We sought kinase domain (KD) mutations at the start of treatment with dasatinib in 46 chronic myeloid leukemia (CML) patients resistant to or intolerant of imatinib. We identified BCR-ABL mutant subclones in 12 (26%) cases and used pyrosequencing to estimate subsequent changes in their relative size after starting dasatinib. Four patients lost their mutations, which remained undetectable, 3 patients retained the original mutation or lost it only transiently, 3 lost their original mutations but acquired a new mutation (F317L), and 2 developed another mutation (T315I) in addition to the original mutation within the same subclone. This study shows that expansion of a mutant Ph-positive clone that responds initially to a second generation tyrosine kinase inhibitor may be due either to late acquisition of a second mutation in the originally mutated clone, such as the T315I, or to acquisition of a completely new mutant clone, such as F317L.
Introduction
A significant minority of newly diagnosed patients with chronic myeloid leukemia (CML) in chronic phase (CP) fail to respond adequately or lose their response to imatinib mesylate (IM) after an initial reduction in leukemia cell load. Some of these patients will still respond to one or other of the newer tyrosine kinase inhibitors (TKI). Resistance to IM is due to various possible mechanisms,1,,–4 but perhaps the best characterized is expansion of a subclone bearing a BCR-ABL kinase domain (KD) mutation that impairs IM binding.5,,–8 Here we report finding KD mutations in 12 patients judged to have failed imatinib. The kinetics of the mutant subclones evolved very differently after the start of dasatinib.
Methods
Patients
We studied 46 consecutive CML patients judged to be resistant to or intolerant of imatinib, who started treatment with dasatinib at the Hammersmith Hospital in London between April 2005 and July 2006. Dasatinib was administered at a dose of 70 mg twice daily by mouth, but dosage was reduced if necessary in the presence of myelosuppression or for other toxic effects. The majority of patients were treated with dasatinib in a phase 2 multicenter study sponsored by the manufacturer and approved by the Research Ethics Committee of the Hammersmith Hospital. All patients gave informed consent for their participation in accordance with the Declaration of Helsinki.
RQ-PCR for BCR-ABL transcripts and kinase domain mutation analysis
cDNA was synthesized from peripheral blood or bone marrow aspirates and subjected to real-time quantitative polymerase chain reaction (RQ-PCR) as previously described.9 cDNA from samples from patients with evidence of resistance to TKI were screened for KD mutations by direct sequencing and subjected to quantitative single nucleotide polymorphism (Q-SNP) analysis by pyrosequencing if appropriate, as detailed previously.10 If a patient's therapy was changed but he/she achieved no lasting response, cDNA was rescreened by direct sequencing and subjected to Q-SNP analysis as necessary.10
Cloning of the BCR-ABL kinase domain
When the results of Q-SNP studies suggested that 2 mutations were present in the same allele, an 863-bp amplicon containing the entire KD generated by nested PCR was cloned using TOPO TA Cloning kit (Invitrogen, Glasgow, United Kingdom) in accordance with recommendations from the manufacturer. Following transformation of competent cells followed by 2-mL overnight cultures, the plasmid DNA was extracted using SV Minipreps (Promega, Southampton, United Kingdom) as recommended by the manufacturer. DNA was subsequently subjected to Sanger sequencing reaction as described previously10 to sequence the KD.
Restriction fragment length polymorphism analyses
The loss or gain of a restriction site due to base substitution was used to confirm the coexistence of the 2 mutations in a single mutant subclone. These studies were performed on 450-bp amplicon from residues 220 to 370 amplified by nested PCR, using standard methods and endonuclease in accordance with manufacturer's recommendations.
Results and discussion
We detected KD mutations by direct sequencing at the time of starting dasatinib in 12 of the 46 patients, of whom 6 were in CP and 6 in more advanced phases (Table 1). In the remaining34 patients no mutations were found at the time of starting dasatinib or thereafter. Of the 12 patients with mutations, 8 were still taking dasatinib at the time of this analysis for a median period of more than 11 months (range 6+ to 25+), including 5 of the 6 patients still in CP when they started dasatinib. The drug had been discontinued in 4 patients.
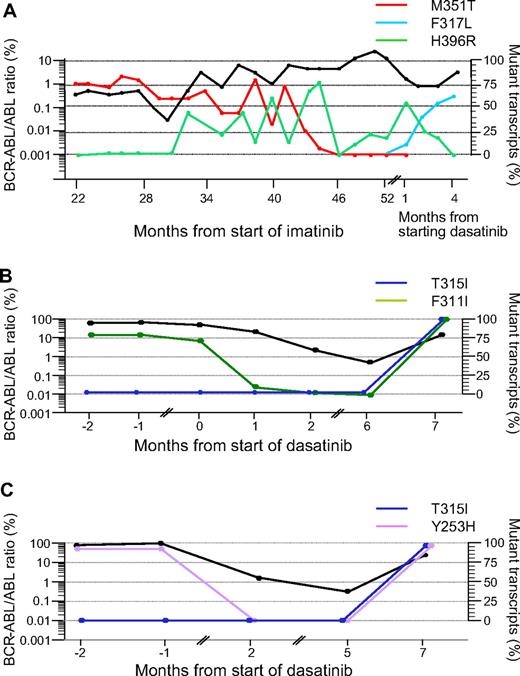
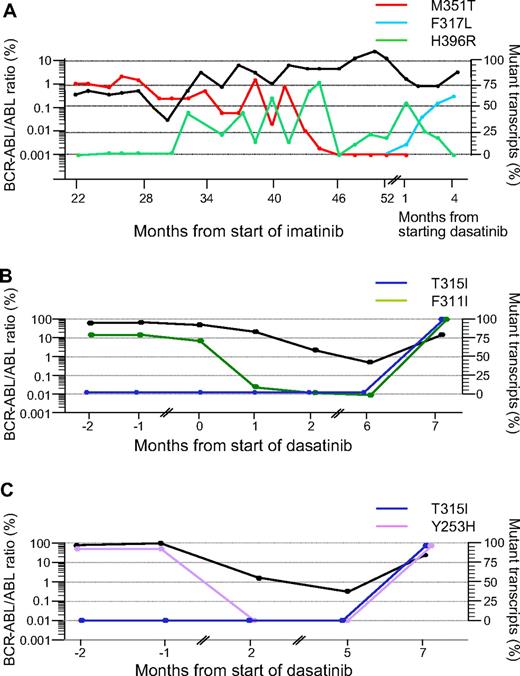
In these 12 patients we identified 4 patterns of evolution after starting dasatinib: (1) In 4 patients (nos. 1–4) the mutant clone identified before starting dasatinib became undetectable and remained so during the duration of follow-up. This was associated with a clinical response and a more than 2-log reduction in BCR-ABL transcripts compared with the predasatinib transcript level. (2) In 3 patients (nos. 5–7) the mutant clone identified before the start of dasatinib persisted during treatment or became transiently undetectable and then recurred. The occurrence of a mutation does not always precede clinical relapse. (3) In 3 patients (nos. 8–10) the original mutated clone disappeared, but a new mutant clone was acquired, which was F317L in each case (Figure 1A. (4) In 2 patients (nos. 11 and 12) a new mutation (T315I) appeared in addition to the original mutation in the same clone (Figure 1B,C).
Kinetics of mutations in mutant subclones in 3 representative patients. The kinetics of the BCR-ABL transcripts measured by RQ-PCR are represented by the bold black lines in all 3 panels (expressed as a ratio to the ABL control transcripts × 100%9 ). The proportions of the subclones with the various mutations measured at different time points are represented by the colored lines. (A) Patient no. 10. A 55-year-old female with CML in chronic phase was started on 400 mg daily IM. After 30 months from start of IM the M351T mutant clone had decreased from 80% to 50%, but an additional mutation, H396R, was identified. Subsequently, the 2 mutations evolved discordantly consistent with 2 distinct subclones. After starting dasatinib only H396R mutation was detectable. One month from start of dasatinib therapy, H396R became undetectable, a third mutant subclone, F317L, was detected by Q-SNP. (B) Patient no. 11. A 53-year-old female achieved a partial cytogenetic response to 400 mg daily IM but despite increasing the dose to 600 mg, she entered myeloblastic transformation. Dasatinib was subsequently introduced at 70 mg twice daily, which induced a reduction in BCR-ABL transcripts over the next 6 months, and the F311I mutation became transiently undetectable by Q-SNP. Subsequently, the BCR-ABL levels increased, and the patient was found to have T315I mutation in addition to F311I. The fact that the levels of the 2 mutations coincided closely in the terminal phase of her disease suggests that they were present in the same allele. This was confirmed by RFLP studies and sequencing of cloned PCR products. The patient eventually died in blastic transformation. (C) Patient no. 12. A 23-year-old woman achieved partial cytogenetic response with IM therapy but failed to maintain the response. She then progressed rapidly to lymphoblastic transformation and underwent sibling allogeneic hematopoietic stem cell transplant (HSCT). Four months after HSCT she relapsed to blastic phase, at which time Y253H mutation was detected. She was then started on dasatinib, which induced a 2-log reduction in BCR-ABL transcript numbers, and the Y253H mutant clone became transiently undetectable. Thereafter tumor load increased and T315I was detected in addition to resurgent Y253H mutation. The Q-SNP data implied both mutations were in the same allele; this was confirmed by RFLP studies.
Kinetics of mutations in mutant subclones in 3 representative patients. The kinetics of the BCR-ABL transcripts measured by RQ-PCR are represented by the bold black lines in all 3 panels (expressed as a ratio to the ABL control transcripts × 100%9 ). The proportions of the subclones with the various mutations measured at different time points are represented by the colored lines. (A) Patient no. 10. A 55-year-old female with CML in chronic phase was started on 400 mg daily IM. After 30 months from start of IM the M351T mutant clone had decreased from 80% to 50%, but an additional mutation, H396R, was identified. Subsequently, the 2 mutations evolved discordantly consistent with 2 distinct subclones. After starting dasatinib only H396R mutation was detectable. One month from start of dasatinib therapy, H396R became undetectable, a third mutant subclone, F317L, was detected by Q-SNP. (B) Patient no. 11. A 53-year-old female achieved a partial cytogenetic response to 400 mg daily IM but despite increasing the dose to 600 mg, she entered myeloblastic transformation. Dasatinib was subsequently introduced at 70 mg twice daily, which induced a reduction in BCR-ABL transcripts over the next 6 months, and the F311I mutation became transiently undetectable by Q-SNP. Subsequently, the BCR-ABL levels increased, and the patient was found to have T315I mutation in addition to F311I. The fact that the levels of the 2 mutations coincided closely in the terminal phase of her disease suggests that they were present in the same allele. This was confirmed by RFLP studies and sequencing of cloned PCR products. The patient eventually died in blastic transformation. (C) Patient no. 12. A 23-year-old woman achieved partial cytogenetic response with IM therapy but failed to maintain the response. She then progressed rapidly to lymphoblastic transformation and underwent sibling allogeneic hematopoietic stem cell transplant (HSCT). Four months after HSCT she relapsed to blastic phase, at which time Y253H mutation was detected. She was then started on dasatinib, which induced a 2-log reduction in BCR-ABL transcript numbers, and the Y253H mutant clone became transiently undetectable. Thereafter tumor load increased and T315I was detected in addition to resurgent Y253H mutation. The Q-SNP data implied both mutations were in the same allele; this was confirmed by RFLP studies.
In the 2 group 4 patients one mutation was identified in the earliest available sample, but the second mutation, T315I, was identified after initial response to dasatinib. The fact that in both cases the level of the second mutation paralleled closely the level of the first mutation provided strong evidence that the second mutation actually occurred in the subclone that carried the original mutation. This was confirmed by restriction fragment length polymorphism (RFLP) data, which yielded fragment sizes consistent with sequencing data showing that in each case the 2 mutations were within the same allele. It is interesting to note that Shah and colleagues also have described the coexistence of 2 or more mutations in the same BCR-ABL mRNA, a phenomenon that they term a “compound mutation,” in 4 of their 13 IM-resistant CML patients treated subsequently with dasatinib.11 In contrast, Q-SNP kinetic data for the 2 mutations in patient number 10, while on IM, strongly suggested the presence of 2 distinct subclones.
Our findings support the notion that dasatinib, being appreciably smaller than imatinib, may have a greater affinity for the active kinase conformation and may therefore be able to inhibit a significant number of imatinib-resistant mutant clones.12,,–15 Furthermore, the data support reports suggesting that contact site mutants, notably T315I and F317L, may both be especially resistant to dasatinib.12,–14 These observations imply that in patients numbers 6 and 7 with mutant subclones G250R and M244V, respectively, neither of whom ever achieved MMR despite continuing dasatinib, dasatinib resistance was not directly attributable to the mutant subclone but to other undefined mechanism(s). In this series no patient who lacked a mutation when starting dasatinib subsequently acquired one, which suggests that a specific subgroup of CML patients may have a particular predisposition to acquire new BCR-ABL KD mutations.
These findings raise the issue of when precisely the T315I subclone originated in the leukemia cell population in patient numbers 11 and 12. The fact that the dasatinib-resistant clone appeared to develop abruptly in both patients implies either that the T315I mutation developed de novo after start of dasatinib or that it preexisted the dasatinib treatment but was held in check by another unidentified clonal population that was in turn then suppressed by dasatinib. Whichever the case, the 2 mutations may act in synergy to confer a major proliferative advantage over the unmutated leukemia cell population.
The precise mechanism that links a TKI-resistant KD mutation and subsequent events is unclear. First, what is the basis of the proliferative advantage of that subclone over the nonmutated clone, and second, what is the basis of the possible progression to advanced phase disease?16 One can only speculate that a given mutant clone is permitted to expand “selectively” when the choice, dose, and duration of TKI therapy are all “suboptimal.” This expansion would presumably allow an increase in the size of a population of leukemia cells especially susceptible to acquisition of additional genetic changes, either in the BCR-ABL gene or equally credibly in other genes, and this in turn would expedite progression to a more aggressive phenotype for the leukemia. This supposition highlights the need for early detection of an emerging resistant clone with the aim of eradicating it before it becomes refractory to further treatment.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the various staff members responsible for the clinical care of the patients described in this report.
This work was supported by the funds for research and clinical service provided by the Hammersmith Hospitals Trust. Ms Mehta was undertaking a medical student elective and was supported by bursaries from Cancer Research UK, the Leukemia Research Fund (United Kingdom), the Pathological Society of Great Britain and Northern Ireland, and the Wellcome Trust.
Authorship
Contribution: J.S. Khorashad, M.A., A.B., J.S. Kaeda, and S.G. performed RQ-PCR for BCR-ABL transcripts and quantitation of the size of the mutant subclones; A.G.R. and V.D.M. carried out FISH studies and contributed to molecular studies; H.d.L. and P.M. collected clinical data; J.F.A., D. Marin, E.O., and D. Milojkovic supervised care of patients in the clinic and wrote various parts of the manuscript; and J. S. Kaeda, J. S. Khorashad, D. Milojkovic, D. Marin, E.O., J.M.G., and J.F.A. prepared the final manuscript for submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Prof John M. Goldman, Department of Haematology, Imperial College - Hammersmith Hospital Campus, London W12 ONN, United Kingdom; e-mail: j.goldman@imperial.ac.uk.