other or to the microenvironment. (I,I′) DIC (I) and DIC+ fluorescence overlay (I′) images of the first CD41-GFPlow immigrants in the left thymus anlage at 56 hpf. (J) Left thymus at 4.5 dpf. (K,L) Pronephros area in dorsal view at 5 dpf (K) and dorso-lateral view at 8 dpf (L). da indicates dorsal aorta; pcv, posterior cardinal vein (axial vein); ca, caudal artery; cv, caudal vein; y, yolk sac; CHT, caudal hematopoietic tissue; s, somite muscle; ntc, notochord; pd, pronephric duct; phs, primary head sinus; ba, branchial arches; oe, otic epithelium; t, thymus; pf, pectoral fin; g, pronephric glomerulus; and sb, swimbladder. Bars, 20 μm in (B,C,H); 10 μm in (E,G); 50 μm in (F,J,K).
High-speed spinning disk confocal fluorescence imaging was performed either on a PerkinElmer system15 (PerkinElmer Life and Analytical Sciences, Waltham, MA) (Video S3) or an Andor Revolution XD system16 (Andor Technology, South Windsor, CT) (Videos S6,S9,S10).
Image stacks were processed using the ImageJ software (National Institutes of Health, Bethesda, MD). Cell tracking and extraction of cell migration velocities were performed with the MetaMorph software (Molecular Device, Sunnyvale, CA).
In vivo cell tracing
Cell tracing through laser-mediated uncaging of caged fluorescein dextran was done as described previously.4 The tracing of CD41-GFP cells by uncaging of caged rhodamine-dextran 10.000 (Invitrogen) was done in the same way, except that that a 5-fold more diluted concentration of the tracer (10 mg/mL, instead of 50 mg/mL for caged fluorescein dextran) was injected in the 1- to 4-cell stage embryos, for higher amounts proved to be toxic.
To prevent any unwanted uncaging of the tracer as a result of the light required for extended in vivo follow-up of the traced cells, an additional 455-nm long-pass filter (GG455; Schott, Mainz, Germany) was inserted in both the epifluorescence and transmitted light paths.
Results
CD41-gfp transgene expression recapitulates the journey of definitive blood precursors from AGM to the successive hematopoietic organs
The CD41-gfp transgenic zebrafish line was generated by Lin et al8 to visualize the development of thrombocytes in zebrafish. They indeed found brightly fluorescent cells that start to accumulate by 2 dpf in the ventral tail between the caudal artery and caudal vein (CV; ie, in the CHT that we characterized recently4 ) and from 3 dpf onward, increasing numbers of circulating GFPhigh cells—presumably thrombocytes. They also noted the presence in the ventral tail of more weakly fluorescent cells that might represent early thrombocyte progenitors or even earlier hematopoietic progenitors.
We therefore focused on these GFPlow cells and searched for their site and stage of emergence in the embryo. Through confocal fluorescence imaging of live embryos, we first detected GFPlow cells by 33 to 35 hpf as sparse single cells along the trunk, inserted in the DP joint, the very site where we and others had demonstrated the presence of multipotent hematopoietic precursors4,7 (Figure 1A-C). Among various embryos, these cells appeared at any rostrocaudal level of the DP joint, except for the first 2 to 3 positive cells, which more often appeared at its rostral end.
We could obtain virtually simultaneous transverse and longitudinal optical sections of the same live embryo through confocal scanning sequentially in the xy and xz modes, using Bodipy TR as a red fluorescent vital histologic stain14 (Figure 1B,C; Video S1). This revealed that the GFPlow cells were arranged not just between the DA and PCV but also slightly more laterally along the dorsal part of the PCV.
At the same stage, a few cells of similarly low or slightly higher fluorescence (Table S1) were observed in the circulation and in the CHT (Figure 1D); these cells displayed a typical early hematopoietic progenitor morphology, with very scant cytoplasm (Figure 1E,E′), and were frequently found undergoing mitosis (Supplemental Figure S1B).
In addition, a bilateral lining of GFPlow cells was present in the trunk along the dorsal side of the pronephric ducts both at this and later stages (Figure 1F, asterisks), but these cells did not correlate with the expression pattern of hematopoietic markers (eg, scl, c-myb, ikaros, runx1), and they remained associated with the pronephric ducts throughout our time-lapse fluorescence movies (see below).
By 48 hpf, the number of CD41-GFPlow cells all along the DP joint had increased (Figure 1F). Circulating HSC-like GFPlow cells had become frequent in the circulation (Figure S2) and displayed rolling behavior (Figure 1G; Video S2), with frequent stalling, only on the walls of veins, as opposed to arterial vessels, similar to the circulating myeloid cells (macrophages and granulocytes) of the embryo (data not shown). One such circulating GFPlow cell was observed undergoing mitosis in the duct of Cuvier, with a stalled macrophage pulling one of the 2 daughter cells (Figure S2).
By 48 to 56 hpf, The CHT contained GFPlow and GFPhigh cells (Figure 1H; Table S1). The latter could be recognized as prothrombocytes, with characteristic spindle shape and scant cytoplasm. They displayed a tether linking them to one another or to the microenvironment (Figure 1H arrows). These prothrombocytes often entered the circulation as sister cells linked by a tether that could transiently extend to many cell diameters (Video S3). Once in the circulation, their behavior was very different from that of the GFPlow HSC-like cells; they did not roll in the vessels (Video S2), but stopped occasionally, hanging to the vessel wall through a tether emanating from their tapered end, as they did before in the CHT.
Strikingly, GFPlow HSC-like cells were already found to colonize the nascent thymus by this stage, 54 to 56 hpf (Figure 1I,I′) (ie, 16-18 hours before the first reported detection of lymphoid cells in the thymus).17 Some were near the nascent thymus, often on its caudal side, just where our previous cell tracing data suggested a migration path for early immigrants to the thymus by 5 dpf.4
The number of GFPlow HSC-like immigrants in the thymus increased steadily over the next hours and days (Figure 1J), whereas their fluorescence per cell decreased gradually past 3 dpf (Table S1), suggesting a decrease of CD41-gfp transgene expression.
By 4.5 dpf, GFPlow hematopoietic precursors were detected in the kidney around the lateral and caudal sides of the pronephric glomerulus, in an arrangement identical to that of the c-myb+ hematopoietic precursors previously detected there at that stage by in situ hybridization,4,7 and their number increased over the next days (Figure 1K,L).
The CD41-GFPlow cells in the DP joint/AGM are definitive blood precursors
To trace directly the fate of the GFPlow cells found in the zebrafish AGM, we injected a conditional (ultraviolet photoactivatable) red fluorescent cell tracer, caged rhodamine-dextran 10.000, in 1-cell stage embryos. Then by 48 hpf, we laser-uncaged the rhodamine in specific GFPlow cells of the DP joint, thus conferring them an additional red fluorescence. Then we followed up the fate of these initially red + green fluorescent cells in the developing embryos. Figure 2A shows the uncaging of the rhodamine tracer in 2 GFPlow cells in the DP joint. 17 hours later, many red cells were found in the CHT (Figure 2B). In Figure 2C, the tracer was uncaged in 4 GFPlow cells of the posterior DP joint. Twelve hours later, 1 red cell was still detected there, another in the underlying PCV lumen, 1 rolling in the circulation, several in the caudal hematopoietic tissue, including likely sister cells (Figure 2D,E). After 1 more day (80 hpf), 2 labeled cells were detected in the thymus (Figure 2F). Finally, Figure S3 shows the uncaging of a small group of GFPlow cells at the trunk-tail junction. Seventeen hours later, several red-labeled cells were detected further caudally in the CHT, and 3 others in the thymus (Figure S3B,C). By 5.5 dpf, many red labeled cells were found in the thymus (Figure S3D), still numerous cells in the CHT, and several rolling in the circulation.
CD41-GFPlow cells from the AGM seed the CHT and thymus. Fate of CD41-GFPlow AGM cells labeled at 48 hpf by laser-uncaging of caged rhodamine-dextran. All images are left lateral views, rostral to the left. (A) Rhodamine uncaging in 2 GFPlow AGM cells, led 17 hours later to (B) numerous rhodamine labeled cells in the caudal-most part of the CHT. (C-F) Rhodamine uncaging in 4 GFPlow cells in the AGM (C) led 12 hours later in the CHT to (D) a pair of double labeled cells (arrows and asterisks) linked by a tether (arrowheads), as sister cells usually are,4 and (E) several labeled cells including another a pair of sisters (arrow and asterisks); (F) By 80 hpf, 2 labeled cells were found in the thymus (arrows). Isv indicates intersomatic vessel. Scale bars, 20 μm.
CD41-GFPlow cells from the AGM seed the CHT and thymus. Fate of CD41-GFPlow AGM cells labeled at 48 hpf by laser-uncaging of caged rhodamine-dextran. All images are left lateral views, rostral to the left. (A) Rhodamine uncaging in 2 GFPlow AGM cells, led 17 hours later to (B) numerous rhodamine labeled cells in the caudal-most part of the CHT. (C-F) Rhodamine uncaging in 4 GFPlow cells in the AGM (C) led 12 hours later in the CHT to (D) a pair of double labeled cells (arrows and asterisks) linked by a tether (arrowheads), as sister cells usually are,4 and (E) several labeled cells including another a pair of sisters (arrow and asterisks); (F) By 80 hpf, 2 labeled cells were found in the thymus (arrows). Isv indicates intersomatic vessel. Scale bars, 20 μm.
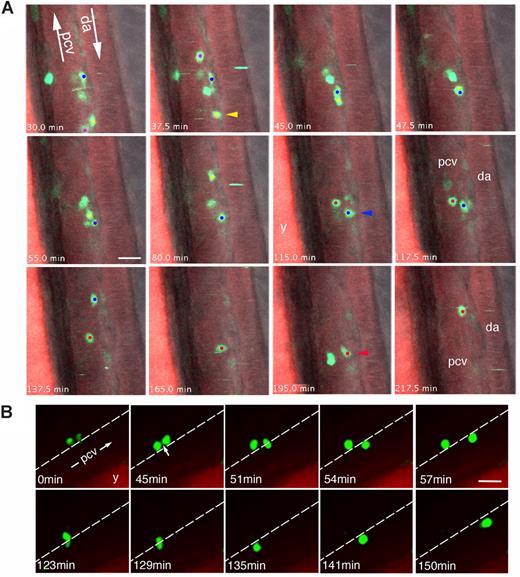
Behavior of CD41-GFPlow definitive blood precursors in the zebrafish AGM. Time-lapse confocal fluorescence imaging of live CD41-gfp embryos pre-incubated with Bodipy TR (lateral views of the trunk). (A) 54 hpf, combined fluorescence from 2 focal planes (1 and 2) 6 μm apart, overlaid with transmitted light image (see Videos S4,S5). Rostral is to the top. Three cells (red, blue, and yellow dots) initially all in focal plane 1—in the abluminal mesenchyme dorsal (yellow and blue cells) or lateral (red cell) to the PCV—move to the deeper plane 2 one after another (arrowheads) to enter the PCV lumen and circulation. These successive moves in depth can be directly seen in Video S4. The yellow cell moves to plane 2 at t = 37.5 minutes.; it and the blue cell (still in plane 1) move toward eachother (still in the DP joint) until the yellow one passes below the blue (t = 45 to 47.7 minutes), then moves straighter within the PCV lumen (between 65 and 80 minutes), after which it is taken by the blood flow. The blue cell moves to plane 2 at 115 to 117.5 minutes, then is in the PCV lumen, in which it is last seen at t = 137.5 minutes. Finally the red cell moves to plane 2 at 195 minutes, after which it is in the PCV lumen, where it is last visible at 217.5 minutes. (B) 48 hpf; 2 (likely sister) GFP+ cells from the DP joint, initially linked by a tether (arrow), enter one after the other into the PCV lumen and circulation, in a PU.1 Mo injected embryo (Video S6). Scale bars, 20 μm. y, yolk tube.
Behavior of CD41-GFPlow definitive blood precursors in the zebrafish AGM. Time-lapse confocal fluorescence imaging of live CD41-gfp embryos pre-incubated with Bodipy TR (lateral views of the trunk). (A) 54 hpf, combined fluorescence from 2 focal planes (1 and 2) 6 μm apart, overlaid with transmitted light image (see Videos S4,S5). Rostral is to the top. Three cells (red, blue, and yellow dots) initially all in focal plane 1—in the abluminal mesenchyme dorsal (yellow and blue cells) or lateral (red cell) to the PCV—move to the deeper plane 2 one after another (arrowheads) to enter the PCV lumen and circulation. These successive moves in depth can be directly seen in Video S4. The yellow cell moves to plane 2 at t = 37.5 minutes.; it and the blue cell (still in plane 1) move toward eachother (still in the DP joint) until the yellow one passes below the blue (t = 45 to 47.7 minutes), then moves straighter within the PCV lumen (between 65 and 80 minutes), after which it is taken by the blood flow. The blue cell moves to plane 2 at 115 to 117.5 minutes, then is in the PCV lumen, in which it is last seen at t = 137.5 minutes. Finally the red cell moves to plane 2 at 195 minutes, after which it is in the PCV lumen, where it is last visible at 217.5 minutes. (B) 48 hpf; 2 (likely sister) GFP+ cells from the DP joint, initially linked by a tether (arrow), enter one after the other into the PCV lumen and circulation, in a PU.1 Mo injected embryo (Video S6). Scale bars, 20 μm. y, yolk tube.
Such cell tracing demonstrated the traveling of CD41-GFPlow cells from the zebrafish AGM to the CHT then to the thymus. We were not surprised to detect less numerous labeled progenies for these cells than we had previously found for single cells randomly photolabeled in the DP joint through the uncaging of caged fluorescein-dextran.4 The latter tracer was indeed detected by enzyme-coupled immunohistochemistry, a much more sensitive method (unavailable for caged rhodamine dextran) that made it possible to detect labeled progeny even after dilution of the tracer through several cell divisions.
Unlike primitive hematopoiesis, the generation of definitive HSCs in mammalian embryos is runx1- and Notch-dependent.9 So is the appearance of c-myb+ precursors in the zebrafish AGM and of subsequent rag1+ cells in the thymus.5 Likewise, morpholino-mediated knock-down of runx1 expression in CD41-gfp embryos suppressed the appearance of CD41-GFP+ cells in the AGM, then in the CHT and thymus (Figure S4A-L; note that the nonhematopoietic GFP+ cells adherent to the pronephric ducts were not affected), as did early treatment of embryos with the γ-secretase inhibitor DAPT (Figure S4M-P), which prevents Notch signaling.5 In contrast, injection of an anti-PU.1 morpholino dose that suppressed myeloid differentiation in the embryo up to 2 dpf11 (D. LeGuyader and P.H., unpublished data, September 2006) had no effect on the appearance and dynamics of GFP+ cells in the AGM, CHT, and thymus (data not shown, but see Figure 3B and Video S6).
Because CD41-GFPlow precursors appear in the DP joint by 33–35 hpf, we expected that they may arise within the c-myb+ cell cord present there already by 26 hpf. A simultaneous detection of the CD41-gfp and c-myb mRNAs by 2-color fluorescent in situ hybridization at 36 hpf confirmed that all gfp mRNA-positive cells detected there were also c-myb+ (Figure S5).
Dynamics of multipotent hematopoietic precursors in the AGM
Because this was the first opportunity to observe the behavior of definitive blood precursors in the AGM of a live, intact vertebrate embryo, we investigated the dynamics of the CD41-GFPlow cells of the AGM by fluorescence confocal time-lapse microscopy.
The same cell behaviors were observed in various time-lapse sequences spanning from 36 to 56 hpf. The CD41-GFPlow cells of the DP joint could change shapes rapidly, from round to elongated and vice versa (Video S4); occasionally one underwent mitosis during the sequence (Figure S1A). Most often they entered the circulation within 2 to 3 hours of observation (28/43 cells followed, from 10 different embryos), and they always did so through the axial PCV, never through the dorsal aorta (Figure 3; Videos S4Video 5 (=movie 4-stereo). Intravasation of CD41-GFPlow cells from the AGM into the PCV at 54 hpf (MOV, 3,762 KB)–S6). Their fluorescence often increased during these events (Video S6). They often entered into the vein lumen as pairs of likely sister cells, one after another (Figure 3B arrow; Video S6). Intriguingly, in most cases, their intravasation into the PCV seemed to involve little cell deformation; the cells did not seem to have to traverse an endothelial wall. In Video S4, 3 cells at initially distinct locations in the abluminal mesenchyme successively enter the vein at the same site (this is best seen through stereoscopic viewing, in Video S5), suggesting a discrete entry point through the endothelial wall.
An electron microscopic analysis at 26, 36, and 48 hpf revealed similar features at all 3 stages and at any rostrocaudal level of the DP joint. A narrow band of mesenchymal tissue extended between the DA and PCV, containing pale, irregular mesenchymal cells, some laden with lipid droplets, melanophores (Figure 4A,D), and sparse hematopoietic progenitors displaying primitive features: electron dense, with scant cytoplasm devoid of membranous organelles but filled with free ribosomes (Figure 4A-C). Inspection of the vessel walls revealed that unlike the DA endothelium, the PCV endothelium consists in cells that overlap each other (Figure 4E). Although some cell junctions can be observed in these overlapping areas (Figure 4F,G arrows), they appear less strong than those joining the cells of the dorsal aorta (Figure 4H arrows), which moreover show interdigitations (Figure 4I). Stromal reticular cells often line the PCV wall (Figure 4J), and appear at some places to bridge a small gap between adjacent endothelial cells (Figure 4K,L), thus creating a potentially even more favorable site for hematopoietic cell exit into the PCV lumen. These 2 configurations of the PCV dorsal wall probably facilitate the intravasation of hematopoietic precursors into the PCV.
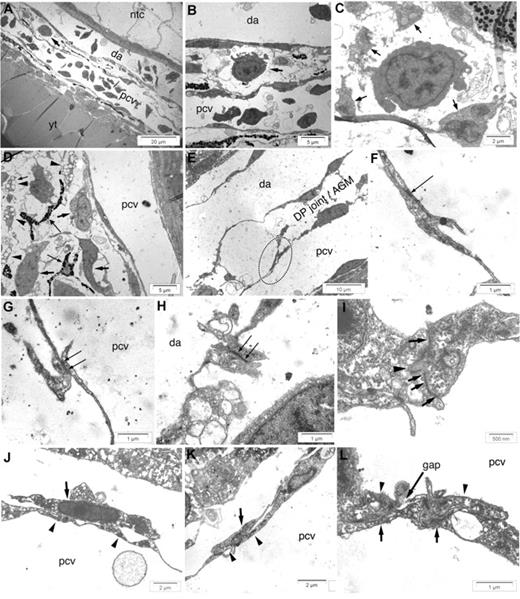
Ultrastructure of the zebrafish AGM. Electron microscopy of longitudinal sections of the DP joint at 48 hpf (A-D), 26 hpf (E-H,K,L), and 36 hpf (I,J). (A,B) An hematopoietic early precursor (arrows) visible in the DP joint (A) is shown at higher magnifications in (B). (C) A similar precursor, at higher magnification, surrounded by processes of mesenchymal cells (arrows). (D) Structure of the DP joint mesenchyme, with irregular mesenchymal cells (arrows), lipid-laden cells (arrowheads), and melanin-laden pigment cells (thin arrows). (E) Typical overlap (dotted ellipse) between adjacent endothelial cells of the PCV dorsal wall, in contrast with the continuous appearance of the DA endothelial wall. (F,G) Higher magnification of the thin overlapping processes of adjacent PCV endothelial cells, showing cell junctions between them (arrows), though shorter and less dense than those between endothelial cells of the DA (H, arrows). (I) Adjacent DA endothelial cells also show interdigitations penetrating each other (arrowhead), in addition to cell junctions (arrows). (J) Intimate apposition between a stromal reticular cell (arrow) and PCV endothelium (arrowheads). (K) A stromal reticular cell (arrow) bridging a short gap between 2 adjacent PCV endothelial cells (arrowheads). (L) Two endothelial cells (arrowheads) keeping a gap, underlaid by 2 stromal reticular cells (arrows), one of which covers the gap. Ntc indicates notochord; and yt, yolk tube.
Ultrastructure of the zebrafish AGM. Electron microscopy of longitudinal sections of the DP joint at 48 hpf (A-D), 26 hpf (E-H,K,L), and 36 hpf (I,J). (A,B) An hematopoietic early precursor (arrows) visible in the DP joint (A) is shown at higher magnifications in (B). (C) A similar precursor, at higher magnification, surrounded by processes of mesenchymal cells (arrows). (D) Structure of the DP joint mesenchyme, with irregular mesenchymal cells (arrows), lipid-laden cells (arrowheads), and melanin-laden pigment cells (thin arrows). (E) Typical overlap (dotted ellipse) between adjacent endothelial cells of the PCV dorsal wall, in contrast with the continuous appearance of the DA endothelial wall. (F,G) Higher magnification of the thin overlapping processes of adjacent PCV endothelial cells, showing cell junctions between them (arrows), though shorter and less dense than those between endothelial cells of the DA (H, arrows). (I) Adjacent DA endothelial cells also show interdigitations penetrating each other (arrowhead), in addition to cell junctions (arrows). (J) Intimate apposition between a stromal reticular cell (arrow) and PCV endothelium (arrowheads). (K) A stromal reticular cell (arrow) bridging a short gap between 2 adjacent PCV endothelial cells (arrowheads). (L) Two endothelial cells (arrowheads) keeping a gap, underlaid by 2 stromal reticular cells (arrows), one of which covers the gap. Ntc indicates notochord; and yt, yolk tube.
Unlike what has been amply documented in mammals and birds, our video-enhanced DIC microscopic scrutiny of the whole region never revealed any “intra-aortic cluster” of cells (regardless of GFP expression) or even single cells anchored intraluminally to the ventral wall of the dorsal aorta, at any stage of development.
Dynamics of cell release from the AGM to the CHT: CD41-gfp expression marks competency to leave the AGM
Cell tracing data have indicated that definitive blood precursors are present all along the trunk DP joint already at the onset of blood circulation.7 Because CD41-GFPlow cells were detected there only from 30 to 35 hpf onward, it was conceivable that some of these blood precursors may already leave the AGM before or regardless of their expression of the CD41-gfp transgene. To investigate this point, we uncaged fluorescein-dextran all along the DP joint at 26 hpf (Figure 5A,B) and scored in real time for the first seeding of the blood and CHT by the labeled cells. This happened only 6 to 8 hours later (32-34 hpf); 50% of the embryos had 1 to 5 labeled cells in the tail (Figure 5C-E) and the same embryos also had 2 to 5 labeled cells in the circulation. Fourteen hours later they were many more, with more diverse morphologies (Figure S6A-D). In contrast, when we uncaged the AGM cells at 37 to 38 hpf, 2 hours later all embryos already had 1 to 10 cells in the CHT (Figure S6E-G) and several in the circulation. Finally, when AGM cells were uncaged at 50 hpf, 2 hours later the CHT contained 8 to 12 labeled cells (data not shown). Whether uncaging was performed at 26, 37, or 50 hpf, the first labeled cells detected in the CHT all had early precursor morphology and were stalled in the CV lumen, whereas 8 to 12 hours later, the labeled cells were in both the CV lumen and abluminal mesenchyme (Figure S6B-D), and none was detected in the thymus yet. These data indicate that although already present all along the DP joint at 26 hpf, the definitive blood precursors first enter the circulation by 32 to 34 hpf (depending on the embryo), and by this route immediately seed the CHT; they are released from the AGM at rates of approximately 2.5 AGM cells/hour by 35 hpf and approximately 5 cells/hour by 48 hpf. Thus the overall dynamics and rate of cell release from the AGM to the CHT correlated precisely with the dynamics of CD41-GFP expression in the AGM and the observed intravasation of CD41-GFPlow cells from the AGM into the PCV within 2 to 3 hours of imaging.
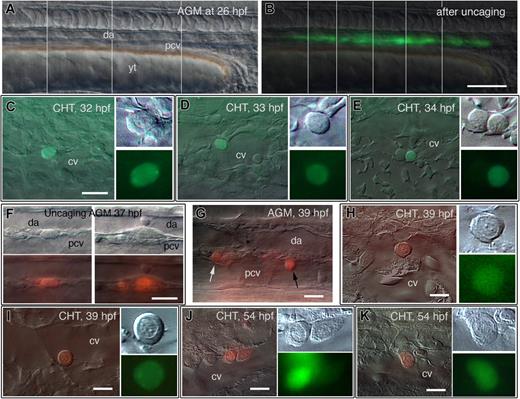
Dynamics of cell release from AGM to CHT: CD41-gfp expression marks competency to leave the AGM. (A-E) Live follow-up of AGM cells labeled by laser-mediated uncaging of caged fluorescein at 26 hpf; left lateral views, rostral to the left. (A,B) DIC (A) and fluorescence (B) image of the trunk just after uncaging fluorescein all along the DP joint; vertical lines separate the parts captured at a slightly different focus. (C-E) Depending on the embryo, the first photolabeled cell is detected in the CHT at 32 hpf (C), 33 hpf (D), or 34 hpf (E), all with hematopoietic progenitor morphology, and in the CV lumen. (F-K) Follow-up of AGM cells labeled by laser-mediated uncaging of caged rhodamine in CD41-gfp transgenic embryos. (F) Uncaging of cells along the DA-PCV joint at 37 hpf. (G) Two hours later, some of these labeled cells, still in the trunk, have rounded up (white arrow) and/or entered the PCV (black arrow). (H,I) At the same time, the very first red labeled cells are detected in the CHT; these cells are all GFP+, and with similar early progenitor morphology, including sharply defined dot-like nucleoli. (J,K) Seventeen hours post uncaging, most red labeled cells in the CHT are still GFP+, with more diverse morphologies. Scale bars, 100 μm (B), 20 μm (C-E,F), 10 μm (G-K).
Dynamics of cell release from AGM to CHT: CD41-gfp expression marks competency to leave the AGM. (A-E) Live follow-up of AGM cells labeled by laser-mediated uncaging of caged fluorescein at 26 hpf; left lateral views, rostral to the left. (A,B) DIC (A) and fluorescence (B) image of the trunk just after uncaging fluorescein all along the DP joint; vertical lines separate the parts captured at a slightly different focus. (C-E) Depending on the embryo, the first photolabeled cell is detected in the CHT at 32 hpf (C), 33 hpf (D), or 34 hpf (E), all with hematopoietic progenitor morphology, and in the CV lumen. (F-K) Follow-up of AGM cells labeled by laser-mediated uncaging of caged rhodamine in CD41-gfp transgenic embryos. (F) Uncaging of cells along the DA-PCV joint at 37 hpf. (G) Two hours later, some of these labeled cells, still in the trunk, have rounded up (white arrow) and/or entered the PCV (black arrow). (H,I) At the same time, the very first red labeled cells are detected in the CHT; these cells are all GFP+, and with similar early progenitor morphology, including sharply defined dot-like nucleoli. (J,K) Seventeen hours post uncaging, most red labeled cells in the CHT are still GFP+, with more diverse morphologies. Scale bars, 100 μm (B), 20 μm (C-E,F), 10 μm (G-K).
Therefore, we performed the same experiment but now in CD41-gfp embryos, using caged rhodamine-dextran as the tracer. After uncaging of the red tracer, either at 37 or 52 hpf, all along the DP joint, the first rhodamine-labeled cells appeared in the CHT again within the next 2 hours (Figure 5F-I; Figure S6H,I). All of them were also GFPlow and displayed a remarkably homogeneous early progenitor morphology, which diversified in the ensuing 17 hours (Figure 5J,K). These data demonstrate that all cells that are released from the AGM to the CHT are CD41-GFPlow early precursors.
Routes of early immigrants to the thymus
Because the CD41-gfp transgene is still expressed in the progeny of AGM cells as they seed the nascent thymus to become thymocytes, this enabled us to visualize directly their migration routes to the nascent thymus, again through time-lapse confocal microscopy. Figure 6A-G and Video S7 (preferably its stereoscopic version, Video S8) give an overall view of this process over 13 hours, starting at 54 hpf (18 hours before the first reported expression of rag1 in the thymus). The main feature was that CD41-GFPlow cells migrated a long way through the mesenchyme to reach the thymus, and they did so from widely diverse locations, both rostral and caudal to the thymus, indicating that most of them first probably traveled through the blood but then extravasated at quite diverse locations relative to their final target, the thymus. In Videos S7 and S8 and Figure 6A-G, 2 cells can be followed in 7- and 9-hr long journeys, respectively, through the mesenchyme to the thymus (Figure 6A blue/purple and white tracks). Cell 1 (blue track and arrowheads) is already visible at the beginning of the sequence (54 hpf, Figure 6B) adjacent to the optic vein; it first moves deeper, along the medial side of the ear, then to the more superficial mesenchyme rostroventral to the thymus (Video S8; Figure 6C,D); there it pauses for 1 hour, during which it undergoes mitosis (Figure 6E purple arrowheads), and the 2 daughter cells move first separately then together to the thymus (Figure 6F,G purple arrowheads). Cell 2 appears in the sequence at 56.7 hpf (Figure 6C white arrowhead), 350 μm caudal to the thymus, on the caudal border of the left common cardinal vein (CCV); it skirts round the CCV to a more superficial location (Video S8; Figure 6D,E), where it moves to the thymus in a straighter route that passes between the thymus-proximal muscle and epidermis.4 Two other cells (Figure 6C,E-G yellow and cyan arrowheads; Video S7) can be followed on the last portion of their journey (approximately 2.5 hours) to the thymus, both from its caudal side. One appeared at 408 minutes (61 hpf) at the caudal border of the ear, next to the posterior cerebral vein (PceV), and followed Cell 2 along the same final route (Video S7 yellow arrowheads). We were surprised to find that once at the thymus, all these first immigrants did not slow down but kept moving in and out, making loops up to 100 μm long (Video S7). Overall, their cruising speed ranged from 1 to 3.5 μm/min, whether they were still distant from the thymus or had already reached it.
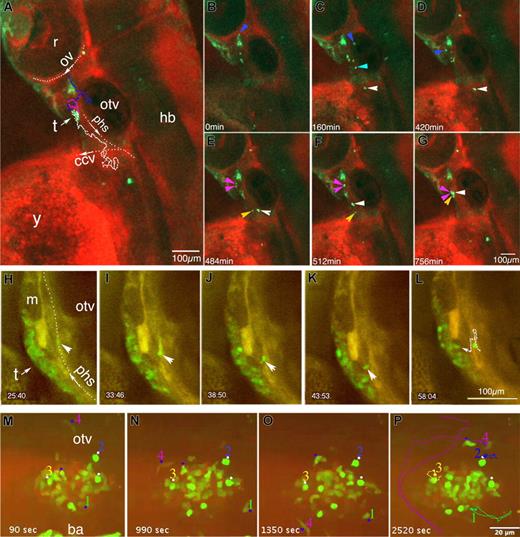
Behavior of CD41-GFPlow immigrants to the nascent thymus. Time-lapse fluorescence imaging of CD41-gfp embryos incubated with Bodipy TR, performed by conventional (A-G) or spinning disk (H-P) confocal microscopy. (A-G) Routes of GFPlow immigrants toward the nascent left thymus, lateral view; z-projection images of an embryo scanned through a depth of 80 μm (11 planes) every 4 minutes. Between 54 and 67 hpf (Videos S7 and S8); asterisk, GFP+ nonhematopoietic mesenchymal tissue; (A) Trajectories of 2 cells and their progeny in this 13 hour sequence: cell 1 (white dots), and cell 2 (blue dots) which divided at t = 450 minutes, giving 2 daughter cell routes (purple dots). (B-G) Some relevant time points; arrowheads point at the tracked cells with the same color code as in (A). (H-L) Right thymus at 96 hpf, lateral view; scanning performed every minute on a selected confocal plane (see Video S9); time is indicated in minutes and seconds; a GFP+ cell (arrowhead) in the PHS stops (H), moves back against the blood flow (I) and extravasates (J,K) to join the thymus (L). The dotted line in L recapitulates its trajectory; another circulating GFP+ cell also stops in this part of the PHS at t = 50 minutes (L), but then circulates again 18 minutes later. (M-P) Dynamics of CD41-GFPlow cells in and around the thymus at 5 dpf, ventrolateral view (Video S11); scanning performed every 90 seconds, time indicated in seconds. Dots and numbers point at cells whose trajectory during this 42-minute sequence was traced and is recapitulated in (P). White arrows indicate the position and sense of blood flow of the primary head sinus (phs), common cardinal vein (ccv), and optic vein (ov); r indicates retina; hb, hindbrain; y, yolk sac; m, muscle; and t, thymus.
Behavior of CD41-GFPlow immigrants to the nascent thymus. Time-lapse fluorescence imaging of CD41-gfp embryos incubated with Bodipy TR, performed by conventional (A-G) or spinning disk (H-P) confocal microscopy. (A-G) Routes of GFPlow immigrants toward the nascent left thymus, lateral view; z-projection images of an embryo scanned through a depth of 80 μm (11 planes) every 4 minutes. Between 54 and 67 hpf (Videos S7 and S8); asterisk, GFP+ nonhematopoietic mesenchymal tissue; (A) Trajectories of 2 cells and their progeny in this 13 hour sequence: cell 1 (white dots), and cell 2 (blue dots) which divided at t = 450 minutes, giving 2 daughter cell routes (purple dots). (B-G) Some relevant time points; arrowheads point at the tracked cells with the same color code as in (A). (H-L) Right thymus at 96 hpf, lateral view; scanning performed every minute on a selected confocal plane (see Video S9); time is indicated in minutes and seconds; a GFP+ cell (arrowhead) in the PHS stops (H), moves back against the blood flow (I) and extravasates (J,K) to join the thymus (L). The dotted line in L recapitulates its trajectory; another circulating GFP+ cell also stops in this part of the PHS at t = 50 minutes (L), but then circulates again 18 minutes later. (M-P) Dynamics of CD41-GFPlow cells in and around the thymus at 5 dpf, ventrolateral view (Video S11); scanning performed every 90 seconds, time indicated in seconds. Dots and numbers point at cells whose trajectory during this 42-minute sequence was traced and is recapitulated in (P). White arrows indicate the position and sense of blood flow of the primary head sinus (phs), common cardinal vein (ccv), and optic vein (ov); r indicates retina; hb, hindbrain; y, yolk sac; m, muscle; and t, thymus.
The primary head sinus (PHS) is the main vein collecting the blood bilaterally from the head past 2 dpf, and it runs just along the thymus.18 Figure 6H-L and Video S9 show the extravasation of a CD41-GFPlow immigrant from the right PHS into the right thymus. The cell stopped on the venous wall, moved back against the blood flow for 2.5 cell diameters, and extravasated (the whole process took 18 minutes.). Even though this was a priori the quickest route to the thymus for a circulating cell, it did not seem to be predominant. Indeed, when we selectively occluded the left PHS upstream of the left thymus through laser-mediated cell ablation (Video S10), we observed no delay in colonization of the left thymus relative to the right thymus or to the thymi of sibling embryos (Figure 7).
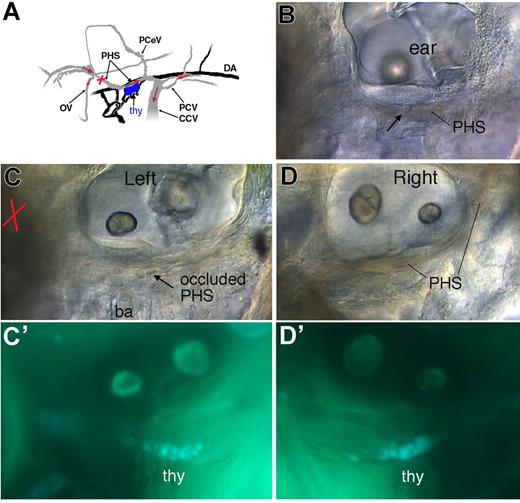
Occlusion of circulation in the PHS does not affect early thymus colonization. At 52 hpf, just before the onset of thymus colonization by CD41-GFPlow cells, circulation in the PHS along the left thymus was selectively suppressed by laser-ablating many erythrocytes at the upstream position indicated by a red cross, until the cell debris stopped the blood flow. At 70 hpf, the embryos in which circulation in this vessel was still blocked were sorted, and the content in GFPlow cells of the left thymus was compared with that of the right thymus and with the left thymus of control siblings. (A) Scheme of the venous (gray) and arterial (black) circulation in the region of interest at 60 hpf, left lateral view,18 with the sense of relevant venous circulation indicated by red arrows, and the nascent thymus in blue. (B-F) Live CD41-gfp 70 hpf embryos. (B) Control embryo, left side; arrow points at a leukocyte rolling in the PHS, with the fast flow of erythrocytes evidenced by a reddish shade. (C) Sibling (left side) in which the left PHS was occluded 18 hours earlier (52 hpf) at the position indicated by the red cross; arrow points at stagnant erythrocytes in the occluded PHS (see also Video S10); (D) Right side of the same embryo, with full circulation in the right PHS; (C′,D′) Corresponding CD41-GFPlow cells in the left (C′) and right (D′) thymi. PHS indicates primary head sinus; OV, optic vein; CCV, common cardinal vein; PceV, posterior cerebral vein; thy, thymus; and ba, branchial arches.
Occlusion of circulation in the PHS does not affect early thymus colonization. At 52 hpf, just before the onset of thymus colonization by CD41-GFPlow cells, circulation in the PHS along the left thymus was selectively suppressed by laser-ablating many erythrocytes at the upstream position indicated by a red cross, until the cell debris stopped the blood flow. At 70 hpf, the embryos in which circulation in this vessel was still blocked were sorted, and the content in GFPlow cells of the left thymus was compared with that of the right thymus and with the left thymus of control siblings. (A) Scheme of the venous (gray) and arterial (black) circulation in the region of interest at 60 hpf, left lateral view,18 with the sense of relevant venous circulation indicated by red arrows, and the nascent thymus in blue. (B-F) Live CD41-gfp 70 hpf embryos. (B) Control embryo, left side; arrow points at a leukocyte rolling in the PHS, with the fast flow of erythrocytes evidenced by a reddish shade. (C) Sibling (left side) in which the left PHS was occluded 18 hours earlier (52 hpf) at the position indicated by the red cross; arrow points at stagnant erythrocytes in the occluded PHS (see also Video S10); (D) Right side of the same embryo, with full circulation in the right PHS; (C′,D′) Corresponding CD41-GFPlow cells in the left (C′) and right (D′) thymi. PHS indicates primary head sinus; OV, optic vein; CCV, common cardinal vein; PceV, posterior cerebral vein; thy, thymus; and ba, branchial arches.
Two days later (5 dpf), CD41-GFP+ cells arriving at the thymus were still actively moving in and out of it, as bees around their hive (Figure 6M-P; Video S11).
Finally, a double immunodetection of GFP and phosphohistone H3 followed by confocal fluorescence analysis revealed CD41-GFPlow cells undergoing mitosis in both the nascent thymus and kidney (Figure S1C).
Discussion
As in mouse embryos, a low level of CD41 expression characterizes nascent HSCs in the AGM,3 so we found that a low level of CD41-gfp transgene expression characterizes the definitive blood precursors in the zebrafish homologue of the AGM, the DP joint. This and other common phenotypic and functional traits4,7 make it highly likely that these blood precursors are nascent HSCs. The possibility in the zebrafish embryo to follow up the cells in real-time allowed us to go further and uncover that the rise in CD41-gfp expression in these cells occurs at a particular stage of their maturation, namely just before they become released into the circulation.
As for the definitive HSCs in mammals, the generation of these CD41-GFPlow definitive HSC precursors in zebrafish is runx1 and Notch dependent, unlike the generation of primitive blood cells.
The continuous release of CD41-GFPlow precursors from the DP joint to the CHT between 34 hpf and 3 dpf implies that the transgene is turned on asynchronously within a larger pool of less mature HSC precursors, which the cell tracing data indicate to be already present all along the DP joint at the onset of blood circulation. Double labeling identified this pool as the c-myb+ population present there at that stage as a continuous cell cord.
Thus, CD41-gfp expression rises up asynchronously and seemingly randomly among the c-myb+ precursors in the DP joint from approximately 32 hpf onward. This rise seems to correlate with acquisition of competency to leave the DP joint and enter the circulation, which they then do within 2 hours, to immediately home to the CHT. Through time, more precursors turn on the CD41-gfp transgene and leave. Obviously, such a dynamic link between CD41-gfp expression and cell release from the AGM could not have been suspected without a real time follow-up of the cells involved.
As the expression of the CD41-gfp transgene appears to reflect faithfully that of the endogenous CD41 gene,8 the dynamic link uncovered could suggest an involvement of the CD41 integrin in the intravasation of the emerging HSCs into the axial vein.
A novelty of the zebrafish relative to mammals and birds is that the generation and release of definitive blood precursors in the embryo does not involve intra-aortic clusters (IACs) of hematopoietic precursors traversing the DA ventral wall, locally interrupting the endothelial lining. We detected no single hematopoietic cells anchored intraluminally to the DA ventral wall or any discontinuity in the latter at the ultrastructural level. CD41-GFPlow HSC precursors appeared in the subaortic mesenchyme, similar in that to the sub-aortic CD41+ HSC precursors identified in the mouse AGM.3 From there, however, they entered circulation not through the aorta but through the axial PCV, apparently without having to squeeze through an endothelial wall. We found that this intravasation is facilitated by a special configuration of the PCV dorsal endothelium, whereby adjacent endothelial cells are not contiguous but overlapping, a configuration reminiscent of what has been described for lymphatic vessels.19
The progeny of the CD41-GFPlow precursors that colonize the thymus to become thymocytes, and the pronephros to seed definitive hematopoiesis still express GFP. This allowed us first to show that they can undergo mitosis at any step in their journey to the successive hematopoietic organs: not only in the AGM, CHT, thymus, and kidney, but also in transit—in the blood and in the mesenchyme on their way to the thymus.
Second, we could determine that the first CD41-GFPlow immigrants arrive in the thymus by 54 hpf, whereas previously, thymocytes had been first detected by 72 hpf, by the onset of rag1 expression. Fifty-four hours postfertilization is actually the earliest time at which the thymic epithelium anlage was detected histologically,20 suggesting that it starts to secrete chemoattractants as soon as it forms from the branchial arch epithelium. We found that the CD41-GFPlow HSC precursors rolled in the veins, but not in arterial vessels. In mammals, this is a physiologically crucial feature, which reflects the presence on the venous wall of specific molecules, whose interaction with circulating leukocytes mediates their rolling.21,22 Our observations indicate that this functional difference between arterial and venous vessels is not only conserved in zebrafish but already operational from the onset of embryonic blood circulation, visible by then through the rolling of primitive macrophages (data not shown). Because the rolling behavior is a prerequisite to leukocyte extravasation, we expected the circulating CD41-GFPlow precursors to leave the blood to the thymus from veins. Our in vivo follow-up is consistent with this notion, as each cell that we tracked migrating through the mesenchyme to the thymus first appeared adjacent to a vein. We thus predict that such a condition will also prevail for the extravasation of circulating hematopoietic precursors in mammalian embryos. Unexpectedly, only some of the CD41-GFPlow precursors took what would appear as the most direct route to the thymus: extravasation from the nearest vein, the PHS that runs just along the nascent thymus. Most immigrants rather traveled through the mesenchyme from much farther locations, both rostral and caudal to the thymus, indicating that they extravasated from more remote veins. Our tracking of individual cells migrating over 12 hours through the mesenchyme revealed directional migration to the nascent thymus from a distance of at least 350 μm. This strongly suggests the presence of a chemoattractant(s) gradient that extends and can be perceived over several hundred micrometers from the source.
Once they have reached the thymus, the early immigrants do not stop but keep moving in and out of it as bees around their hive, within a 80-μm range. Various molecular explanations may account for such a behavior (eg, a cycle of desensitization/resynthesis of a chemokine receptor or downstream intracellular signaling component). In addition, when exposed to hill-shaped chemokine gradients in vitro, neutrophils were found not to stop at the site of maximum concentration but to go further and come back.23 An additional factor of local movement could be that not only the thymus rudiment but also one or more other tissues in its vicinity contribute chemoattractants, as found in the mouse.24,25 This could make the long-range chemoattraction to the thymus more efficient but the short range more chaotic.
This restless in-and-out movement of CD41-GFPlow immigrants still occurs by 5 dpf, 2 days after the first detection of rag1 expression. Because rag1 is expressed from the beginning strictly within the confines of the thymus,4 one implication is that the onset of rag1 expression in these immigrants, which probably marks their irreversible commitment to a lymphoid, then T-cell fate,17 correlates with a clear behavioral transition, from the bees-around-the-hive to a stiller phenotype. We suggest this stiller behavior reflects their closer interaction with the thymic epithelium, an interaction that will drive both their progress through the T-cell differentiation sequence, and the maturation of the thymic epithelium itself.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Isabelle Godin, Thomas Boehm, and Jean-Pierre Levraud for their critical reading of the manuscript; Mark Cooper and Iain Johnson for the Bodipy TR; Valérie Briolat for her kind assistance with CD41-gfp zebrafish and plasmids; Hui-Feng Lin for the gfp probe matching the CD41-gfp transgene; and Anne Danckaert for the quantification of cell trajectories with the MetaMorph software.
This work was supported by an internal grant from the Stem Cells Horizontal Program of the Pasteur Institute. K.K. and E.M. were supported by the European Commission through the FP6 Integrated Project ZF-Models.
Authorship
Contribution: K.K., E.M., A.Z., and P.H. designed and performed experiments, analyzed data, and checked or improved the manuscript. A.C. performed experiments. E.P. and C.M. analyzed data. P.H. drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Philippe Herbomel, Unité Macrophages et Développement de l'Immunité, Institut Pasteur, 25 rue du Dr Roux, 75724 Paris cedex 15, France; e-mail: herbomel@pasteur.fr.
References
Author notes
K.K. and E.M. contributed equally to this work.