In the current issue of Blood, Kissa and colleagues provide the first live imaging of hematopoietic stem cell (HSC) migration between different sites of hematopoiesis in zebrafish.
The process of HSC generation and subsequent migration to hematopoietic organs during mammalian development has been a topic of intense study for many years.1 In mice, these studies relied on ex vivo cell cultures or transplantation schemes to demonstrate stem-cell potential of sorted cell populations. However, these approaches pose inherent problems. For example, observed differences in engraftment potential of hematopoietic cells from the yolk sac likely reflect variations in experimental set-up and are thus not entirely conclusive.1 An animal model in which HSC generation and migration can be directly visualized in vivo is therefore desirable. The zebrafish (Danio rerio), with its embryonic transparency, rapid development, and hematopoietic sites equivalent to those of mammals (see figure) presents just such a model. Recent work by the group led by Phillipe Herbomel2 identified the previously uncharacterized zebrafish fetal liver equivalent—caudal hematopoietic tissue (CHT)—using in vivo uncaging techniques in a CD41:eGFP transgenic line that labels HSCs during early development.3 A similar strategy has identified the earliest definitive progenitors in the zebrafish posterior blood island.4
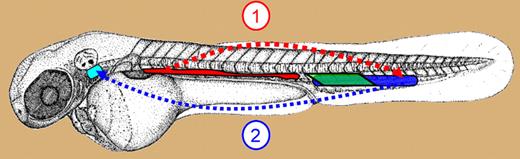
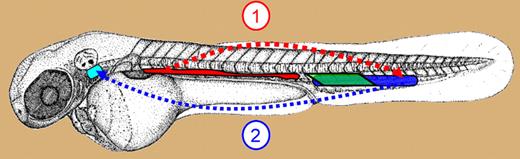
Sites of definitive hematopoiesis in zebrafish embryo. Schematic showing the anatomic location of the AGM/DP joint (red), PBI (green), and CHT (blue) in zebrafish embryo. Cells are visible in the AGM at 26 hours after fertilization (hpf). AGM starts releasing CD41 expressing, migration-competent HSCs at 32 hpf into the PCV (dotted red arrow). HSCs in the CHT uncaged after 44 hpf migrate (dotted blue arrow) to thymus (turquoise) via PCV and mesenchyme. Cells with erythroid and myeloid potential are seen in the PBI between 24 hpf and 40 hpf.
Sites of definitive hematopoiesis in zebrafish embryo. Schematic showing the anatomic location of the AGM/DP joint (red), PBI (green), and CHT (blue) in zebrafish embryo. Cells are visible in the AGM at 26 hours after fertilization (hpf). AGM starts releasing CD41 expressing, migration-competent HSCs at 32 hpf into the PCV (dotted red arrow). HSCs in the CHT uncaged after 44 hpf migrate (dotted blue arrow) to thymus (turquoise) via PCV and mesenchyme. Cells with erythroid and myeloid potential are seen in the PBI between 24 hpf and 40 hpf.
Kissa and colleagues couple in vivo DIC video microscopy with uncaging of HSCs in the CD41:eGFP transgenic line, extending their previous work to provide novel and surprising findings that differ from observations in mice. For example, intraaortic cell clusters were not observed; rather, HSCs develop in the subaortic mesenchyme within the AGM/DP joint from where they enter circulation by intravasation through the axial vein (PCV). Competence for migration into the PCV coincides with CD41 expression by c-myb+ HSCs in the AGM/DP joint. Following their entry into the PCV, HSCs reach the CHT via circulation. However, they migrate to the thymus from the CHT primarily through the mesenchyme, and less frequently after extravasation from thymus-proximal venules (see figure). Once they reach the thymus, CD41:eGFP cells move both in and out of the thymus “as bees around their hive” until they reach a more stagnant state, presumably when rag expression begins and they become committed to the T-cell fate.
The data raise interesting questions that will help advance our understanding of HSC biology. For example, does the coincidence of HSC migration competency and CD41 expression in the AGM/DP joint indicate a role for the CD41 integrin in HSC migration? The PCV is “open” several hours before HSCs leave the AGM/DP joint, but c-myb+ HSCs cells enter circulation only once they express CD41, suggesting that this process may require CD41. Morpholino-mediated CD41 gene knockdown is a straightforward experiment in zebrafish and may provide a speedy answer. Another question raised by these data are whether the CD41+ cells in the thymus maintain multipotency similar to fetal liver-derived progenitors previously described in mice.5 Kissa et al elegantly demonstrate the power of in vivo HSC imaging in zebrafish and underscore the strength of the zebrafish model for research in hematopoiesis.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■