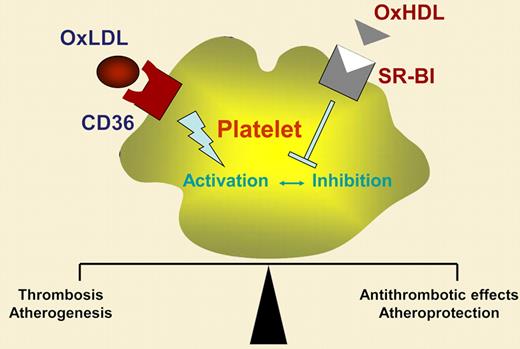
Platelets play a critical role in thrombotic diseases such as myocardial infarction or ischemic stroke. Beyond their role in thrombosis, platelets are the major trigger in boosting atherosclerosis.2 In patients with hypercholesterolemia, platelets are hyperreactive, indicating that circulating lipoproteins in blood influence platelet properties. Lipid-lowering drugs result in a reduction of platelet reactivity in these patients. Low-density (LDL), very low density (VLDL), and especially oxidized low-density lipoprotein (OxLDL), which all contain apoprotein B-100, enhance platelet activation, whereas high-density lipoprotein (HDL) has known antiplatelet effects.3 Lipoproteins bind to the platelet surface and are internalized via specific scavenger receptors. A variety of scavenger receptors have been detected on platelets, including CD36, SR-BI, and lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1).4,5 OxLDL preferentially binds to CD36 and LOX-1, and activates platelets via these scavenger receptors. SR-BI has been recognized as a receptor primarily for HDL, an interaction that exerts antiplatelet activity. Thus, platelet-derived scavenger receptors may play a crucial role in thrombosis and atheroprogression.
Valiyaveettil and colleagues have now identified an important mechanism allowing HDL to attenuate platelet activation. The authors' idea was based on previous findings from others indicating that HDL inhibits platelet function and that reduced levels of the HDL-binding scavenger receptor SR-BI are associated with increased platelet aggregation.6 In their unique work, Valiyaveettil and coworkers convincingly show that oxidized, but not native, HDL inhibits agonist-induced platelet activation. The inhibitory potential of OxHDL was dependent on the agonist used to activate platelets in vitro. Whereas adenosine diphosphate (ADP)– or thrombin-induced platelet activation was substantially reduced, collagen-dependent platelet aggregation was only marginally attenuated. In further studies with knock-out mice, the authors identified SR-BI as the platelet scavenger receptor that mediates the antiplatelet activity of OxHDL. Interestingly, the antiplatelet effect of OxHDL/SR-BI communication was independent of the eNOS/Akt pathway, known to play a role in HDL/SR-BI–induced eNOS activation in endothelial cells. Although the work by Valiyaveettil and colleagues implicated OxHDL as an antithrombotic strategy, data validating their findings (eg, in mouse models of thrombosis) are lacking so far.
In summary, OxHDL, but not native HDL, has potent antiplatelet effects via platelet scavenger receptor SR-BI. This is a novel and intriguing finding that broadens our knowledge of how platelet-mediated hemostasis and thrombosis are regulated, especially in patients with hypercholesterolemia. The authors' findings open a new research area focusing on platelet scavenger receptors as promising antiplatelet targets. Understanding the (patho)physiologic role of platelet scavenger receptors (see figure) may not only be of interest to scientists in the field of antithrombotic research. Because platelets are the major trigger of atherosclerosis, targeting platelet scavenger receptors may offer a promising strategy to treat and attenuate atheroprogression.
Hypothelial role of OxHDL/CD36 and OxHDL/SR-BI interaction in regulation of platelet function.
Hypothelial role of OxHDL/CD36 and OxHDL/SR-BI interaction in regulation of platelet function.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■