Abstract
To program pluripotent cells into blood, a knowledge of the locations of precursors during their journey through the embryo and the signals they experience would be informative. The anterior (a) and posterior (p) ventral blood islands (VBIs) in Xenopus are derived from opposite sides of the pregastrula embryo. The aVBI goes through a “hemangioblast” state, characterized by coexpression of blood and endothelial genes at neurula stages, whereas the pVBI expresses these genes in a nonoverlapping fashion several hours later, after commitment to either a blood or an endothelial fate. We describe a novel role for fibroblast growth factor (FGF) in controlling the timing of Scl, Lmo2, and Runx1 expression in the 2 VBI compartments. Blocking FGF signaling during gastrulation expands expression at neurula stages into posterior regions. We show, by lineage labeling, explant analysis, and targeted blocking of FGF signaling, that this is due to the pVBI prematurely expressing these genes with the timing of the aVBI. In contrast, overexpression of FGF in aVBI precursors eliminates the anterior hemangioblast program. Using this information, we have recapitulated the anterior hemangioblast program in pluripotent cells in vitro by inhibiting FGF signaling in anterior mesoderm induced by activin and exposed to bone morphogenetic protein (BMP) signaling.
Introduction
During early vertebrate embryogenesis, the first tissues to develop are blood and endothelium located in the blood islands of yolk sac mesoderm.1 The blood islands are the source of primitive erythropoiesis and vasculogenesis, which supply the early embryo with a circulatory system carrying primarily erythroid and some myeloid cells.2
As a result of early observations in chick, a common precursor to blood and endothelium, called the hemangioblast, was proposed.3,4 Evidence for the existence of the hemangioblast comes from the observations that many genes are coexpressed and/or required for the very early stages of blood and endothelial development.1,5 In addition, transient mouse embryonic stem cell (ESC)–derived blast colony forming cells (BL-CFCs) and more recently equivalent human colonies, give rise to both lineages and are considered to be the in vitro equivalent of hemangioblasts.6,7 Comparable colonies have been detected in cultures from mouse embryos, suggesting that such bipotential precursors may also exist in vivo.8,9 Very recently, clonal analysis of progenitor contributions to yolk sac populations using mouse ESCs,10 and single cell resolution fate mapping in zebrafish late blastula/gastrula embryos11 have arrived at contradictory conclusions on the existence of the hemangioblast. The fish study verifies a common progenitor for blood and endothelium, whereas the mouse study claims that few, if any, hemangioblasts exist. Further fate mapping may be required to resolve the issue.
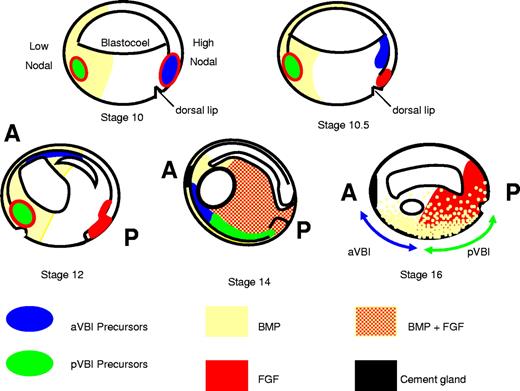
In Xenopus, blood and endothelium of the primitive lineage are located in the ventral blood island (VBI) of the tailbud stage embryo (approximately 26-36 hours after fertilization [hpf]). Fate mapping has demonstrated that the VBI is composed of 2 compartments, the anterior (a) and posterior (p) VBI, which are derived from opposite sides of the 32-cell stage embryo.12 The precursors of the anterior VBI (aVBI) and posterior VBI (pVBI), therefore, take very different routes through the embryo during gastrulation, and the 2 compartments finally meet during early neurula stages (stage 14, approximately 16 hpf; Figure 1). It is at this time that the earliest signs of blood and endothelial gene expression are detected. In the anterior of the neurula embryo, a population of cells lying just below the cement gland coexpress blood and endothelial genes, and lineage labeling has shown that this population contributes later to mature vessels and the blood cells circulating within them.13 The hemangioblast-like population of cells are the precursors of the aVBI. In contrast, the pVBI compartment develops more slowly and shows nonoverlapping blood and endothelial gene expression from stage 25 onward. In addition, the aVBI and pVBI compartments differ in their potential. The aVBI gives rise to myeloid cells, but these are not generated by the posterior compartment. Cells expressing myeloid genes are seen during tailbud stages in the pVBI, but these have migrated from the aVBI.14 A second wave of myeloid cells seen well after tailbud stages is currently of unknown origin.15
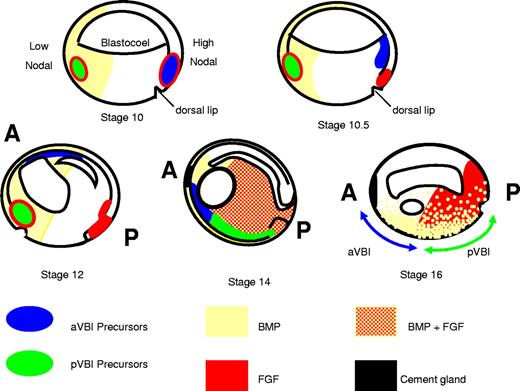
A model for programming the VBI. aVBI precursors in the dorsal lip at stage 10 rapidly escape FGF signaling by crawling over the blastocoel roof toward the animal pole where they encounter BMP signaling in the ventral animal pole ectoderm and commit to a hemangioblast fate (stage 12). They continue to migrate anteriorly and ventrally, and by neurula stages (14-16) they are found in the anterior ventral mesoderm just below the cement gland. pVBI precursors migrate much more slowly and experience prolonged exposure to FGF and BMP signaling through to tailbud stages. By stage 14, the anterior and posterior VBI compartments have met in the ventral midline; and although the aVBI experiences relatively low FGF and BMP signaling, the pVBI region is high in both signals. A block to FGF signaling allows the premature expression of erythroid genes in the posterior, but the high BMP levels do not permit premature expression of endothelial and myeloid genes posteriorly (for more details of the model, see “Discussion”). A indicates anterior; and P, posterior. Distribution of FGF/MAPK and BMP/pSmad1 as in Schohl and Fagotto.31
A model for programming the VBI. aVBI precursors in the dorsal lip at stage 10 rapidly escape FGF signaling by crawling over the blastocoel roof toward the animal pole where they encounter BMP signaling in the ventral animal pole ectoderm and commit to a hemangioblast fate (stage 12). They continue to migrate anteriorly and ventrally, and by neurula stages (14-16) they are found in the anterior ventral mesoderm just below the cement gland. pVBI precursors migrate much more slowly and experience prolonged exposure to FGF and BMP signaling through to tailbud stages. By stage 14, the anterior and posterior VBI compartments have met in the ventral midline; and although the aVBI experiences relatively low FGF and BMP signaling, the pVBI region is high in both signals. A block to FGF signaling allows the premature expression of erythroid genes in the posterior, but the high BMP levels do not permit premature expression of endothelial and myeloid genes posteriorly (for more details of the model, see “Discussion”). A indicates anterior; and P, posterior. Distribution of FGF/MAPK and BMP/pSmad1 as in Schohl and Fagotto.31
Signaling requirements for establishing blood and endothelial programs have been examined, and BMP, Wnt, Notch, and VEGF have all been implicated.16,17 Reported roles for fibroblast growth factor (FGF) in blood and endothelial development, however, have been contradictory. Thus, in chick erythroid progenitors, basic FGF (bFGF) supports proliferation over differentiation.18 Again, in chick, FGF blocks a late step in primitive erythroid differentiation and promotes endothelial development.19 In Xenopus, FGF blocks blood and promotes somite fate20 or blocks blood in favor of endothelial development.21 In contradiction to these negative roles in blood development, FGF21 plays an essential role in a late step in erythropoiesis in fish.22 Furthermore, in mouse ESCs, addition of bFGF during embryoid body differentiation increases BL-CFC (hemangioblast) frequency, whereas addition of bFGF during the growth of hemangioblast colonies from these BL-CFCs is inhibitory.23 Thus, FGF appears to have 2 opposing effects on hemangioblast development: enhancing their generation but interfering with their subsequent development. The supportive role played by FGF in hemangioblast generation may reflect the requirement for FGF in mesoderm induction, patterning, and migration.24,25
Here we investigate the role played by FGF signaling in the specification of primitive blood and endothelium in Xenopus anterior and posterior VBI compartments. We find that, in the pVBI, FGF holds back expression of the blood program until the cells are committed to either the blood or the surrounding vitelline vessel programs at tailbud stages. In contrast, aVBI precursors, located near the dorsal lip of the blastopore at the start of gastrulation, escape FGF signaling there by crawling away, and we find that prolonging their exposure suppresses the hemangioblast program. Armed with this information and with the knowledge that BMP is required at least for Scl expression,13 we have recapitulated the anterior hemangioblast program in naive animal cap cells and shown that it requires induction of anterior mesoderm by activin, a block to FGF signaling, and exposure to BMP. Because this has been achieved in a simple serum-free medium, the signals required for hemangioblast generation are more clearly delineated than has been possible in the majority of ESC studies to date.
Methods
This study was approved by the Faculty of Clinical Medicine, Local Ethical Review Committee, University of Oxford.
Embryo and explant manipulations
Xenopus embryos were obtained and cultured as described previously.26 SU5402 (Merck, Nottingham, United Kingdom)–treated embryos and explants were incubated in the presence of 25-100 μM SU5402 dissolved in dimethylsulfphoxide (DMSO; final concentration 0.5% in 0.1× modified Barth saline [MBS]) and cultured to the desired stage; SU5402 treatment results in open blastopores, and this was used as proof of the efficacy of the treatment in combination with Western blot analysis for pMAPK (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). RNAs were injected in water (4 nL) into the animal pole or marginal zone as indicated in figure legends. For overexpression studies, 200 to 400 fg embryonic FGF (eFGF) mRNA (levels chosen by titration for activity) was injected into the marginal zone at the 4-cell stage and the embryos were grown to neurula stages for in situ hybridization analysis.13 Animal cap and VMZ explants were dissected at stages 8 and 10, respectively, and cultured in 1 × MBS27 to neurula stages. A titration of activin mRNA was performed to determine the optimal concentrations required to induce dorsoanterior mesoderm (Figure S2). For sectioning, animal caps were fixed in 4% paraformaldehyde/phosphate-buffered saline for at least 3 weeks at 4°C, and groups of 10 were embedded in wax and cut into 10-μm sections.
RNA analysis
In situ hybridization of whole mounted and sectioned embryos or explants was performed using digoxigenin-labeled probes and BM-Purple substrate. Probes for Scl, Fli1, and Runx1 were synthesized as described previously.13 Antisense Mpo probe was synthesized by linearising pBluescript SK(-) (ID XL038023, National Institute for Basic Biology [NIBB], Okasaki, Japan) with Sac1, Lmo2 by linearizing pCMV-Sport6 (ID 4174203, IMAGE; MRC Gene Service, Cambridge, United Kingdom) with EcoR1 and SpiB by linearizing pCMV-Sport6 (ID 5537169, MRC Gene Service) with EcoR1 followed by in vitro transcription with T7 in each case. In situ hybridization on wax sections was performed as described previously.12 For lineage analysis, β-galactosidase RNA was injected into the 2 ventral or dorsal blastomeres of 4-cell embryos and assayed for enzyme activity at neurula stages as described previously.12
Quantitative RT-PCR
The isolation and quantitation of RNAs were determined by real-time reverse transcription–polymerase chain reaction (RT-PCR) as described previously.28,29 Amounts relative to the housekeeping RNA, ornithine decarboxylase (Odc), were expressed as a ratio to uninjected VMZs or animal caps. Experiments were repeated 3 times, and average values and standard deviations (shown as error bars) were calculated. Table 1 indicates the sequences of primers and probes.
Photography
Images of whole and sectioned embryos were captured using a Nikon SMZ 1500 dissecting microscope (1× HR Plan Apo objective, NA 0.13) with a Nikon DXM1200 digital camera driven by Act-1 version 2.12 software or a Nikon eclipse E600 microscope with a Nikon digital camera DXM1200C using NIS elements BR2.30 SP4 imaging software (all from Nikon UK, Kingston-upon-Thames, United Kingdom).
Results
Blocking FGF signaling during gastrulation increases the number of cells expressing Scl, Lmo2, and Runx1
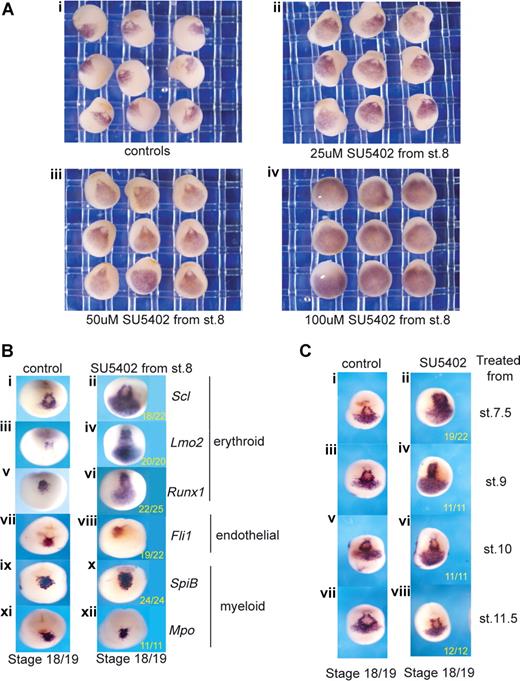
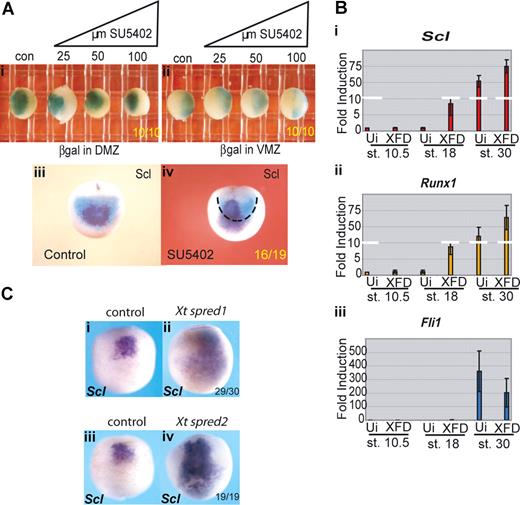
To explore the FGF requirement early in the programming of primitive blood and endothelium, Xenopus embryos were treated from blastula stages (7.5-8.5, approximately 5 hpf) with the inhibitor, SU5402, which blocks all FGF signaling at the level of the receptors,30 and the effects on the expression of blood and endothelial genes were examined approximately 13 hours later at neurula stages (18/19). At this time, blood and endothelial genes are coexpressed in a population of hemangioblast-like cells located anteriorly just below the cement gland13 (Figures 1,2). This “hemangioblast” population represents the progenitors of the blood and endothelium of the anterior VBI (aVBI), together with the endocardium and vessels connected to the heart. In contrast, no expression of blood or endothelial genes is detected at this time in the presumptive posterior VBI (pVBI). We first titrated the inhibitor, SU5402, over a range of 25 to 100 μM (Figure S1, resulting pMAPK levels) and probed for Scl as a representative hemangioblast marker (Figure 2A). The expression of Scl was expanded posteriorly and laterally in a dose-dependent manner until, at the top end of the titration, Scl expression occupied the entire ventral region of the neurula stage embryo. We then proceeded to examine the effects of SU5402 treatment on the expression of other blood and endothelial markers.
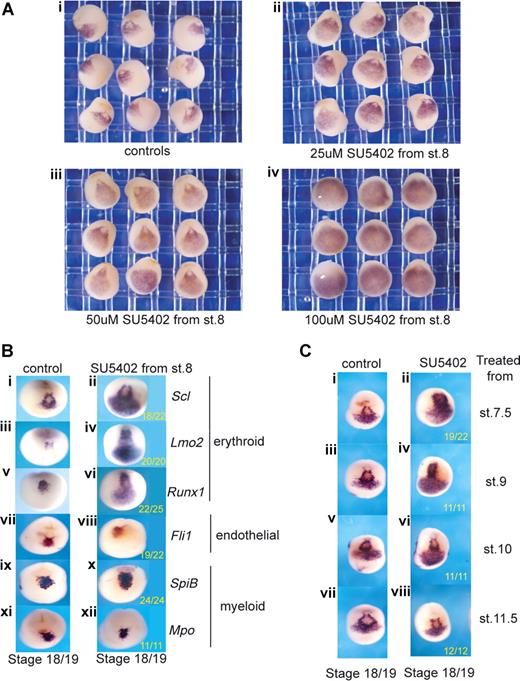
Posterior expansion of erythroid gene expression when FGF signaling is inhibited during gastrulation. (A) Blocking FGF signaling expands Scl expression into posterior regions in a dose-dependent manner (ventral views). Embryos were treated from stage 8 with increasing concentrations of SU5402: 25 μM (ii), 50 μM (iii), and 100 μM (iv), grown to stage 18/19 and probed along with control embryos (i) by whole-mount in situ hybridization for Scl expression. Control siblings were treated with 0.1× MBS containing 0.5% DMSO. (B) Blocking FGF signaling expands erythroid gene expression posteriorly but leaves endothelial and myeloid gene expression intact. Embryos were treated with 50 μM SU5402 from stage 8 and grown to stage 18/19 (anterior ventral views). Control embryos (i,iii,v,vii,ix,xi) in 0.1× MBS plus 0.5% DMSO and treated embryos (ii,iv,vi,viii,x,xii) were then probed by whole-mount in situ hybridization for Scl (i,ii), Lmo2 (iii,iv), Runx1 (v,vi), Fli1 (vii,viii), SpiB (ix,x), and Mpo (xi,xii). Numbers of embryos assayed is recorded in bottom right of each panel. (C) FGF signaling must be blocked during gastrulation for the expansion of Scl expression (anterior ventral views). Embryos were treated with 50 μm SU5402 from stages 7.5, 9, 10, and 11.5 (ii,iv,vi,viii) and grown along with untreated embryos (i,iii,v,vii) to stage 18/19. Embryos were probed for Scl expression by whole-mount in situ hybridization.
Posterior expansion of erythroid gene expression when FGF signaling is inhibited during gastrulation. (A) Blocking FGF signaling expands Scl expression into posterior regions in a dose-dependent manner (ventral views). Embryos were treated from stage 8 with increasing concentrations of SU5402: 25 μM (ii), 50 μM (iii), and 100 μM (iv), grown to stage 18/19 and probed along with control embryos (i) by whole-mount in situ hybridization for Scl expression. Control siblings were treated with 0.1× MBS containing 0.5% DMSO. (B) Blocking FGF signaling expands erythroid gene expression posteriorly but leaves endothelial and myeloid gene expression intact. Embryos were treated with 50 μM SU5402 from stage 8 and grown to stage 18/19 (anterior ventral views). Control embryos (i,iii,v,vii,ix,xi) in 0.1× MBS plus 0.5% DMSO and treated embryos (ii,iv,vi,viii,x,xii) were then probed by whole-mount in situ hybridization for Scl (i,ii), Lmo2 (iii,iv), Runx1 (v,vi), Fli1 (vii,viii), SpiB (ix,x), and Mpo (xi,xii). Numbers of embryos assayed is recorded in bottom right of each panel. (C) FGF signaling must be blocked during gastrulation for the expansion of Scl expression (anterior ventral views). Embryos were treated with 50 μm SU5402 from stages 7.5, 9, 10, and 11.5 (ii,iv,vi,viii) and grown along with untreated embryos (i,iii,v,vii) to stage 18/19. Embryos were probed for Scl expression by whole-mount in situ hybridization.
Genes later expressed in erythroid (Scl, Lmo2, Runx1), endothelial (Fli1), and myeloid (SpiB, Mpo) cells in Xenopus embryos are all expressed in the putative hemangioblast population at stages 18/19 (Figure 2B). The later lineage affiliations of these genes are different in the mouse (see “Discussion”), but for the Xenopus embryo they are used here as a shorthand to refer to subsets with differing responses to perturbed signaling. The continued expression of all these anterior hemangioblast genes in SU5402-treated embryos suggests that none of them requires FGF for their induction (Figure 2B). However, in addition to expression in the anterior, the erythroid genes (Scl, Lmo2, Runx1) were also expressed more posteriorly (Figure 2Bi-vi). In contrast, expression of the myeloid and endothelial genes was not expanded (Figure 2Bvii-xii). These results suggest that the restriction of early expression of erythroid genes to the anterior region of the neurula embryo may be partly because of repression in posterior regions by the relatively high levels of FGF activity located there.31,32
To define the time when FGF signaling must be blocked for this expansion of erythroid gene expression, we applied SU5402 at various times and found that, whereas stage 10 was early enough, stage 11.5 was too late, indicating that the block must occur during gastrulation (Figure 2C).
Posterior regions prematurely adopt the erythroid program of the anterior embryo when FGF signaling is blocked
The posterior expansion of erythroid gene expression might be the result of proliferation of anterior precursors into posterior regions or to posterior cells prematurely expressing the erythroid program. To distinguish between these 2 possibilities, we carried out lineage tracing using targeted injection of β-galactosidase (β-gal) mRNA into the aVBI precursors, the so-called dorsal marginal zone (DMZ), or into the pVBI precursors, the so-called ventral marginal zone (VMZ), of the embryo at the 4-cell stage. Embryos were treated from stage 7.5 with a range of concentrations of SU5402 from 25 to 100 μM, grown to neurula stages and processed for β-gal activity. Whichever side of the marginal zone was injected with β-gal RNA, the boundaries of activity remained unchanged at all concentrations of inhibitor examined (Figure 3Ai,ii). When DMZ-injected embryos were subsequently probed for Scl expression at stage 18, as expected, the Scl signal was seen contained within the β-gal positive domain (Figure 3Aiii). However, after administration of 25 μM SU5402, we were able to detect Scl expression beyond the β-gal territory (Figure 3Aiv). Taken together, these results suggest that there has been no proliferation of anterior cell types into posterior regions but rather that the posterior half of the embryo has adopted the erythroid program with the timing of the anterior hemangioblast.
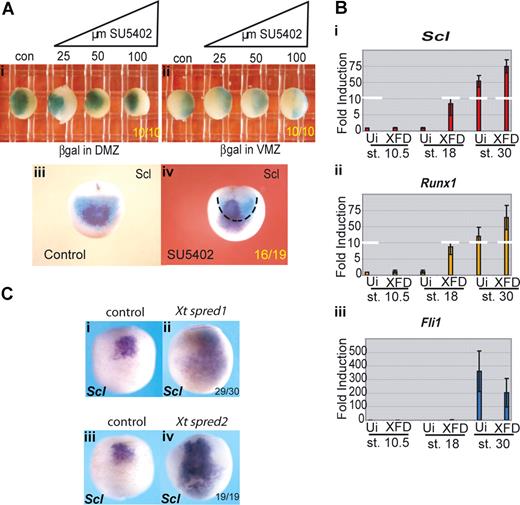
Erythroid gene expression in the pVBI is suppressed by FGF signaling. (A) The anterior/posterior boundary within the VBI is unchanged by SU5402 treatment, but Scl expression is expanded into posterior territory. Embryos were injected at the 4-cell stage with 200 pg β-galactosidase mRNA into (i) both DMZ blastomeres or (ii) both VMZ blastomeres. Embryos were treated from stage 8 with increasing concentrations of SU5402 (25-100 μM) and grown along with controls (con) to stage 18/19 then stained for β-galactosidase. (iii,iv) Embryos injected with 200 pg β-galactosidase RNA per DMZ blastomere were treated with 25μM SU5402 from stages 8-18. Embryos were stained for βgal and subsequently probed for Scl by whole-mount in situ hybridization. Black dashed line in subpanel iv delineates the limit of β-gal staining (DMZ territory) at stage 18. Subpanels i and ii are ventral views; and subpanels iii and iv, anterior views. Embryo numbers are in the bottom right corner of each subpanel. (B) Injection of a dominant-negative FGF receptor induces precocious erythroid gene expression in VMZ explants; 200 to 400 pg XFD mRNA (levels that strongly block MAPK activity, Figure S3b) was injected into both VMZ blastomeres of 4-cell stage embryos. Embryos were grown to stage 10 when VMZ explants were excised and cultured until sibling embryos reached stages 18 and 30. Explants were processed for real time RT-PCR probing for Scl (i), Runx1 (ii), and Fli1 (iii). Precocious expression of Scl and Runx1 at stage 18 was observed in XFD injected explants compared with uninjected (Ui) control VMZ explants, whereas expression of the endothelial gene Fli1 was not precociously induced. Values represent the average of 3 experiments; error bars represent SD. (C) FGF antagonists Spred 1 and 2 mimic the effects of SU5402. Embryos at the 2-cell stage were injected in the marginal zone with 1ng of Xt Spred 1 (ii) or 2ng Xt Spred 2 (iv) mRNAs, levels previously shown to block MAPK activation efficiently,34 and grown alongside uninjected controls (i,iii) to neurula stages when they were probed for expression of Scl by whole-mount in situ hybridization. Expansion of Scl expression into posterior and lateral regions was seen as for SU5402 treatment. Embryo numbers are in the bottom right corner of each subpanel.
Erythroid gene expression in the pVBI is suppressed by FGF signaling. (A) The anterior/posterior boundary within the VBI is unchanged by SU5402 treatment, but Scl expression is expanded into posterior territory. Embryos were injected at the 4-cell stage with 200 pg β-galactosidase mRNA into (i) both DMZ blastomeres or (ii) both VMZ blastomeres. Embryos were treated from stage 8 with increasing concentrations of SU5402 (25-100 μM) and grown along with controls (con) to stage 18/19 then stained for β-galactosidase. (iii,iv) Embryos injected with 200 pg β-galactosidase RNA per DMZ blastomere were treated with 25μM SU5402 from stages 8-18. Embryos were stained for βgal and subsequently probed for Scl by whole-mount in situ hybridization. Black dashed line in subpanel iv delineates the limit of β-gal staining (DMZ territory) at stage 18. Subpanels i and ii are ventral views; and subpanels iii and iv, anterior views. Embryo numbers are in the bottom right corner of each subpanel. (B) Injection of a dominant-negative FGF receptor induces precocious erythroid gene expression in VMZ explants; 200 to 400 pg XFD mRNA (levels that strongly block MAPK activity, Figure S3b) was injected into both VMZ blastomeres of 4-cell stage embryos. Embryos were grown to stage 10 when VMZ explants were excised and cultured until sibling embryos reached stages 18 and 30. Explants were processed for real time RT-PCR probing for Scl (i), Runx1 (ii), and Fli1 (iii). Precocious expression of Scl and Runx1 at stage 18 was observed in XFD injected explants compared with uninjected (Ui) control VMZ explants, whereas expression of the endothelial gene Fli1 was not precociously induced. Values represent the average of 3 experiments; error bars represent SD. (C) FGF antagonists Spred 1 and 2 mimic the effects of SU5402. Embryos at the 2-cell stage were injected in the marginal zone with 1ng of Xt Spred 1 (ii) or 2ng Xt Spred 2 (iv) mRNAs, levels previously shown to block MAPK activation efficiently,34 and grown alongside uninjected controls (i,iii) to neurula stages when they were probed for expression of Scl by whole-mount in situ hybridization. Expansion of Scl expression into posterior and lateral regions was seen as for SU5402 treatment. Embryo numbers are in the bottom right corner of each subpanel.
Blocking FGF signaling with a dominant negative FGF receptor or the FGF antagonists, Spred 1 and 2, mimic the effects of SU5402
The experiments so far suggest that FGF signaling delays the expression of erythroid genes posteriorly. However, because SU5402 has been shown to also inhibit PDGF receptor function, albeit weakly,30 we next inhibited FGF signaling using either a dominant negative FGF receptor, XFD,33 or the natural inhibitors, Spred 1 and 2.34 mRNA coding for XFD was injected into the VMZ at the 4-cell stage, which includes the future pVBI population, and VMZ explants were excised at stage 10 and analyzed by quantitative RT-PCR at stages 10.5, 18, and 32. As with SU5402 treatment of whole embryos, blocking FGF in isolated VMZs led to premature expression of the erythroid genes, Scl and Runx1, at neurula stages, whereas expression of the endothelial gene, Fli1, was not induced (Figure 3Bi-iii). Furthermore, whereas the expression of the erythroid genes continued to be enriched at tailbud stages in explants injected with XFD, Fli1 expression was slightly repressed. Expansion of erythroid at the expense of endothelial cell fate by blocking FGF has been reported previously,21 but this is the first demonstration that this is the result of premature expression of the erythroid program in this region. Expression of the myeloid markers, SpiB and Mpo, was not detected in control or XFD injected explants (data not shown). These results therefore mirror the results in whole embryos treated with SU5402, with premature expression of erythroid genes in the presumptive pVBI and no parallel induction of endothelial or myeloid gene expression.
Spred 1 and 2 are feedback antagonists of FGF signaling and have been shown to work downstream of the FGF receptor via the MAPK pathway.34 We injected mRNAs for Spred 1 and 2 into the marginal zone of 2-cell embryos and monitored Scl expression at neurula stages. Scl expression was expanded posteriorly and laterally (Figure 3Cii,iv) at a time when uninjected embryos expressed Scl in the anterior region only (Figure 3Ci,iii), thus phenocopying the activity of SU5402 (Figure 2A). This experiment further suggests that, of the pathways activated downstream of the FGF receptor, MAPK is responsible for the effects we have reported here.
Induction of the anterior hemangioblast population requires the absence of FGF signaling
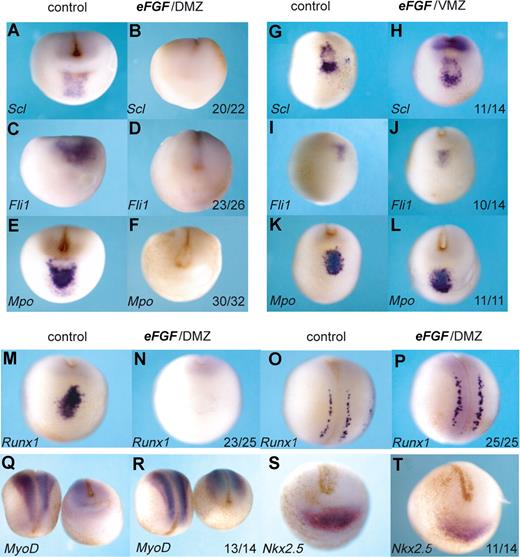
The precursors of the anterior hemangioblast population originate from the most anterior region of the 32-cell embryo.13 At the beginning of gastrulation, FGF signaling is high in this “dorsal lip” region of the embryo31,32 (Figure 1), but the precursors of the aVBI quickly move away toward the animal pole, which is relatively free of FGF signaling. To ask whether this is a requirement for their specification, we exposed these cells to continued FGF signaling by injecting eFGF mRNA into the presumptive aVBI, the DMZ, at the 4-cell stage. Not only erythroid (Scl, Runx1) but also endothelial (Fli1) and myeloid (Mpo) gene expression was eliminated (Figure 4A-F,M,O). This effect was specific to the anterior hemangioblast program because Runx1 expression in the Rohan Beard cells of the neural plate was unaffected (Figure 4O,P), and expression of the somite and heart markers, MyoD and Nkx2.5, was also undisturbed (Figure 4Q-T). The lack of effect on MyoD expression emphasizes the differing requirements for FGF signaling seen here for the anterior blood compartment compared with that reported previously for the posterior blood compartment, where FGF favors muscle differentiation at the expense of blood.20 As expected, injection of eFGF mRNA into the VMZ, which represents precursors of the pVBI and where FGF levels remain relatively high throughout gastrulation, had no effect on expression of erythroid, endothelial, or myeloid markers in the anterior hemangioblast (Figure 4G-L). Taken together, these results suggest that specification of the full anterior hemangioblast program requires the absence of FGF signaling.
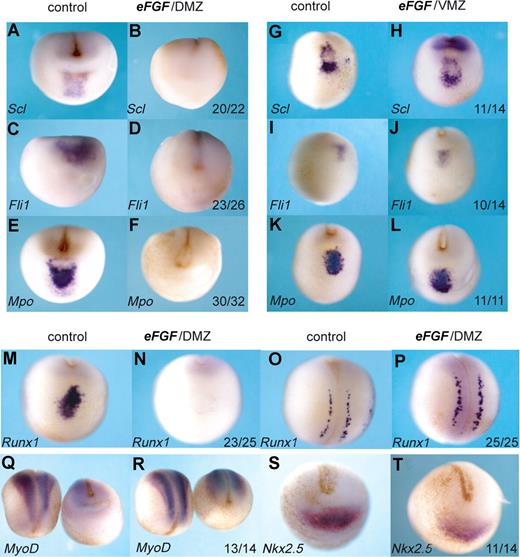
Overexpression of FGF in the DMZ, but not the VMZ, eliminates the anterior hemangioblast program. Embryos at the 4-cell stage were injected with 400 fg eFGF mRNA either into the DMZ (B,D,F,N,P,R,T) or the VMZ (H,J,L). Control embryos (A,C,E,G,I,K,M,O,Q,S) were water injected. Embryos were grown to stage 18/19 and then probed by in situ hybridization for Scl, Fli1, Mpo, Runx1, MyoD, or Nkx2.5. Probes used are indicated in the bottom left hand corner, and numbers of embryos probed are indicated in the bottom right-hand corner of each box. Panels M,N and O,P are anterior-ventral and dorsal views, respectively, of the same embryo probed for Runx1. Panels Q and R show dorsal views for left-hand embryos and anterior-ventral views for right hand embryos. All other embryos show anterior-ventral views.
Overexpression of FGF in the DMZ, but not the VMZ, eliminates the anterior hemangioblast program. Embryos at the 4-cell stage were injected with 400 fg eFGF mRNA either into the DMZ (B,D,F,N,P,R,T) or the VMZ (H,J,L). Control embryos (A,C,E,G,I,K,M,O,Q,S) were water injected. Embryos were grown to stage 18/19 and then probed by in situ hybridization for Scl, Fli1, Mpo, Runx1, MyoD, or Nkx2.5. Probes used are indicated in the bottom left hand corner, and numbers of embryos probed are indicated in the bottom right-hand corner of each box. Panels M,N and O,P are anterior-ventral and dorsal views, respectively, of the same embryo probed for Runx1. Panels Q and R show dorsal views for left-hand embryos and anterior-ventral views for right hand embryos. All other embryos show anterior-ventral views.
Recapitulation of the anterior hemangioblast program in pluripotent cells
The data from the embryo predict that the induction of the full anterior hemangioblast program in naive cells will require the absence of FGF. To test this, we made use of animal caps from Xenopus blastulae. Excised animal caps if left untreated will form atypical epidermis but if treated with growth factors such as activin or FGF they respond by forming mesodermal or endodermal derivatives in a dose-dependent manner.35-37
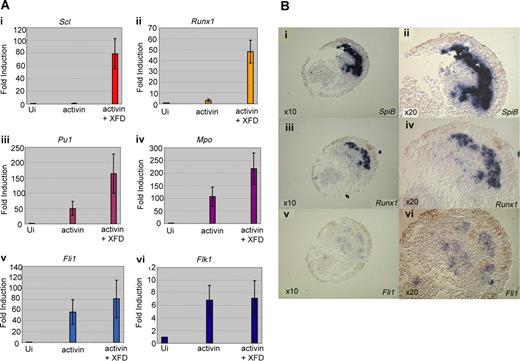
The animal poles of 1-cell embryos were injected with mRNA for activin B (to induce anterior mesoderm) and XFD (to block FGF signaling). Optimal doses of activin and XFD were determined by monitoring cap elongation and phosphorylation of MAPK (Figures S2,S3). Animal caps were excised at stage 8 (early blastula) and cultured in a simple salt solution without serum to stage 18/19 when they were collected for analysis of hemangioblast transcripts by quantitative RT-PCR (Figure 5A). As predicted from the embryo and explant data, expression of the erythroid genes, Scl and Runx1, were strongly induced (10- to 100-fold) in caps injected with mRNAs for activin and XFD, compared with caps injected with activin alone (Figure 5Ai,ii). In contrast, whereas expression of the myeloid genes, SpiB and Mpo, and the endothelial genes, Fli1 and Flk1, was also strongly induced by the combination of activin and XFD, blocking FGF resulted in a much more minor increase (1- to 3-fold) over activin alone (Figure 5Aiii-vi), consistent with the whole embryo results where expression of these genes was much less sensitive to a block in FGF signaling (Figures 2B,3B). Thus, the response of animal caps to activin and XFD appears to recapitulate the programming of neurula stage anterior hemangioblasts in embryos.
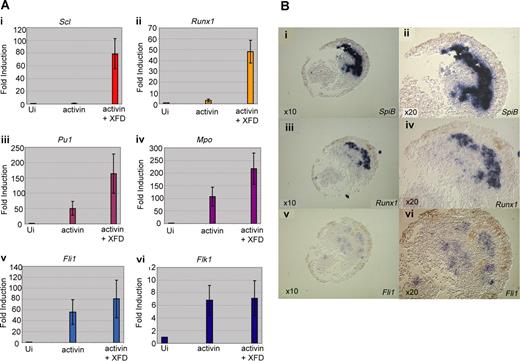
Induction of the anterior hemangioblast program in animal caps requires signaling by activin in the absence of FGF. (A) Embryos at the 1-cell stage were injected in the animal pole with 200 to 400 fg activin mRNA either alone or in combination with 400 pg dominant-negative FGF receptor (XFD) mRNA. Embryos were grown to stage 8, and animal caps were excised and cultured in 1 × MBS to stage 18 and then processed for real-time RT-PCR analysis, probing for Scl (i), Runx1 (ii), SpiB (iii), Mpo (iv), Fli1 (v), and Flk1 (vi). Values represent the average of 3 experiments; error bars represent SD. (B) SpiB, Runx1, and Fli1 expression overlap in animal caps in which the anterior hemangioblast program has been induced by injection of activin and XFD mRNA; 400 fg activin plus 400 pg XFD mRNA were injected into the animal pole at the 1-cell stage, caps were excised at stage 8 and cultured to stage 18/19 as judged by sibling embryos. Caps were fixed, embedded, and sectioned in wax. Alternate 10 × 10-μm sections were probed for SpiB (i,ii), Runx1 (iii,iv), and Fli1 (v,vi) by in situ hybridization on sections. Original magnification ×10 for subpanels i, iii, and v. Original magnification ×20 for panels ii, iv, v, and vi (the same sections).
Induction of the anterior hemangioblast program in animal caps requires signaling by activin in the absence of FGF. (A) Embryos at the 1-cell stage were injected in the animal pole with 200 to 400 fg activin mRNA either alone or in combination with 400 pg dominant-negative FGF receptor (XFD) mRNA. Embryos were grown to stage 8, and animal caps were excised and cultured in 1 × MBS to stage 18 and then processed for real-time RT-PCR analysis, probing for Scl (i), Runx1 (ii), SpiB (iii), Mpo (iv), Fli1 (v), and Flk1 (vi). Values represent the average of 3 experiments; error bars represent SD. (B) SpiB, Runx1, and Fli1 expression overlap in animal caps in which the anterior hemangioblast program has been induced by injection of activin and XFD mRNA; 400 fg activin plus 400 pg XFD mRNA were injected into the animal pole at the 1-cell stage, caps were excised at stage 8 and cultured to stage 18/19 as judged by sibling embryos. Caps were fixed, embedded, and sectioned in wax. Alternate 10 × 10-μm sections were probed for SpiB (i,ii), Runx1 (iii,iv), and Fli1 (v,vi) by in situ hybridization on sections. Original magnification ×10 for subpanels i, iii, and v. Original magnification ×20 for panels ii, iv, v, and vi (the same sections).
To check that the gene expression recorded by RT-PCR reflects coexpression in a population of putative hemangioblasts, induced animal caps were monitored by in situ hybridization to alternate 10μm serial sections (Figure 5B). We found that a significant proportion of the cells expressing any of the markers, SpiB, Runx1, or Fli1, were expressing all 3 genes, as seen for the anterior hemangioblast population in the embryo. However, only in 2 of 10 caps were hemangioblast genes strongly induced, weak induction was seen in another 3 caps, and the remaining 5 were negative for all hemangioblast gene expression. Clearly, conditions are still far from optimal with respect to the proportion of cells induced; nevertheless, we conclude that the response to activin and XFD of at least a subpopulation of animal cap cells does indeed recapitulate the programming of anterior hemangioblasts in the embryo.
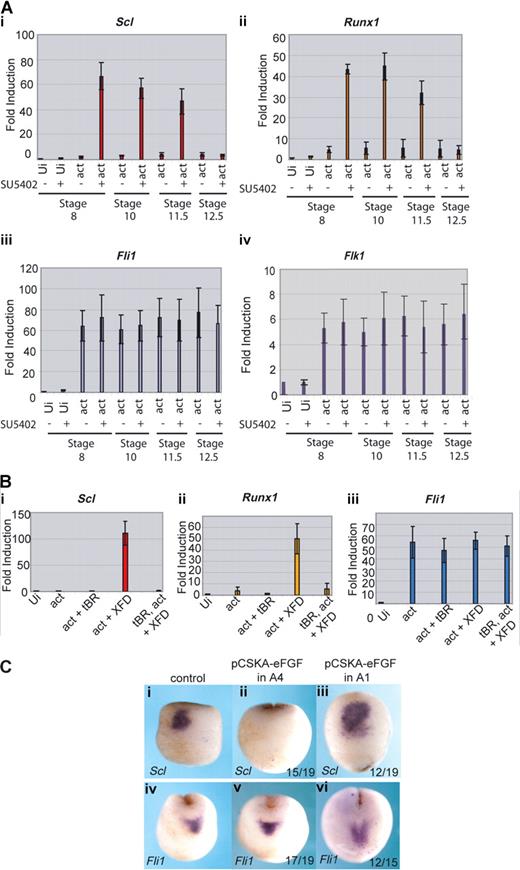
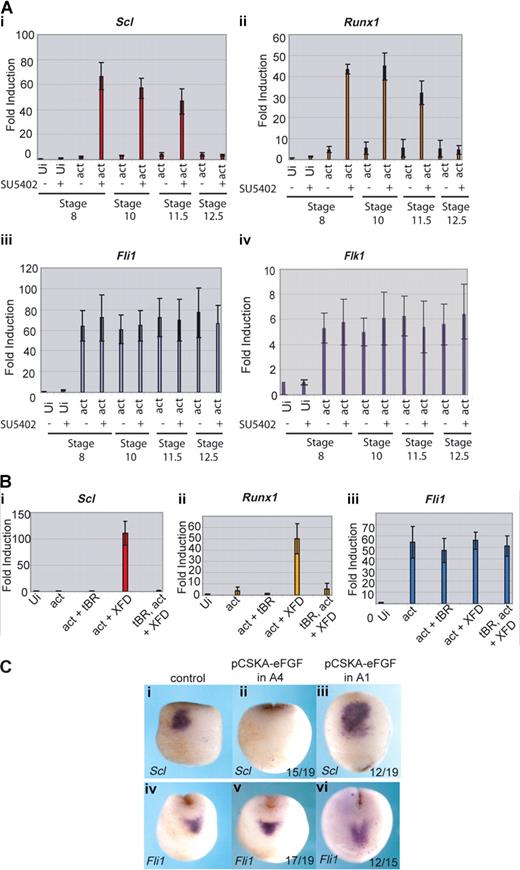
We have shown that, in the whole embryo, the block to FGF signaling needs to occur during gastrulation for the posterior embryo to express erythroid genes with the timing of the anterior embryo. To determine whether this is true of induction of the anterior hemangioblast program in animal caps, the inhibitor SU5402 was applied to the caps at stages 8, 10, 11.5, or 12.5. The activities of the activin RNA and the SU5402 inhibitor were monitored by cap morphology: activin elongated the caps and addition of SU5402 inhibited the elongation38 (data not shown). Quantitative RT-PCR shows that Scl and Runx1 were induced by activin plus the FGF inhibitor, as long as the inhibitor was present before or at stage 11.5 (Figure 6Ai,ii). However, if inhibitor was added at the end of gastrulation (at stage 12.5), erythroid genes were no longer induced in the caps. As controls, we monitored the endothelial genes, Fli1 and Flk1, whose expression in the anterior embryo does not depend on FGF signaling, and they were unaffected by inhibitor treatment at all time points (Figure 6Aiii,iv). Thus, as predicted from the embryo, the full anterior hemangioblast program induced in animal caps requires FGF signaling to be blocked during gastrulation.
FGF blocks erythroid gene expression by inhibiting its induction by BMP. (A) Erythroid gene expression is induced in caps when FGF signaling is blocked during gastrulation; 400 fg activin mRNA was injected into the animal pole of single cell embryos. At stage 8, animal caps were excised and cultured in the presence of 50 μM SU5402 or buffer from stage 8, 10, 11.5, or 12.5 until sibling embryos reached stage 18. Caps were processed for real-time RT-PCR probing for Scl (i), Runx1 (ii), Fli1 (iii), and Flk1 (iv). Ui- indicates uninjected; act, activin. Values represent the average of 3 experiments; error bars represent SD. (B) The induction of Scl and Runx1 by activin + XFD is BMP-dependent; 400 fg activin mRNA was injected with or without 1 ng tBR mRNA and 400 pg XFD mRNA. Animal caps were excised at stage 8 and cultured until sibling embryos reached stage 18. Explants were snap frozen and processed for real-time RT-PCR probing for Scl (i), Runx1 (ii), or Fli1 (iii). Ui indicates uninjected; tBR, truncated dominant negative BMP receptor; act, activin. Values represent the average of 3 experiments; error bars represent SD. (C) FGF expressed in presumptive ventral ectoderm late during gastrulation blocks Scl but not Fli1 expression in the anterior hemangioblast. Embryos were injected with 10 ng pCSKAefgf DNA either into both A4 blastomeres (ii,v) or both A1 blastomeres (iii,vi) at the 32-cell stage. Correctly targeted embryos were identified using coinjected GFP mRNA, and these embryos were grown to stage 17 alongside uninjected controls (i,iv) then probed for Scl (i,iii) or Fli1 (iv,vi) expression by whole-mount in situ hybridization. Scl, but not Fli1, expression was blocked when A4 was targeted (ii,v), whereas expression of both genes was unaffected when A1 was targeted (iii,vi). Numbers of embryos assayed is recorded in bottom right of each panel.
FGF blocks erythroid gene expression by inhibiting its induction by BMP. (A) Erythroid gene expression is induced in caps when FGF signaling is blocked during gastrulation; 400 fg activin mRNA was injected into the animal pole of single cell embryos. At stage 8, animal caps were excised and cultured in the presence of 50 μM SU5402 or buffer from stage 8, 10, 11.5, or 12.5 until sibling embryos reached stage 18. Caps were processed for real-time RT-PCR probing for Scl (i), Runx1 (ii), Fli1 (iii), and Flk1 (iv). Ui- indicates uninjected; act, activin. Values represent the average of 3 experiments; error bars represent SD. (B) The induction of Scl and Runx1 by activin + XFD is BMP-dependent; 400 fg activin mRNA was injected with or without 1 ng tBR mRNA and 400 pg XFD mRNA. Animal caps were excised at stage 8 and cultured until sibling embryos reached stage 18. Explants were snap frozen and processed for real-time RT-PCR probing for Scl (i), Runx1 (ii), or Fli1 (iii). Ui indicates uninjected; tBR, truncated dominant negative BMP receptor; act, activin. Values represent the average of 3 experiments; error bars represent SD. (C) FGF expressed in presumptive ventral ectoderm late during gastrulation blocks Scl but not Fli1 expression in the anterior hemangioblast. Embryos were injected with 10 ng pCSKAefgf DNA either into both A4 blastomeres (ii,v) or both A1 blastomeres (iii,vi) at the 32-cell stage. Correctly targeted embryos were identified using coinjected GFP mRNA, and these embryos were grown to stage 17 alongside uninjected controls (i,iv) then probed for Scl (i,iii) or Fli1 (iv,vi) expression by whole-mount in situ hybridization. Scl, but not Fli1, expression was blocked when A4 was targeted (ii,v), whereas expression of both genes was unaffected when A1 was targeted (iii,vi). Numbers of embryos assayed is recorded in bottom right of each panel.
BMP requirements for hemangioblast induction in pluripotent cells
We have previously shown that, in the anterior hemangioblast, erythroid (Scl) expression is dependent on BMP signaling whereas endothelial (Fli1) expression is not.13 To determine whether the induction of hemangioblast genes in animal caps reported here is dependent on BMP signaling, we used a dominant negative BMP receptor (tBR) to inhibit endogenous BMP in induced caps. Coexpression of tBR in caps injected with activin and XFD mRNAs ablated the expression of Scl and Runx1 (Figure 6Bi,ii), whereas expression of Fli1 occurred in the absence of BMP signaling (Figure 6Biii). Thus, the BMP requirements for hemangioblast induction in animal caps recapitulate those of the whole embryo.
FGF blocks erythroid gene expression in the anterior hemangioblast by antagonizing BMP signaling
One mechanism by which FGF might block erythroid gene expression is by blocking BMP signaling.39,40 To test this hypothesis, we used a construct expressing eFGF under the control of the cytoskeletal actin promoter (pCSKAefgf), which drives expression during gastrulation.32 We targeted the A4 blastomeres of the 32-cell embryo, which are thought to be the source of the BMP signal that induces the anterior hemangioblast population during gastrulation41 and separately to the A1 blastomeres, which are devoid of BMP as a control. Scl expression in the anterior hemangioblast at neurula stages was repressed when A4 blastomeres (Figure 6Cii) but not A1 blastomeres (Figure 6Ciii) were targeted; however, Fli1 expression was unaffected in the same cells (Figure 6Cv,vi). The same phenotype was observed when BMP signaling was blocked.13 These observations are consistent with a model whereby FGF exerts temporal control over erythroid gene expression in the posterior VBI by antagonizing BMP.
Discussion
A novel role for FGF in blood development
We show here that FGF signaling controls the timing of expression of Scl, Lmo2, and Runx1 in embryonic hemangioblasts. In Xenopus embryos, the expression of these genes continues only in the erythroid derivatives of these cells, and we have therefore referred to them as “erythroid” genes. However, they have a broader affiliation in lineages derived from the mouse yolk sac: for example, Runx1 is also expressed in myeloid derivatives, and Scl and Lmo2 in endothelial cells.42-44 Nevertheless, in both organisms, these genes are master regulators of hematopoiesis; therefore, the embryonic signals controlling their expression is of considerable interest.
The role reported here for FGF in blood development is distinct from that reported by Kumano and Smith,20 where FGF controls the boundary between blood and somite in posterior mesoderm, and blocking FGF expands blood into somite territory. At low levels of the inhibitor SU5402 (25-50 μM), we see an expansion of erythroid markers only in the ventral midline, a region that is never fated to be somite (Figure 2A). This activity therefore does not involve a fate change between blood and somite, rather a timing change between anterior and posterior midline blood compartments. Furthermore, overexpression of FGF anteriorly does not result in expansion of MyoD at neurula stages (Figure 4), again emphasizing that we are not dealing with a fate change between blood and somite. The 2 activities might overlap at high levels of inhibitor when ectopic erythroid expression begins to be expressed laterally as well as posteriorly (Figure 2A). In the study by Kumano and Smith,20 although they demonstrate ectopic expression of a leading edge mesoderm marker (blood is leading edge mesoderm) in somite territory at gastrula stages, this is not maintained in the later embryo; and although somite development is compromised (indistinct chevrons), there is no blood expression in somite regions. Because we know that somite development is dependent on FGF,45 we might expect a disruption to somite morphology independent of a fate change to blood.
Timing of Scl, Lmo2, and Runx1 expression in the posterior blood island
We have shown that, for the posterior VBI to turn on the erythroid program at the same time as the anterior VBI, FGF signaling must be blocked during gastrulation. The precursors of the posterior VBI are thought to be induced by low levels of nodal signaling and to involute very slowly during gastrulation (Figure 1). They are therefore exposed to prolonged FGF signaling in the vicinity of the blastopore,46 which prevents the cells from progressing with their erythroid program. Because FGF is thought to promote posterior identity during embryonic development,33 the premature expression of the erythroid program might be seen as a fate change whereby posterior tissues adopt a more anterior fate when FGF signaling is blocked. Lineage tracing of Scl-expressing cells supported this view because the anterior/posterior boundary within the VBI remained unchanged even though Scl expression had expanded (Figure 3A). However, a complete transformation from posterior to anterior fate would require the induction of myeloid expression posteriorly, and we did not see that. Premature endothelial gene expression did not occur either. We conclude that FGF temporal control is specific to the erythroid program posteriorly.
Recently, Isaacs et al reported an expansion of Scl expression as a result of blocking FGF signaling.47 No other blood or endothelial genes were examined. MyoD was measured but at a different time point from Scl. A reduction of MyoD expression at stage 12.5, alongside the expansion of Scl at stage 21 (∼10 hours later), was interpreted as reflecting the dorsoventral patterning activity of FGF. However, the 2 results need not be connected. The loss of MyoD is to be expected because FGF is needed for somite development and the expansion of Scl can be explained by our timing model. Indeed, when we measured the expression of MyoD and Scl simultaneously, there was no reciprocity between the 2, albeit in an overexpression study (Figure 4).
We and others have shown previously that erythroid gene expression in the VBI is positively regulated by BMP signaling.13,48-52 One mechanism, therefore, by which FGF could prevent the progression of the erythroid program is by antagonism of BMP signaling.39,40 In support of this, ectopic expression of FGF at a time and place in the embryo when and where the anterior hemangioblast program is induced by BMP, repressed the erythroid program without affecting the endothelial (Figure 6C). Such a mechanism could also explain why the expansion of erythroid expression resulting from a block to FGF signaling is dose-dependent, such that expansion occurs posteriorly at low levels of SU5402 and both posteriorly and laterally at higher levels (Figure 2A). At the highest concentration of drug, even prospective somite, located dorsolaterally, may be rendered competent to become blood as reported previously.20 A plausible explanation would be that FGF control over the erythroid program is most sensitive to inhibition at the ventral midline where BMP signaling is high, requiring higher levels of inhibitor more laterally where BMP signaling is lower31 (Figure 1).
When expression of erythroid and endothelial genes finally commences posteriorly, their expression is not colocalized in the same cells (ie, no posterior hemangioblast population is seen). A likely explanation for this is that the high level of BMP signaling posteriorly is incompatible with expression of the endothelial program, even in the presence of FGF/MAPK signaling (Figure 1). At later times, during late neurula and early tailbud, when FGF signaling is dropping, BMP signals in a concentration gradient emanating laterally from the ventral midline.31 The high concentration at the ventral midline, together with the falling level of FGF signaling, drives erythroid differentiation, whereas the reduced level of BMP signaling in more lateral cells permits expression of the endothelial program, which is favored by FGF.19,21 In this view, BMP acts to regulate emergence of the erythroid and endothelial programs both temporally, to allow expansion of the progenitor pool, and spatially to generate blood surrounded by endothelium.
A model for induction of the anterior hemangioblast program
The conclusion that FGF controls the emergence of the erythroid program posteriorly led to the prediction that recapitulation of the full anterior hemangioblast program in the animal cap system would require the inhibition of FGF signaling. The successful testing of this prediction is consistent with the following model for induction of this population of cells in the embryo (Figure 1). The anterior hemangioblasts derive from blastomeres C1 and D1 of the 32-cell embryo,13 whose progeny at the beginning of gastrulation are located at the dorsal blastopore lip, where they experience high levels of FGF and nodal signaling, and low levels of BMP resulting from the presence of BMP antagonists.53 These cells are the first to involute at the start of gastrulation and crawl quickly away from the source of BMP antagonists and FGF. Their journey takes them over the blastocoel roof where, around mid to late gastrulation, they encounter a burst of BMP signaling from animal pole ectoderm,13,41 which can be blocked by ectopic expression of FGF (Figure 6C). Thus, we propose that high levels of activin (nodal) induce anterior leading edge mesendoderm, which, early during gastrulation, involutes, escapes FGF signaling and, after receiving a BMP signal from animal cap ectoderm, becomes committed to the hemangioblast program.
The role of FGF in hemangioblast development
It has long been thought that induction of mesoderm by activin/nodal requires FGF signaling.38,54 Blood and endothelium are mesodermal derivatives, so the induction of hemangioblast mesoderm in caps by activin in the presence of the dominant negative FGF receptor (XFD) seems at first contradictory. The mesodermal marker, Brachyury (Xbra in Xenopus), is an immediate early and direct target of activin,55 and the maintenance of Xbra expression is dependent on an auto-regulatory loop between eFGF and Xbra.32,56 However, in the absence of FGF signaling, Xbra is transitorily expressed,56 suggesting that mesoderm can be induced in the absence of FGF signaling. Nevertheless, in view of the presence of active MAPK signaling in the dorsal leading edge mesendoderm at the time of involution and also in animal caps when they are cut,57 we conclude that brief but not prolonged exposure to FGF/MAPK signaling is compatible with, and likely required for, hemangioblast induction. The transient requirement for FGF/MAPK signaling is consistent with observations of differentiating mouse ESCs, where it favors hemangioblast generation but not their subsequent development,23 and where hemangioblast induction is optimal from mesoderm expressing the lowest levels of Brachyury.58 The observations made here show that this proposed pathway of differentiation mimics the embryo and that it can be used to program pluripotent cells in vitro in the absence of serum. Thus, these observations contribute to the goal of achieving fully defined conditions for the differentiation of pluripotent cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Aldo Ciau-Uitz, Feng Liu, and Tessa Peterkin (Oxford University, Oxford, United Kingdom) for critical reading of the manuscript, Aldo Ciau-Uitz for help with the SpiB and Mpo probes, Naoto Ueno (NIBB, Xenopus laevis Expression Sequencing Tag [EST] project) for the Mpo plasmid, Betsy Pownall (York University, York, United Kingdom) for pCSKAefgf, and Enrique Amaya (Birmingham University, Birmingham, United Kingdom) for Xt Spred 1 and 2.
This work was supported by the Medical Research Council (United Kingdom).
Authorship
Contribution: M.W. designed the project, performed experiments, prepared figures, and cowrote the paper; D.C. performed research, prepared figures, and contributed to the writing; and R.P. conceptualized and guided the project and cowrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Roger Patient, MRC Molecular Haematology Unit, Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Headington, Oxford OX3 9DS, United Kingdom; e-mail: roger.patient@imm.ox.ac.uk.