Abstract
Lumiliximab is a chimeric macaque-human monoclonal antibody to CD23, a protein expressed on virtually all chronic lymphocytic leukemia (CLL) cells. We examined the ability of lumiliximab to mediate apoptosis, antibody-dependent cellular cytotoxicity, and complement-dependent cytotoxicity against primary CLL cells and CD23-expressing B-cell lines. Our data suggest that lumiliximab kills CLL cells and CD23-expressing B cells predominantly by apoptosis, which occurs through the intrinsic pathway. Lumiliximab-induced apoptosis was accompanied by the down-regulation of antiapoptotic proteins Bcl-2, Bcl-XL, and XIAP, activation of Bax, and release of cytochrome c from the mitochondria. We also found that the addition of lumiliximab to rituximab or fludarabine results in synergistic cytotoxicity of primary CLL cells and CD23-expressing B-cell lines. We investigated the in vivo activity of lumiliximab in a human disseminated CD23+ B-cell lymphoma SCID mouse model and found greater antitumor activity with it than with control antibody. We also found that paralysis-free survival was greater with lumiliximab plus rituximab or fludarabine than with any of those agents alone. These results suggest that lumiliximab may be an effective treatment alone or in combination with rituximab or chemotherapy agents in CLL or other CD23-overexpressing B-cell malignancies.
Introduction
B-cell chronic lymphocytic leukemia (CLL), the most commonly diagnosed leukemia in the United States, accounts for 25% of all cases of leukemia in adults.1 A distinguishing feature of CLL is the accumulation of small, mature-appearing CD5+, CD19+, and CD23+ lymphocytes in the blood, bone marrow, and lymphoid tissues, which results from their failure to undergo programmed cell death or apoptosis.2,3 The survival of B cells in CLL is promoted by several signals that alter the intrinsic or extrinsic pathways of apoptosis. CLL cells inherently express high levels of the antiapoptotic protein Bcl-2, which contributes to their resistance to apoptosis and to poor clinical responses to chemotherapy.4 Signals from the microenvironment through CD44 or stromal cell–derived factor-1 (SDF1) have been shown to result in the up-regulation of the antiapoptotic protein Mcl-1, and signals through CD40 ligation result in the up-regulation of the antiapoptotic protein Bcl-XL.5,6 Thus, agents that can induce apoptosis in CLL cells, by either the direct stimulation of apoptotic pathways7,8 or the modification of the balance of antiapoptotic and proapoptotic proteins, are of great interest for therapeutic intervention in CLL.9
Studies showing that response rates were higher and progression-free survival was greater10 and that clinical remission rates were higher11 with fludarabine compared with conventional therapy using alkylating agents have led to fludarabine's use in first-line therapy for CLL. A recent phase 3 study has shown that response rates and progression-free survival are greater with the combination of fludarabine and cyclophosphamide than with fludarabine alone but also that the combination has greater hematotoxicity.12 Concurrent with efforts to increase therapeutic efficacy by combining chemotherapies with different modes of action has been the introduction of therapeutic antibodies for CLL, including rituximab and alemtuzumab. Rituximab, a chimeric monoclonal antibody to CD20, has modest activity as a single agent in CLL. Preliminary studies have shown higher complete response rates and greater progression-free survival with rituximab plus fludarabine13 or plus fludarabine and cyclophosphamide14,15 than with chemotherapy alone in historical controls. The addition of rituximab to fludarabine-based regimens has not been associated with significantly greater toxicity or immunosuppression.13-15 These combination strategies are now being studied in large phase 3 studies in symptomatic patients with previously treated or untreated CLL. The results with rituximab in CLL have led to interest in other B cell–specific antibodies for use in CLL.
CD23 is another potential target for antibody therapy in CLL. The CD23 antigen (also termed FcϵRII), a 45-kDa type II transmembrane glycoprotein that is expressed on several hematopoietic cell types, functions as a low-affinity receptor for IgE.16-18 CD23 has been shown to play a role in modulating the production of IgE by B cells.16 The CD23 protein is a member of the C-type lectin family, and it contains an alpha-helical coiled-coil stalk between the extracellular lectin-binding domain and the transmembrane region that oligomerizes membrane-bound CD23 as a trimer.17,18 CD23 can undergo proteolytic cleavage in the stalk region, resulting in the release of soluble forms of CD23 from the cell surface.17,19 In addition to its involvement in regulating the production of IgE, it has been postulated that CD23 has various biologic functions, including promoting the survival of germinal center B cells.20 The expression of CD23 is highly up-regulated in normal activated follicular B cells and in CLL B cells.21-23
Lumiliximab is a primatized monoclonal anti-CD23 antibody that contains cynomolgus macaque variable regions and human constant regions (IgG1, κ)24 that was developed to inhibit the production of IgE by activated human peripheral blood B cells.25,26 In studies in allergic rhinitis and asthma, lumiliximab had minimal toxicity but modest efficacy.27 Because of the broad expression of CD23 in CLL and the success with other B cell–selective antibodies such as rituximab and alemtuzumab in CLL, we sought to investigate whether lumiliximab could promote killing of primary CLL cells and related CD23+ transformed B-cell lines by initiating apoptosis, antibody-dependent cellular cytotoxicity (ADCC), or complement-dependent cytotoxicity (CDC). Furthermore, because of the widespread use of therapies such as rituximab and fludarabine in CLL, we sought to determine the efficacy of lumiliximab with each of those agents in vitro and in vivo, using a well-characterized xenograft model of lymphoma.
Methods
CLL cells
Blood was obtained from patients with CLL who gave informed consent after approval of the study protocol by the Institutional Review Board at the Scripps Cancer Center, La Jolla, CA, or Ohio State University, Columbus, OH. All patients had immunophenotypically defined CLL as defined by the modified 1996 National Cancer Institute criteria.28 CLL cells were isolated from peripheral blood mononuclear cells (PBMCs) using Ficoll-Hypaque gradient centrifugation. Briefly, 20 mL of blood was layered over 15 mL of Histopaque-1077 (Sigma-Aldrich, St Louis, MO) and was centrifuged at 300g for 20 minutes at room temperature. The gradient interface was harvested and was diluted 3-fold with Hank's Balanced Salt Solution (Biowhittaker, Walkersville, MD). The cell suspension was washed 3 times by repeated centrifugation at 300g for 10 minutes and was resuspended in Hank's Balanced Salt Solution. Cell viability by trypan blue dye exclusion was between 95% and 100%. The isolated cells were incubated (37°C and 5% CO2) in RPMI 1640 media (Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen), 2 mM l-glutamine (Invitrogen), and 100 U/mL penicillin and 100 μg/mL streptomycin (Sigma-Aldrich) for up to 18 hours before being used in the experiments. Freshly isolated CLL B cells, normal T cells, or normal PBMCs were used in all of the experiments.
Cell lines and mice
SKW6.4, SB, Daudi, Raji, Ramos, and DHL-4 B-lymphoma cells (American Type Culture Collection, Manassas, VA) were cultured in complete media: RPMI 1640 (Irvine Scientific, Santa Ana, CA) supplemented with 10% heat-inactivated fetal bovine serum (Hyclone, Logan, UT), 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. Severe combined immunodeficiency (SCID) mice were obtained from Simonsen Laboratories (Gilroy, CA).
Antibodies and chemicals
Lumiliximab is a chimeric macaque-human monoclonal antibody to human CD23 that contains human γ1 heavy chains and human κ light chains.24 Rituximab is a chimeric mouse-human γ1/κ antibody to human CD20.29 CE9.1 is a chimeric macaque-human antibody to human CD4 with human γ1 heavy chains and human κ light chains that is used as an isotype control for lumiliximab. The anti-CD52 antibody alemtuzumab (Genzyme Pharmaceuticals, Cambridge, MA) and the humanized anti–HER2-receptor monoclonal antibody trastuzumab, isotype IgG1κ (Genentech, San Francisco, CA) were obtained commercially. Phycoerythrin-conjugated anti–human CD23, phycoerythrin-conjugated anti–human CD20, and fluorescein isothiocyanate-conjugated anti–human CD19 were from BD Pharmingen (San Diego, CA). Fluorescein isothiocyanate-conjugated F(ab′)2 goat anti–human IgG γ chain-specific antibodies were obtained from Southern Biotechnology (Birmingham, AL). F(ab′)2 goat anti–human IgG Fc γ chain-specific antibodies were obtained from Jackson ImmunoResearch (West Grove, PA). Pharmaceutical-grade fludarabine (Fludara; Berlex Laboratories, Richmond, CA) was prepared as recommended by the supplier.
Cell treatment
CLL cells (106 cells/mL) or SKW6.4 cells (0.5 × 106 cells/mL) were incubated with lumiliximab or rituximab (0.01 μg/mL to 10 μg/mL) in assay medium (RPMI 1640 supplemented with 5% fetal bovine serum, 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin) for 1 hour on ice. Cells were centrifuged, resuspended in assay medium, incubated in the presence of F(ab′)2 goat antihuman IgG Fc γ chain-specific antibodies (15 μg/mL), and incubated in for 18 hours at 37°C. The different parameters for cell treatment were independently optimized. In the combination studies, lumiliximab and rituximab were added together or fludarabine was added when the secondary crosslinking antibody was added.
Apoptosis assays
Apoptosis was assessed using a flow cytometry–based caspase-3 assay. Cultured cells were harvested, washed, and fixed on ice in Cytofix (Cytofix/Cytoperm Kit, BD Pharmingen) according to the manufacturer's instructions. Flow cytometry data were acquired on a FACScan (BD Biosciences, San Jose, CA) and were analyzed using WinList software (Verity Software House, Topsham, ME) or on a FACSCalibur (BD Biosciences) and analyzed using CellQuest software (BD Biosciences).
For caspase inhibitor experiments, CLL cells were pre-incubated with 100 μM caspase inhibitor or dimethyl sulfoxide control for 30 minutes at 37°C, before treatment. After incubation and washing to remove unbound primary antibody, 100 μM caspase inhibitor was readded to the assay medium containing the secondary crosslinking antibody. Apoptosis was then measured by staining with annexin V. To calculate caspase inhibition of lumiliximab, maximal apoptosis was calculated in the presence of control dimethyl sulfoxide by subtracting apoptosis induced in the presence of the isotype control antibody (background apoptosis) from apoptosis induced in the presence of lumiliximab. For each patient sample, apoptosis induced in the presence of caspase inhibitors (minus background) was then normalized to this maximal value.
For annexin V assays, the detection of phosphatidylserine exposure was assessed by double-staining cells with fluorescein isothiocyanate-labeled annexin V and propidium iodide (Roche Biosciences, Palo Alto, CA). Flow cytometry data were acquired and analyzed as described above. Apoptotic cells were defined as the percentage of annexin V-positive/propidium iodide-negative cells.
ADCC assays
ADCC activity was determined by standard 4-hour chromium 51-release assays. 51Cr-labeled target CLL cells were incubated with various concentrations of antibodies for 20 minutes at 37°C, the unbound antibody was washed off, and 104 B-cell CLL cells per well were added to 96-well plates (51Cr 0.2 mCi/mL obtained from GE Healthcare, Chalfont St. Giles, United Kingdom). PBMCs from healthy donors were then added to the plates at indicated effector-to-target ratios. After a 4-hour incubation, supernatants were removed and counted in a gamma counter. The percentage of specific cell lysis was defined as % lysis = 100 × (ER[experimental release] − SR[spontaneous release])/(MR[maximal release] − SR). MR was calculated using 1% Triton X-100 to 51Cr-labeled lyse target cells. To test for the effect of IL-2 activation of effector cells on ADCC, effector cells were incubated with IL-2 (100 U/mL; Peprotech, Rocky Hill, NJ) for 18 hours before the ADCC experiments. In separate experiments, the effect of enrichment of the effector cell population for natural killer (NK) cells on ADCC was tested. NK cells from PBMCs were isolated by negative selection using RosetteSep (StemCell Technologies, Vancouver, BC) and Ficoll-Hypaque density centrifugation as described above. NK cells were activated by incubating them with IL-2 (100 U/mL) for 18 hours before the ADCC experiments.
Western blot analysis
Cell lysates were prepared in RIPA lysis buffer: 10 mM Tris (pH 7.4), 150 mM NaCl, 1% Triton X-100, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate, 5 mM EDTA, and protease inhibitors. The amount of protein in each clarified lysate was quantified using the bicinchoninic acid method (Pierce, Rockford, IL). Each sample was normalized for total protein content (12.5 μg/lane) and was subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (12% polyacrylamide gel) followed by transfer to Immobilon P membranes (Fisher Scientific, Pittsburgh, PA). Mouse monoclonal antibodies to human caspase-3 were obtained from Alexis Biochemicals (San Diego, CA). Rabbit polyclonal antibodies to poly(ADP-ribose) polymerase (PARP) and cleaved caspase-9 and mouse monoclonal antibodies to caspase-8 were obtained from Cell Signaling Technology (Beverly, MA). Anti-XIAP monoclonal antibodies were obtained from BD Transduction Labs (San Diego, CA). Anti-Bcl-2 monoclonal antibodies, anti-Mcl-1 monoclonal antibodies, and anticytochrome c monoclonal antibody LH8.2C12 were obtained from BD Pharmingen. Anti-Bax monoclonal antibody YTH-6A7 was obtained from Trevigen (Gaithersburg, MD) and monoclonal antibodies to Bcl-2, Bcl-XL, cIAP-1, and cIAP-2 from Santa Cruz Biotechnologies (Santa Cruz, CA). All antibodies were used at 1:1000 dilutions. Horseradish peroxidase-conjugated goat antimouse IgG and horseradish peroxidase-conjugated goat antirabbit IgG secondary antibodies were obtained from Bio-Rad Laboratories (Richmond, CA) and were used at 1:5000 dilutions. Detection was performed using an enhanced chemiluminescence assay (Amersham, Arlington Heights, IL). Equivalent protein loading was verified by probing the blot with an antibody to β-actin (Sigma-Aldrich).
Immunocytochemistry
Isotype control or lumiliximab-treated CLL cells were deposited onto Biocoat collagen-coated cover slips (Bedford, MA) by gentle centrifugation at 15g for 3 minutes with no brake. Coverslips were washed twice with phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde for 15 minutes, and washed twice with PBS before permeabilization with 0.01% Triton X-100 for 5 minutes. Coverslips were incubated with 10 μg/mL anti-Bax polyclonal antibody in 5% goat serum/PBS for 30 minutes at 37°C. After 3 washes with PBS, samples were incubated with a 1:2000 dilution of antirat IgG FITC secondary antibody and 1 nM DAPI for 30 minutes at RT. Coverslips were washed 3 times with PBS, once with ddH2O, and mounted onto glass slides with Prolong (Molecular Probes, Eugene, OR) before visualization on a BioRad confocal microscope.
Subcellular fractionation
Cells were fractionated by digitonin lysis, as described by Heibein et al.30 Cells were treated briefly with medium alone or lumiliximab, washed once in ice-cold PBS, and resuspended in 200 μL 0.025% digitonin for 5 minutes on ice. Samples were centrifuged for 5 minutes at 20 800 × g at 4°C, and supernatant (cytosol) and pellet (membrane) fractions collected for Western analysis.
Tumor model
A disseminated human lymphoma model in SCID mice was used for in vivo evaluation of antibody therapy. SKW6.4 cells (3 × 106 to 4 × 106) were intravenously injected into the tail veins of 6- to 8-week-old female SCID mice (10 mice per group). Mice in the single-agent treatment groups were given 100 μg, 200 μg, or 400 μg lumiliximab or rituximab. Mice in the combination-agent group were given 200 μg each of lumiliximab and rituximab. All antibodies were injected intraperitoneally in a final volume of 200 μL. Mice in the control group were given 400 μg of CE9.1 (isotype control for lumiliximab) in the same formulation buffer and volume as lumiliximab and rituximab. The antibody injections were given on days 1, 3, 5, 7, 9, and 11 or on days 3, 7, 11, and 15 after tumor inoculation. The doses were based on a minimum half-life of human IgG1 antibodies in SCID mice of 3 days and were calculated to achieve a steady-state trough level of 100 to 300 μg/mL on day 14. Fludarabine (50 mg/kg) was injected on days 5 and 10. A paralytic form of disseminated lymphoma developed in all of the mice before they died. Mice were monitored for the development of the disease and death. Mice that died between observation periods or in which full paralysis or labored breathing developed were killed and scored as dead.
Results
Lumiliximab mediates apoptosis of CLL and CD23+ transformed lymphoma cells
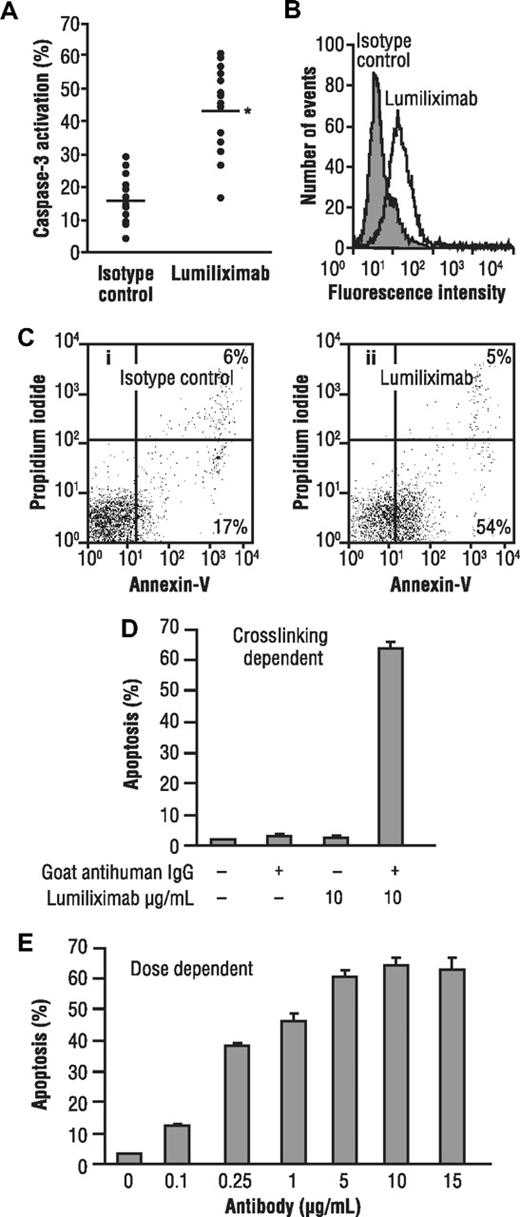
To test the ability of lumiliximab to induce apoptosis, CLL cells from 16 patients and a variety of lymphoma cell lines were incubated in the presence of lumiliximab or control antibody, followed by crosslinking with goat antihuman IgG antibody. Apoptosis was assessed 18 hours later using a flow cytometry-based assay that measures activated caspase-3, a marker for cells undergoing apoptosis. Treating CLL cells with lumiliximab versus control antibody resulted in greater staining for activated caspase-3 in CLL cells, as shown in Figure 1A. The median percent apoptosis induced by lumiliximab was 46% and by the isotype control was 16% (P < .001). No clear correlation was observed between CD23 expression and activation of caspase-3 in CLL patient samples. Data from a representative patient with CLL are shown in Figure 1B, in which the percentage of activated caspase-3–positive cells after treatment with lumiliximab was 55% compared with a background of 20% after treatment with an isotype control. These findings were corroborated by annexin V-propidium iodide staining (Figure 1C). Similar to the apoptosis observed in CLL cells, the transformed B-cell lines SKW6.4 and SB, which both express CD23, undergo apoptosis when treated with lumiliximab. Lumiliximab's ability to induce apoptosis in SKW6.4 B lymphoma cells was tested in the presence or absence of a secondary crosslinking antibody. SKW6.4 cells were incubated with 10 μg/mL lumiliximab with and without the addition of secondary crosslinking goat antihuman IgG antibody. After incubation for 18 hours, cells were analyzed for the presence of activated caspase-3. Induction of apoptosis by lumiliximab was dependent on crosslinking and was not detected in the absence of crosslinking (Figure 1D). The induction of apoptosis was dose dependent (Figure 1E). Lumiliximab-induced apoptosis was detected only in CD23+ B-cell lines (Table 1) and correlated with binding of lumiliximab to cells (data not shown). Of the 6 cell lines tested, 2 (SKW6.4 and SB) were positive for CD23 expression and lumiliximab binding and also for apoptosis induction by lumiliximab. These data show that lumiliximab selectivity induces apoptosis in CD23-expressing cells in vitro.
Induction of apoptosis by lumiliximab in CLL cells from patients with CLL and the transformed B-cell lines SKW6.4 and SB. (A) Freshly prepared CLL cells from 16 patients with CLL were incubated in the presence of 10 μg/mL lumiliximab or isotype control antibody. Apoptosis was measured using a flow cytometry-based assay for activated caspase-3 (*P value in t test, < .001). Data from a representative patient are shown in panels B and C. (B) Flow cytometric analysis of activated caspase-3 (open histogram, lumiliximab-treated; shaded histogram, isotype control). (C) Annexin V/propidium iodide staining: (i) an isotype control-stained sample and (ii) a lumiliximab-stained sample. (D) Induction of apoptosis by lumiliximab is crosslinking dependent. SKW6.4 cells (0.5 × 106 cells/mL) were incubated with 10 μg/mL lumiliximab, with or without F(ab′)2 goat anti–human IgG for 18 hours. (E) Induction of apoptosis by lumiliximab is dose dependent. Incubation with lumiliximab was at the indicated concentrations, and F(ab′)2 goat antihuman IgG was included in all samples for 18 hours. Percentage apoptosis was determined as described in “Apoptosis assays.”
Induction of apoptosis by lumiliximab in CLL cells from patients with CLL and the transformed B-cell lines SKW6.4 and SB. (A) Freshly prepared CLL cells from 16 patients with CLL were incubated in the presence of 10 μg/mL lumiliximab or isotype control antibody. Apoptosis was measured using a flow cytometry-based assay for activated caspase-3 (*P value in t test, < .001). Data from a representative patient are shown in panels B and C. (B) Flow cytometric analysis of activated caspase-3 (open histogram, lumiliximab-treated; shaded histogram, isotype control). (C) Annexin V/propidium iodide staining: (i) an isotype control-stained sample and (ii) a lumiliximab-stained sample. (D) Induction of apoptosis by lumiliximab is crosslinking dependent. SKW6.4 cells (0.5 × 106 cells/mL) were incubated with 10 μg/mL lumiliximab, with or without F(ab′)2 goat anti–human IgG for 18 hours. (E) Induction of apoptosis by lumiliximab is dose dependent. Incubation with lumiliximab was at the indicated concentrations, and F(ab′)2 goat antihuman IgG was included in all samples for 18 hours. Percentage apoptosis was determined as described in “Apoptosis assays.”
Lumiliximab does not mediate ADCC or CDC against CLL cells
Because of the potential importance of other mechanisms of action of therapeutic antibodies, we assessed the ability of lumiliximab to mediate ADCC and CDC against primary CLL cells. As shown in Figure 2, in contrast to rituximab or alemtuzumab, both of which mediate ADCC against primary CLL cells, lumiliximab at all effector cell ratios did not mediate ADCC, showing similar levels of ADCC as the receptor-negative control (ie, trastuzumab). Lumiliximab-mediated ADCC was not enhanced by IL-2 activation of effector cells (data not shown) or by enrichment of the effector cell population with IL-2-activated NK cells (data not shown). In a similar manner, we assessed the ability of lumiliximab to mediate CDC against primary CLL cells. Alemtuzumab but not lumiliximab or rituximab mediated CDC against primary CLL cells in the presence of human plasma (data not shown). Because lumiliximab did not mediate ADCC or CDC, we focused further experiments on the mechanism by which it mediates apoptosis.
Ability of lumiliximab to induce ADCC against primary CLL B cells. Lumiliximab does not induce ADCC against CLL B cells. The ability of trastuzumab, alemtuzumab, rituximab, or lumiliximab to mediate ADCC was evaluated using fresh human PBMCs as effector cells and CD19+ primary CLL B cells as target cells at the indicated effector/target (E/T) ratios. Mean plus or minus SD of 5 patient samples are shown.
Ability of lumiliximab to induce ADCC against primary CLL B cells. Lumiliximab does not induce ADCC against CLL B cells. The ability of trastuzumab, alemtuzumab, rituximab, or lumiliximab to mediate ADCC was evaluated using fresh human PBMCs as effector cells and CD19+ primary CLL B cells as target cells at the indicated effector/target (E/T) ratios. Mean plus or minus SD of 5 patient samples are shown.
Lumiliximab mediates caspase-dependent apoptosis through the mitochondrial death pathway
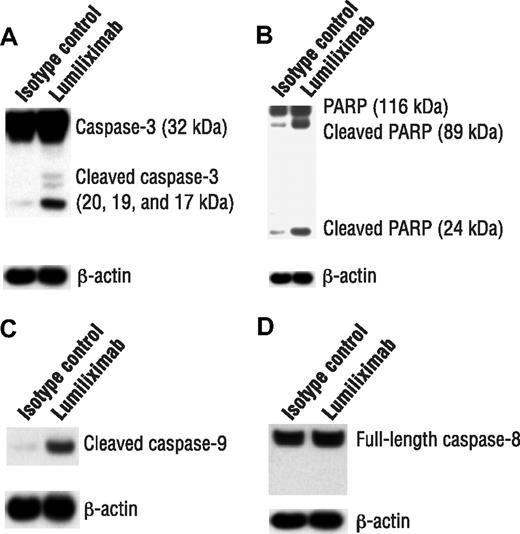
Cell death can occur by necrosis or through caspase-dependent and -independent apoptosis. CLL cells were examined after treatment with lumiliximab for evidence of caspase activation, by immunoblot analysis of caspase-3 zymogen cleavage and processing of PARP. Consistent with the flow cytometry results for caspase-3 activation, treatment with lumiliximab resulted in the cleavage of caspase-3 from its full-length 37-kDa form to its cleaved 20- and 17-kDa forms, indicating activation (Figure 3A). Treating CLL cells also resulted in the cleavage of PARP from its full-length 116-kDa form to its 89-kDa form (Figure 3B).
Induction of apoptosis markers by lumiliximab in CLL cells. CLL cells from 3 patients were assessed for induction of apoptosis markers by lumiliximab. Cells were incubated in the presence of 10 μg/mL lumiliximab or CE9.1 isotype control antibody, followed by crosslinking with F(ab′)2 goat-antihuman IgG. After 18 hours, cells were harvested and subjected to Western blot analysis with (A) anti–caspase-3, (B) anti-PARP, (C) anticleaved caspase-9, and (D) anticaspase-8 antibodies. A representative sample is shown in panels A-D.
Induction of apoptosis markers by lumiliximab in CLL cells. CLL cells from 3 patients were assessed for induction of apoptosis markers by lumiliximab. Cells were incubated in the presence of 10 μg/mL lumiliximab or CE9.1 isotype control antibody, followed by crosslinking with F(ab′)2 goat-antihuman IgG. After 18 hours, cells were harvested and subjected to Western blot analysis with (A) anti–caspase-3, (B) anti-PARP, (C) anticleaved caspase-9, and (D) anticaspase-8 antibodies. A representative sample is shown in panels A-D.
Apoptosis can be induced either through the extrinsic pathway, as in case of death receptor signaling, or through the mitochondrial pathway. The upstream caspase, caspase-8, activates the death receptor pathway, and caspase-9 activates the mitochondrial pathway. To determine which of these 2 upstream caspases was involved in lumiliximab-induced apoptosis, we looked for evidence of processed caspase-8 or caspase-9. A representative experiment is shown that indicates the cleavage of caspase-9 (Figure 3C) but not caspase-8 (Figure 3D) after treatment with lumiliximab. The findings were similar in the SKW6.4 cell line (data not shown). The findings of these studies suggest that lumiliximab induces apoptosis primarily through the mitochondrial pathway by activating caspase-9, which results in the cleavage and activation of caspase-3 and PARP.
Lumiliximab induces Bax activation and the release of proapoptotic mitochondrial proteins
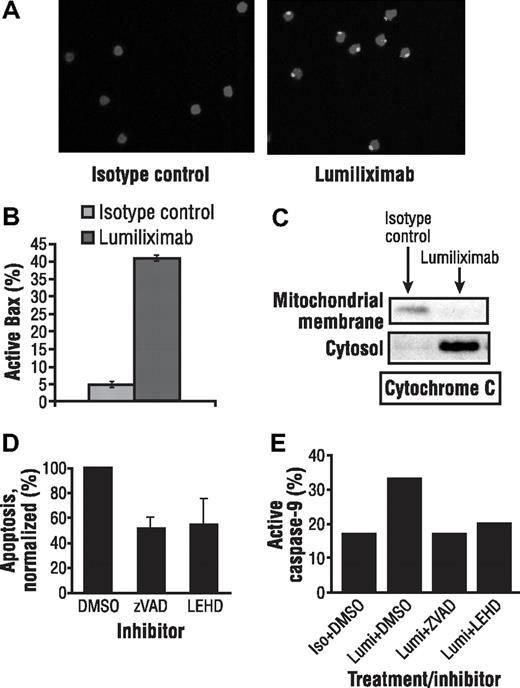
Immunofluorescence experiments using an antibody that recognized the activated conformation of Bax showed the presence of activated Bax in CLL cells treated for 4 hours with lumiliximab, but not in isotype control-treated CLL cells (Figure 4A). The presence of activated Bax was also confirmed using flow cytometric analysis of CLL cells treated with luiliximab or isotype control antibody (Figure 4B).
Lumiliximab induces activation of Bax in CLL cells. (A) CLL cells treated with isotype control or lumiliximab for 4 hours were fixed, permeabilized, stained with anti-Bax NT polyclonal antibody followed by secondary anti–rabbit Ig-FITC, and analyzed by confocal microscopy. (B) Increasing levels of active Bax were detected by intracellular flow cytometry at 2 and 4 hours after treatment with lumiliximab but not in cells treated with isotype control. (C) Lumiliximab treatment induces the translocation of cytochrome c. Cytosol and mitochondria-enriched membrane fractions were prepared as described in “Subcellular fractionation” from isotype control or lumiliximab-treated cells. Samples were analyzed by immunoblot with antibodies against cytochrome c. (D) Summary of caspase inhibition data showing mean plus or minus SD (n = 3). (E) Caspase-9 activation induced by lumiliximab was blocked in the presence of caspase inhibitors z-VAD-fmk (ZVAD) and z-LEHD-fmk (LEHD), confirming the activity of the caspase inhibitors used in these experiments.
Lumiliximab induces activation of Bax in CLL cells. (A) CLL cells treated with isotype control or lumiliximab for 4 hours were fixed, permeabilized, stained with anti-Bax NT polyclonal antibody followed by secondary anti–rabbit Ig-FITC, and analyzed by confocal microscopy. (B) Increasing levels of active Bax were detected by intracellular flow cytometry at 2 and 4 hours after treatment with lumiliximab but not in cells treated with isotype control. (C) Lumiliximab treatment induces the translocation of cytochrome c. Cytosol and mitochondria-enriched membrane fractions were prepared as described in “Subcellular fractionation” from isotype control or lumiliximab-treated cells. Samples were analyzed by immunoblot with antibodies against cytochrome c. (D) Summary of caspase inhibition data showing mean plus or minus SD (n = 3). (E) Caspase-9 activation induced by lumiliximab was blocked in the presence of caspase inhibitors z-VAD-fmk (ZVAD) and z-LEHD-fmk (LEHD), confirming the activity of the caspase inhibitors used in these experiments.
To determine whether Bax activation at mitochondria was accompanied by release of cytochrome c, subcellular fractionation studies were performed as described in “Subcellular fractionation.” Release of cytochrome c was detected by Western analysis in the cytosol of CLL cells treated for 2 hours with lumiliximab but not in cells treated with isotype control antibody (Figure 4C). Antibodies to Rho-GD1 and VDAC were used as markers for cytosolic and membrane fractions, respectively (data not shown). These data indicate that cytochrome c translocates from the membrane fraction to the cytosol after lumiliximab treatment.
Lumiliximab-induced apoptosis was reversed by caspase inhibitors
To investigate if the lumiliximab-induced apoptosis can be reversed using caspase inhibitors, CLL cells were treated with lumiliximab in the presence of the pan caspase inhibitor z-VAD-fmk. On average, in the presence of z-VAD-fmk and z-LEHD-fmk, lumiliximab-induced apoptosis was reduced to 51% and 54% of maximal apoptosis, respectively (Figure 4D). The caspase-inhibitory activity of the z-VAD-fmk and z-LEHD-fmk used in these experiments was confirmed in control experiments in which both inhibitors completely blocked lumiliximab-induced caspase-9 activation (Figure 4E). The results from these caspase inhibitor experiments support the hypothesis that lumiliximab induces apoptosis in a caspase-dependent manner.
Lumiliximab induces changes in the expression of antiapoptotic regulatory proteins
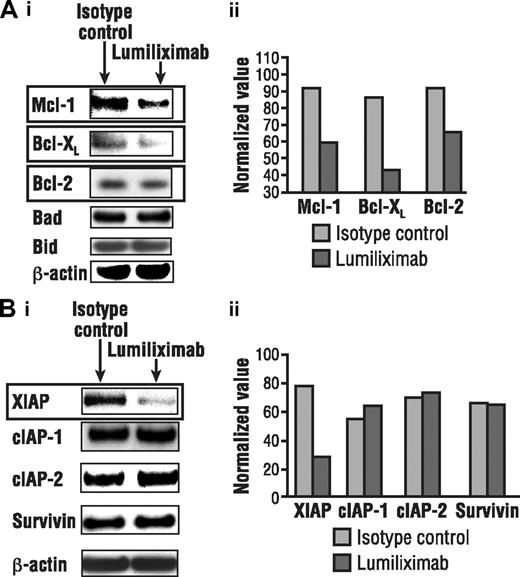
In light of data implicating the role of the mitochondrial pathway in lumiliximab-induced apoptosis, the ability of lumiliximab to modulate the expression of proteins that regulate the release of mitochondrial proteins was investigated. Western analyses revealed that lumiliximab induced the down-regulation of antiapoptotic proteins Bcl-2, Mcl-1, and XIAP. In contrast, the expression of several other antiapoptotic proteins, including cIAP-1, cIAP-2, and survivin, did not change after lumiliximab treatment in CLL cells (Figure 5). Proapoptotic Bcl-2 proteins also were examined; however, no significant changes in the levels of Bax, Bid, Bik, and Bad were observed (data not shown).
Lumiliximab down-regulates antiapoptotic proteins. CLL cells treated with lumiliximab for 18 hours were lysed and subjected to Western analysis for (A) Bcl-2 family and (B) inhibitor-of-apoptosis (IAP) proteins. Films were scanned and quantitated by normalizing to β-actin, using Scion software (Frederick, MD).
Lumiliximab down-regulates antiapoptotic proteins. CLL cells treated with lumiliximab for 18 hours were lysed and subjected to Western analysis for (A) Bcl-2 family and (B) inhibitor-of-apoptosis (IAP) proteins. Films were scanned and quantitated by normalizing to β-actin, using Scion software (Frederick, MD).
Lumiliximab enhances apoptosis mediated by rituximab and fludarabine
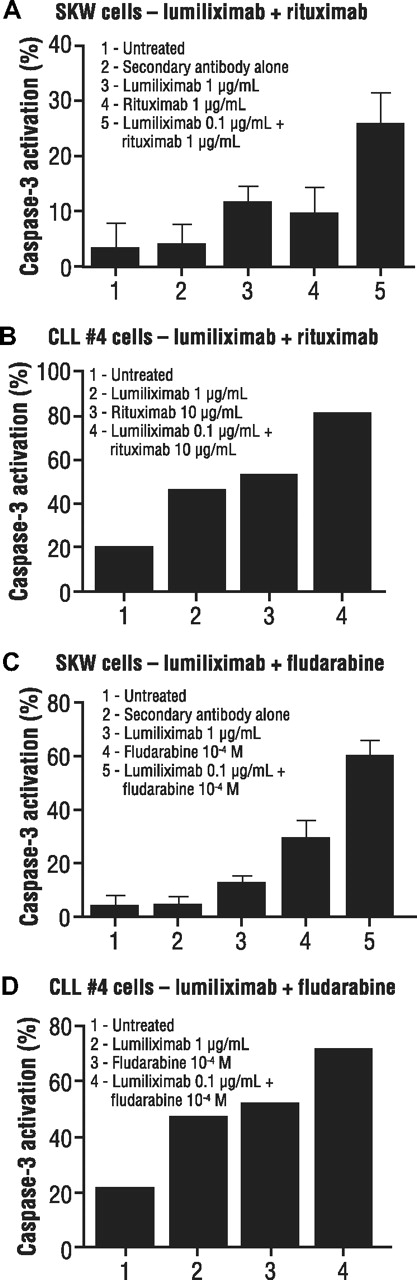
Because lumiliximab strongly induced apoptosis, we hypothesized that this agent might also add to the effectiveness of other agents that are used in treating CLL. Combinations of lumiliximab and rituximab tested in SKW6.4 cells showed greater induction of apoptosis with lumiliximab in combination with various concentrations of rituximab. A representative example is shown in Figure 6A. Studies of lumiliximab plus rituximab in 7 different patient samples demonstrated greater apoptosis induction than either rituximab or lumiliximab alone. A representative sample is shown in Figure 6B.
Induction of apoptosis in SKW6.4 lymphoma cells by combinations of lumiliximab, rituximab, and fludarabine. (A) SKW6.4 cells or (B) CLL cells were incubated in the presence of lumiliximab, rituximab, or both followed by crosslinking with F(ab′)2 goat antihuman IgG, as described in “Cell treatment.” Percentage apoptosis was determined after 18 hours using the caspase-3 flow cytometry–based assay as described in “Apoptosis assays.” (C) SKW6.4 cells or (D) CLL cells were incubated in the presence of lumiliximab, fludarabine, or both, as described in “Cell treatment.” Percent apoptosis was determined after 18 hours, as described in “Apoptosis assays.” In panels A and C, each error bar represents SD.
Induction of apoptosis in SKW6.4 lymphoma cells by combinations of lumiliximab, rituximab, and fludarabine. (A) SKW6.4 cells or (B) CLL cells were incubated in the presence of lumiliximab, rituximab, or both followed by crosslinking with F(ab′)2 goat antihuman IgG, as described in “Cell treatment.” Percentage apoptosis was determined after 18 hours using the caspase-3 flow cytometry–based assay as described in “Apoptosis assays.” (C) SKW6.4 cells or (D) CLL cells were incubated in the presence of lumiliximab, fludarabine, or both, as described in “Cell treatment.” Percent apoptosis was determined after 18 hours, as described in “Apoptosis assays.” In panels A and C, each error bar represents SD.
Combinations of lumiliximab and fludarabine were tested on both SKW6.4 cells and patient samples to determine their effect on apoptosis induction. As shown in Figure 6C, lumiliximab plus fludarabine resulted in greater apoptosis compared with either agent alone. Similar results were observed in CLL cells in which each agent alone induced apoptosis, but there was greater apoptosis with the combination of lumiliximab and fludarabine in all 7 patient samples tested (representative data shown in Figure 6D). This suggests that lumiliximab may synergize with rituximab and fludarabine to induce apoptosis in CD23-expressing cells, including CLL cells.
In vivo antitumor activity of lumiliximab as a single agent or in combination with rituximab or fludarabine
There are at this time no good in vivo models for testing the efficacy of antibodies for the treatment of CLL. We therefore tested the in vivo antitumor effect of lumiliximab in a disseminated lymphoma SCID mouse model using a cell line that expresses CD23. Human SKW6.4 B lymphoma cells are inoculated by tail vein into SCID mice; after inoculation, these cells disseminate throughout the mouse and grow primarily in the lungs and liver. Similar to other disseminated lymphoma models described in the literature, dissemination of SKW6.4 B lymphoma cells results in hind limb paralysis that is indicative of the terminal phase of the disease. Treatment with lumiliximab was begun 1 day after the inoculation and was repeated every 2 days for a total of 6 injections. Per Institutional Animal Care and Use Committee guidelines, mice that developed severe paralysis were killed; thus, we evaluated survival as “paralysis-free survival.”
The antitumor responses with 2 doses of single-agent lumiliximab are shown in Figure 7A. Paralysis-free survival was significantly greater with both doses of single-agent lumiliximab than with control (200 μg, P = .003; 400 μg, P = .001). In addition, lumiliximab 100 μg had similar antitumor activity as the 200-μg dose (data not shown), suggesting that the limiting dose was less than 100 μg. Furthermore, the antitumor response with lumiliximab was comparable with that with rituximab at the same dose and schedule (data not shown for rituximab). The effect of lumiliximab plus rituximab was also evaluated in the SKW6.4 disseminated model. Paralysis-free survival in mice treated with lumiliximab and rituximab was significantly longer than that in mice treated with rituximab 200 μg (P = .041) or lumiliximab 200 μg (P= .039) (Figure 7B). These in vivo experiments demonstrate that lumiliximab alone or in combination with rituximab has activity in the animal model. The enhancement of paralysis-free survival in the lumiliximab plus rituximab group corroborates the in vitro finding of modest synergy with these 2 antibodies (Figure 6).
Paralysis-free survival after treatment with lumiliximab, rituximab, fludarabine, and lumiliximab plus rituximab or lumiliximab plus fludarabine in a disseminated lymphoma mouse model. (A) On day 0, groups of mice (n = 10) were inoculated intravenously with 4 × 106 SKW6.4 cells. Mice in the rituximab or lumiliximab single-agent groups were given 200 μg or 400 μg of lumiliximab. Mice in the control group were given 400 μg isotype control antibody. Antibody injections were given on days 1, 3, 5, 7, 9, and 11 after tumor inoculation. (B) On day 0, groups of mice (n = 10) were inoculated intravenously with 3.6 million SKW6.4 cells. Mice in the lumiliximab single-agent group received 200 μg of lumiliximab intraperitoneally on days 3, 7, 11, and 15. (B) Mice in the rituximab group were given 200 μg of rituximab. Mice in the combination group were injected intraperitoneally with 200 μg each of lumiliximab and rituximab. (C) Mice in the fludarabine group were given 50 mg/kg on day 5 and day 10 after tumor inoculation. Mice in the combination group were injected intraperitoneally with 200 μg of lumiliximab and 50 mg/kg fludarabine according to the regimen described here. Mice in the control group were given 200 μg isotype control antibody according to the regimen described here. Antitumor response in this model is defined as “% Paralysis-free survival,” which represents the fraction of animals surviving without terminal paralysis or severe disease.
Paralysis-free survival after treatment with lumiliximab, rituximab, fludarabine, and lumiliximab plus rituximab or lumiliximab plus fludarabine in a disseminated lymphoma mouse model. (A) On day 0, groups of mice (n = 10) were inoculated intravenously with 4 × 106 SKW6.4 cells. Mice in the rituximab or lumiliximab single-agent groups were given 200 μg or 400 μg of lumiliximab. Mice in the control group were given 400 μg isotype control antibody. Antibody injections were given on days 1, 3, 5, 7, 9, and 11 after tumor inoculation. (B) On day 0, groups of mice (n = 10) were inoculated intravenously with 3.6 million SKW6.4 cells. Mice in the lumiliximab single-agent group received 200 μg of lumiliximab intraperitoneally on days 3, 7, 11, and 15. (B) Mice in the rituximab group were given 200 μg of rituximab. Mice in the combination group were injected intraperitoneally with 200 μg each of lumiliximab and rituximab. (C) Mice in the fludarabine group were given 50 mg/kg on day 5 and day 10 after tumor inoculation. Mice in the combination group were injected intraperitoneally with 200 μg of lumiliximab and 50 mg/kg fludarabine according to the regimen described here. Mice in the control group were given 200 μg isotype control antibody according to the regimen described here. Antitumor response in this model is defined as “% Paralysis-free survival,” which represents the fraction of animals surviving without terminal paralysis or severe disease.
In addition, because of the in vitro synergy observed with lumiliximab and fludarabine or rituximab, we also investigated the combination of lumiliximab plus fludarabine in the SKW6.4 SCID mouse model. Although statistical significance was not achieved in mice treated with lumiliximab and fludarabine, an improved overall trend in paralysis-free survival was observed compared with that in mice treated with fludarabine or lumiliximab alone (Figure 7C). This finding provides additional support for the in vitro data (Figure 6C,D). Taken together, our data provide support for combining lumiliximab with either fludarabine or rituximab.
Discussion
We have shown that lumiliximab, a chimeric macaque-human anti-CD23 antibody, induces apoptosis in both primary CLL cells and CD23+ B-cell lymphoma cell lines. In contrast, ADCC with nonstimulated or stimulated effector cells and CDC were not seen in primary CLL cells. We have also shown that lumiliximab-induced apoptosis in both CLL and CD23+ lymphoma cell lines is caspase-dependent and occurs through the classic mitochondrial caspase-dependent apoptotic pathway. Lumiliximab-induced apoptosis may have contributed to its antitumor activity observed in the SKW6.4 xenograft model of disseminated B-cell lymphoma model, in which paralysis-free survival at all doses of lumiliximab tested was significantly higher than that of the control. Furthermore, lumiliximab plus rituximab or fludarabine showed enhanced paralysis-free survival in the same SKW6.4 xenograft model. These data support further clinical investigation of lumiliximab in the treatment of CD23+ B-cell malignancies, including CLL.
The mechanism by which apoptosis is induced by the ligation of membrane-bound CD23 by lumiliximab merits further investigation. Other therapeutic antibodies, such as rituximab, depend on intracellular signaling, probably through calcium-dependent pathways, to mediate apoptosis in transformed B cells. In our in vitro studies, we found that the induction of apoptosis by lumiliximab required crosslinking with a secondary antibody, similar to that required with both rituximab and alemtuzumab. This also probably occurs in vivo because lumiliximab bound to cell-surface CD23 is expected to be crosslinked by Fcγ receptors on NK cells and other effector cells. Indeed, treating CD23+ B lymphoma cell lines with lumiliximab in vitro in the presence of FcγIII-expressing cells also resulted in the induction of apoptosis.31 The ligation of CD23 on CLL cells by lumiliximab followed by crosslinking may directly induce signals from CD23 that activate the caspase cascade. The induction of negative signaling that is mediated by crosslinking the low-affinity IgG receptor FcγRIIB (CD32) has been attributed to signaling from the immunoreceptor tyrosine-base inhibitory motif (ITIM) in its cytoplasmic domain.32-34 It is possible that lumiliximab-dependent crosslinking of CD23 also induces signaling through the 4-amino acid YSEI sequence ITIM domain in the cytoplasmic portion of CD23.17,18 Studies examining ITIM signaling through both of these pathways are warranted.
Our data show that lumiliximab, unlike alemtuzumab, relies predominantly on inducing apoptosis through the intrinsic pathway of apoptosis. Studies by others and by our group have shown that both fludarabine and rituximab also use the intrinsic pathway of apoptosis35,36 ; however, they may modulate this pathway in different ways. In this study, we found that lumiliximab induced the down-regulation of antiapoptotic proteins Bcl-2, Mcl-1, and XIAP in CLL cells. One of the defining characteristics of CLL is increased resistance to apoptosis arising both from microenvironmental signals as well as intrinsic alterations in the cell,37 which might account for the lack of robust single-agent activity with CLL therapeutics. Thus, the ability of lumiliximab to down-regulate these antiapoptotic proteins may contribute to its favorable activity in combination with other CLL therapeutics.
Regulation of mitochondrial apoptosis is often accompanied by changes in the conformation of Bax, an antiapoptotic member of the Bcl-2 family. Although we did not find any changes in overall Bax expression after lumiliximab treatment in vitro (data not shown), lumiliximab treatment of CLL cells was accompanied by increased levels of activated Bax. The activation of Bax was accompanied by the release of cytochrome c from the mitochondria. These data are consistent with an involvement of the mitochondrial pathway in lumiliximab-induced apoptosis. Because the addition of caspase inhibitors did not completely abrogate apoptosis, it is possible that other caspase-independent pathways are also involved in lumiliximab-induced apoptosis in CLL.
The most important finding of our study is that lumiliximab has synergy both in vitro and in vivo with fludarabine and rituximab. Both of these agents are now used in the first-line treatment of CLL, and phase 2 studies have shown greater complete response rates and progression-free survival in patients treated with them than in historical controls who had been treated with older monotherapy for CLL.13 A phase 1 trial of single-agent lumiliximab in CLL found that it lacked significant myelosuppression, immunosuppression, or toxicity that would preclude its being combined with rituximab or fludarabine.38 Lumiliximab therapy is also associated with a relatively low rate of infusion reactions,27 which may be a result of its lack of ADCC mediation. Developing a combination regimen of lumiliximab, fludarabine, and rituximab, therefore, seems rational. This strategy is supported by findings in the clinic. A recent multicenter dose-escalation phase 1/2 study of lumiliximab combined with fludarabine, cyclophosphamide, and rituximab for relapsed CD23+ B-cell CLL has shown promising results.39 The study was conducted in 31 patients, 8 of whom had advanced-stage disease. The overall response rate was 71% (complete response, 52%; partial response, 10%; and unconfirmed partial response, 10%). The 52% complete response rate compares favorably with the 25% complete response rate that was seen in a study conducted at the M. D. Anderson Cancer Center in 177 patients with relapsed or refractory CLL who were treated with fludarabine, cyclophosphamide, and rituximab.39 Safety was also similar in the 2 studies. The results of the current preclinical study also support combining lumiliximab with fludarabine or rituximab, or both. As the treatment of CLL moves toward combination regimens with both biologic and chemotherapy agents, lumiliximab may be a valuable addition to standard and novel therapies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Loic Scales, Aihua Zou, Tracey Murphy, and Lisa Berquist for their technical assistance.
This work was supported in part by research funding from Biogen Idec (J.B.).
Authorship
Contribution: N.I.P., K.H., P.C., C.C., A.M., and J.B. were responsible for the study design; N.I.P., P.C., C.C., and J.B. performed the research; N.I.P., P.C., and C.C. were responsible for data collection; N.I.P., P.C., C.C., and K.H. analyzed the data; N.I.P. and J.B. wrote the paper.
Conflict-of-interest disclosure: N.I.P., K.H., P.C., and A.M. are or were employed by a company (Biogen Idec) whose potential product was studied in the present work.
Correspondence: Nuzhat I. Pathan, Department of Oncology Cell Signaling, Biogen Idec, 5200 Research Pl, San Diego, CA 92122; e-mail: nuzhat.pathan@biogenidec.com.