Enucleation is the hallmark of erythropoiesis in mammals. Previously, we determined that yolk sac–derived primitive erythroblasts mature in the bloodstream and enucleate between embryonic day (E)14.5 and E16.5 of mouse gestation. While definitive erythroblasts enucleate by nuclear extrusion, generating reticulocytes and small, nucleated cells with a thin rim of cytoplasm (“pyrenocytes”), it is unclear by what mechanism primitive erythroblasts enucleate. Immunohistochemical examination of fetal blood revealed primitive pyrenocytes that were confirmed by multispectral imaging flow cytometry to constitute a distinct, transient cell population. The frequency of primitive erythroblasts was higher in the liver than the bloodstream, suggesting that they enucleate in the liver, a possibility supported by their proximity to liver macrophages and the isolation of erythroblast islands containing primitive erythroblasts. Furthermore, primitive erythroblasts can reconstitute erythroblast islands in vitro by attaching to fetal liver–derived macrophages, an association mediated in part by α4 integrin. Late-stage primitive erythroblasts fail to enucleate in vitro unless cocultured with macrophage cells. Our studies indicate that primitive erythroblasts enucleate by nuclear extrusion to generate erythrocytes and pyrenocytes and suggest this occurs in the fetal liver in association with macrophages. Continued studies comparing primitive and definitive erythropoiesis will lead to an improved understanding of terminal erythroid maturation.
Introduction
It was recognized in the latter half of the 18th century that enucleation was a unique feature of mammalian erythropoiesis.1 Late-stage definitive erythroblasts in the fetal liver and the postnatal marrow of mammals enucleate by nuclear extrusion. Enucleation begins when vimentin intermediate filaments are lost and the nucleus becomes freely movable within maturing erythroid precursors.2 Soon thereafter, the acentric nucleus is extruded with a thin rim of cytoplasm and an enveloping cell membrane.3,,,–7 The “extruded erythroblast nucleus” then loses phosphatidylserine asymmetry of its plasma membrane and is rapidly engulfed by macrophage cells.8,–10
In contrast to definitive erythropoiesis, where erythrocytes enter the circulation after enucleating, primitive erythroid cells emerge from yolk sac blood islands as immature erythroid precursors and progressively mature in the bloodstream.11,12 The circulation of primitive erythroid cells as nucleated cells has long suggested that they are more similar to the nucleated red cells of birds, fish, and amphibians than the red cells of fetal and adult mammals. However, primitive erythroid precursors in the mouse fetus, unlike avian precursors, lose vimentin intermediate filaments.13 We recognized that primitive erythroid cells in the murine embryo ultimately enucleate and continue to circulate for several days after birth,12 an observation recently confirmed by others.14 Importantly, we found that primitive erythroid cells do not decrease in number as they transition from late-stage erythroblasts to erythrocytes between embryonic day (E)12.5 and E16.5, indicating that enucleation is a normal end point of primitive erythropoiesis in the mouse.12 While definitive erythroblasts normally mature and enucleate in association with macrophages in the fetal liver and postnatal bone marrow, it is not clear where and by what mechanism primitive erythroid cells enucleate in the mammalian embryo. Here we show that late-stage primitive erythroblasts in the mouse embryo can physically associate with macrophage cells and that their enucleation leads to a transient population of extruded nuclei (“pyrenocytes”).
Methods
Approval was obtained from the University of Rochester University Committee Animal Resources office for the use of animals in this study.
Tissue collection and processing
Timed pregnant ICR mice (Taconic, Germantown, NY) were mated overnight and vaginal plugs examined in the morning, considered embryonic day 0.3 (E0.3). At specified times during gestation, mice were killed by CO2 inhalation and embryonic tissues were dissected in PB1 (Dulbecco phosphate-buffered saline [PBS], Invitrogen, Carlsbad, CA; 0.3% BSA, Gemini Bio-Products, Sacramento, CA; 0.68 mM CaCl2, Sigma-Aldrich, St Louis, MO; 0.1% glucose; 0.32 mM Na pyruvate).15 Fetal blood was collected as previously described.12 Embryonic livers were either partially dissociated (for ex vivo island cytospins or in vitro erythroblast island reconstitution) or completely dissociated (for culture or ImageStream analysis) by increasing amounts of gentle pipetting. Adult bone marrow was collected in PB1 and single cell suspensions made by gentle trituration. Cytospins were prepared with 100 000 cells spun at 400 rpm for 3 minutes (Cytospin2; Thermo Shandon, Pittsburgh, PA) and either air dried or fixed for 5 minutes in ice-cold methanol. Whole embryos and dissected spleens were fixed overnight in fresh 4% buffered paraformaldehyde, embedded in paraffin, and sectioned.
DNA fragmentation assay
A total of 2 × 106 E15.5 fetal blood cells were washed in PBS and lysed in 100 μL lysis buffer (50 mM Tris, 10 mM EDTA, 0.5% SDS, 1 mg/mL proteinase K, Invitrogen, pH 8.0) at 55°C for 1 hour. DNA was purified by adding an equal volume of water and then extracting twice with 1:1 phenol/chloroform, followed by ethanol precipitation. The DNA was treated with 250 μg/mL RNaseA (Invitrogen) in Tris-EDTA buffer for 1 hour and subjected to electrophoresis on a 1.8% agarose gel. For controls, 2 × 106 murine bone marrow cells and E15.5 fetal blood cells were each resuspended in 1 mL of association media as described below in In vitro reconstitution of erythroblast islands, with the addition of 0.5 μM staurosporine (EMD Biosciences, San Diego, CA) and cultured for 6 or 24 hours at 37°C, 5% CO2.
Generation of anti–ϵγ-globin antibodies
Immunohistochemistry and histologic staining
For βH1- or ϵγ-globin immunohistochemistry, depariffinized sections, methanol-fixed cytospins, or methanol-fixed reconstituted erythroblast islands were pretreated for 15 minutes at 100°C in 0.1 M Tris pH 6.0, incubated with anti-globin antibodies, and visualized using avidin-biotin complex with Vector Red (Vector Laboratories, Burlingham, CA). For double immunohistochemistry, F4/80 antibody (Serotec, Raleigh, NC) staining was performed first, beginning with a 20-minute 3% buffered Triton-X pretreatment. F4/80 antibodies were detected using secondary goat antirat antibodies conjugated to alkaline phosphatase (Jackson ImmunoResearch, West Grove, PA) and visualized with Vector Blue (Vector Laboratories). When DNA visualization was desired, cells were treated for 15 minutes with either HO (Hoechst 33 342, Invitrogen) or DAPI (Invitrogen) at 5 μg/mL. Wright-Giemsa (Sigma Diagnostics, St Louis, MO) staining was carried out on unfixed cytospins according to the manufacturer's instructions. Photography was carried out with a Nikon Optiphot microscope (4×, 20× objectives with NA 0.13 and 0.75, respectively) or an Eclipse TE 2000-S microscope (Nikon 20×, 40× objectives with NA 0.40 and 0.60, respectively) and a SPOT RT-slider digital camera (Diagnostic Instruments, Sterling Heights, MI) or a Hamamatsu Orca 285 digital camera (Hamamatsu Photonics, Hamamatsu City, Japan) with 40× objective, NA 0.60). Images were processed in Photoshop CS2 (Adobe Systems, San Jose, CA) with Fovea Pro quantitative image analysis plug-ins (Reindeer Graphics, Asheville, NC).
Morphometric analysis
Smears of E14.5 blood were analyzed for ϵγ-globin expression and DAPI staining of DNA, photographed using the Hamamatsu Orca digital microscope camera, and total cellular and nuclear areas of primitive erythroblasts determined using IPlab software (BD Biosciences, Rockville, MD).
Amnis ImageStream analysis
Fixed, permeabilized erythroid cells are very sensitive to lysis and hemoglobin loss; therefore, staining of dissociated fetal liver or peripheral blood was carried out using a method optimized for detection of human fetal hemoglobin containing erythrocytes.16 Briefly, high formaldehyde (4%) fixation was followed by rapid permeabilization in −20°C acetone and immediate staining with minimal washing. Post-fixation in 1% formaldehyde stabilized staining by fixing antibodies within erythroid cells. Cells were stained with PE-Ter119 (eBioscience, San Diego, CA) and anti–ϵγ-globin antibodies visualized by binding of a secondary goat antirabbit antibody conjugated to Alexa Fluor 488 (Invitrogen). For annexin V binding studies, unfixed fetal blood or adult marrow cells were stained with PE-Ter119 followed by annexin V-Alexa Fluor 488 (Invitrogen) according to manufacturer's instructions. Draq5 to 10 μM (Biostatus, Shepshed, United Kingdom) was added to fixed cells and both 7-amino actinomycin D (7AAD) to 20 μg/mL (Invitrogen) and Draq5 were added to live samples immediately before analysis on the ImageStream using the Inspire software (Amnis, Seattle, WA). Bone marrow was treated with staurosporine as for DNA fragmentation assay. Spectral compensation was performed as described17,18 and data analysis was performed using the ImageStream Data Exploration and Analysis Software (IDEAS; Amnis). Briefly, debris and cell aggregates were excluded and individual cells identified by gating on size and aspect ratio. Individual cell populations were identified by gating on cells expressing surface markers and confirmed by visual inspection of the fluorescence pattern.
In vitro reconstitution of erythroblast islands with primitive erythroblasts
Isolation and stripping of erythroblast islands.
Partially dissociated fetal liver or bone marrow was pelleted gently (500g for 3 minutes) to enrich for islands and resuspended at 8 × 107 cells/mL in “bottom media” (10% plasma-derived serum [PDS; Animal Technologies, Tyler, TX], IMDM [Invitrogen], 2 mM glutamine [Invitrogen], 0.15 mM monothialglycerol [MTG; Sigma-Aldrich], and 1 ng/mL M-CFS [Peprotech, Rocky Hill, NJ]); 1 mL of dissociated liver or bone marrow was added to the center of chambers of 2-well Lab-tek slides (Nalge Nunc, Rochester, NY) and allowed to adhere for 4 hours at 37°C, 5% CO2. Erythroblasts were stripped from macrophage cells by washing 2 to 3 times in calcium-, magnesium-free PBS allowing the media to sit 30 seconds between washes. Stripping of erythroblasts from attached macrophage cells was confirmed by microscopic observation before proceeding. Occasional unstripped erythroblast islands were observed, particularly near the edge of the chambers. Unstripped definitive erythroblasts were easily distinguished from primitive erythroblasts during the subsequent analysis because of their large nuclear/cytoplasmic ratio and lack of ϵγ-globin staining (Figure S1; available on the Blood website; see the Supplemental Materials link at the top of the online article).
Preparation of fetal blood.
Fetal blood was resuspended at 106 cells/mL in “association media” (bottom media except with 30% PDS, 2 U/mL erythropoietin [Amgen, West Greenwich, RI], 300 μg/mL transferrin [Sigma-Aldrich] and 40 ng/mL IGF-1 [Peprotech]) in plastic dishes. After a 2- to 4-hour incubation at 37°C, 5% CO2 to allow adherent cells to attach, the supernatant was collected for use in reconstitution assays.
Reconstitution and analysis of erythroblast islands.
A total of 1 mL of the nonadherent fetal blood cells was added to the stripped-adherent fetal liver or adult marrow cells and cultured for 24 to 48 hours at 37°C, 5% CO2. The vast excess of fetal blood cells was then removed by aspiration and the slides briefly rinsed in PB1, fixed in ice-cold methanol for 5 minutes, and processed for immunohistochemistry with anti–ϵγ-globin and F4/80 antibodies to identify primitive erythroblasts and macrophage cells, respectively. For adherence assays, the number of primitive erythroid cells attached to each of 100 to 400 macrophages was analyzed per experiment. For enucleation assays, the nucleation status of 100 to 1000 primitive erythroid cells attached to macrophage cells was determined after costaining with HO to identify nuclei.
In control experiments, the culture of nonadherent fetal blood cells or of stripped erythroblast islands alone did not result in formation of erythroblast islands containing primitive erythroid cells. In addition, total cell numbers, viability, and the proportion of primitive vs definitive erythroid cells were similar when fetal blood cells were cultured either in the presence or absence of stripped-adherent liver cells; 8-well Lab-tek slides were also used successfully for reconstitution of erythroblast islands with proportional reductions in volumes per well.
To determine whether macrophage cells had ingested labeled nuclei, fetal blood cells were pre-labeled with CellTracker red CMTPX or CellTracker Green CMFDA (Invitrogen) according to the manufacturer's instructions with an additional rinse into fresh media after preincubation to reduce excess dye, and used to reconstitute erythroblast islands. After a 48-hour incubation, reconstituted erythroblast islands were stripped of erythroblasts using 3 washes with calcium-, magnesium-free PBS, and fixed with 4% formaldehyde.
Reconstitution of erythroblast islands in the presence of α4-integrin blocking antibodies was performed based on Sadahira et al.19 Either α4-integrin blocking antibody (SG31, Santa Cruz Biotechnology, Santa Cruz, CA) or IgG isotype control (BD Biosciences) were added at 10 μg/mL final concentration during the erythroblast island reconstitution with fetal blood cells as described above. After overnight incubation, nonadherent cells were removed by aspiration and fresh association media with 10 μg/mL antibody was added to the chambers for 30 minutes before washing with PB1 and fixation in ice-cold absolute methanol. After immunohistochemical analysis with anti–ϵγ-globin and F4/80 antibodies, reconstituted erythroblast islands were analyzed as described above.
Autonomous enucleation assay
Completely dissociated E14.5 liver cells or fetal blood cells were pelleted and resuspended at 4 × 106 cells/mL in media optimized to support erythroid precursor maturation, but not erythroid or myeloid progenitor expansion or maturation (IMDM, 10% serum replacement [Invitrogen], 5% PDS, 10% protein free hybridoma II medium [Invitrogen], 2 mM glutamine, 0.15 mM MTG, 2 U/mL erythropoietin). After a 2-hour preincubation in plastic dishes to allow macrophage adherence, nonadherent cells were transferred to a fresh dish and cultured for 72 hours. Aliquots of cells were removed at 24-hour intervals and assayed for total cell numbers, viability by trypan blue exclusion, and percent nucleation by staining with 1 μg/mL HO.
Results
Primitive erythroblasts do not enucleate by splenic pitting or by karyolysis
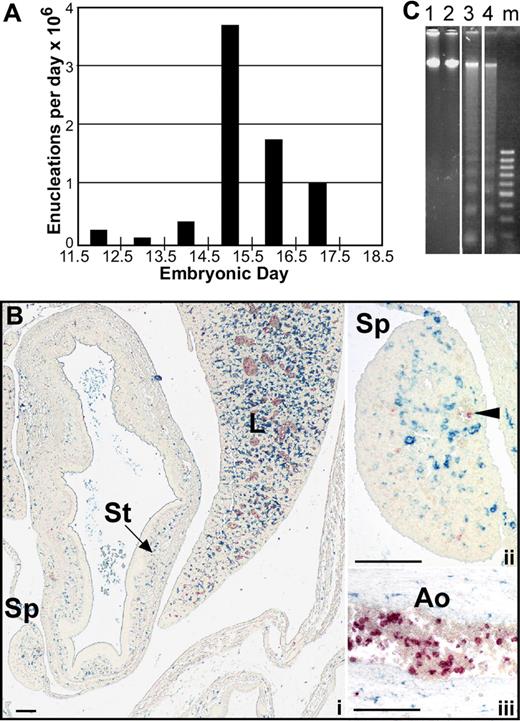
It is now recognized that primitive erythroblasts originate in the yolk sac, mature in the bloodstream as a semisynchronous cohort and subsequently enucleate over a relatively narrow temporal window during development.12,14 Because the number of primitive erythroid cells does not decrease as they transition from late-stage erythroblasts to enucleated erythrocytes,12 we estimated the number of primitive erythroid enucleation events per day in outbred mouse fetuses. As shown in Figure 1A, the large majority of late-stage primitive erythroblasts enucleate between E14.5 and E16.5 of gestation. We therefore focused our studies of the mechanism by which primitive erythroid cells enucleate to these developmental time points.
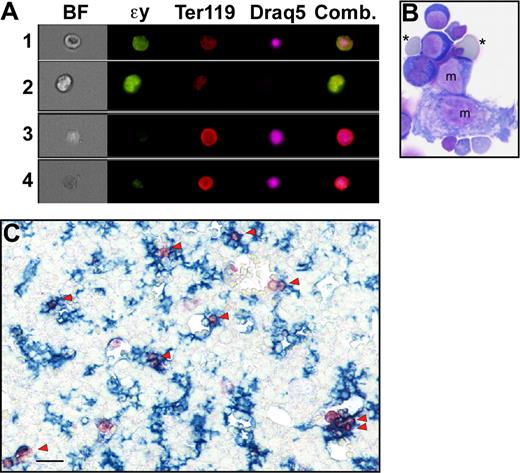
Primitive erythroblasts do not enucleate by splenic pitting or by karyolysis. (A) The estimated number of primitive erythroblasts that undergo enucleation per day between E11.5 and E18.5 of gestation in outbred mice. Enucleation events were estimated using the percentage of enucleated primitive erythroid cells previously determined for each embryonic day, assuming that there are 6.7 × 106 primitive erythroid cells per fetus and that total primitive erythroid cell numbers do not decrease between E12.5 and E17.5 of gestation.12 (B) Immunohistochemistry of transverse section of E15.5 mouse embryo with F4/80 (blue) and anti–ϵγ-globin (red) antibodies reveals the presence of mature macrophage cells and primitive erythroid cells, respectively. At lower power (i), most of the macrophages can be seen in the liver (L) with few in the surrounding body wall and spleen (Sp) or stomach (St). At higher power (ii), infrequent large primitive erythrocytes are seen in vessels in the spleen (arrowhead), but they are not associated with the few splenic macrophages present at this time. Labeled primitive erythroblasts are observed as a subset of blood cells in the aorta (Ao) (iii). Scale bars = 0.1 mm. (C) Analysis of fragmentation of DNA from primitive erythroid cells in E15.5 peripheral blood. Lanes 1 and 2 show no fragmentation of E15.5 peripheral blood DNA in either untreated (lane 1) or after 24 hours of treatment with 0.5 μM staurosporine (lane 2). In contrast, bone marrow DNA shows significant laddering after 6 hours (lane 3) or 24 hours (lane 4) of treatment. Vertical lines have been inserted to indicate repositioned gel lanes.
Primitive erythroblasts do not enucleate by splenic pitting or by karyolysis. (A) The estimated number of primitive erythroblasts that undergo enucleation per day between E11.5 and E18.5 of gestation in outbred mice. Enucleation events were estimated using the percentage of enucleated primitive erythroid cells previously determined for each embryonic day, assuming that there are 6.7 × 106 primitive erythroid cells per fetus and that total primitive erythroid cell numbers do not decrease between E12.5 and E17.5 of gestation.12 (B) Immunohistochemistry of transverse section of E15.5 mouse embryo with F4/80 (blue) and anti–ϵγ-globin (red) antibodies reveals the presence of mature macrophage cells and primitive erythroid cells, respectively. At lower power (i), most of the macrophages can be seen in the liver (L) with few in the surrounding body wall and spleen (Sp) or stomach (St). At higher power (ii), infrequent large primitive erythrocytes are seen in vessels in the spleen (arrowhead), but they are not associated with the few splenic macrophages present at this time. Labeled primitive erythroblasts are observed as a subset of blood cells in the aorta (Ao) (iii). Scale bars = 0.1 mm. (C) Analysis of fragmentation of DNA from primitive erythroid cells in E15.5 peripheral blood. Lanes 1 and 2 show no fragmentation of E15.5 peripheral blood DNA in either untreated (lane 1) or after 24 hours of treatment with 0.5 μM staurosporine (lane 2). In contrast, bone marrow DNA shows significant laddering after 6 hours (lane 3) or 24 hours (lane 4) of treatment. Vertical lines have been inserted to indicate repositioned gel lanes.
Although definitive erythroid cells enucleate by nuclear extrusion in the fetal liver and adult marrow, it is currently not known by what mechanism or even where primitive erythroid cells enucleate. Because circulating nucleated red cells in the adult are thought to be culled by the spleen, we explored whether splenic “pitting” by macrophage cells may be a mechanism of primitive erythroid enucleation. The murine spleen begins to form as an organ at E11.5 to 12.5, and it serves as a site of definitive erythropoiesis in late gestation.20 We examined transverse embryonic sections as well as microdissected spleens for the presence of primitive erythroid cells and macrophage cells from E12.5 to E16.5 of gestation. Dual-label immunohistochemical studies were performed using anti–ϵγ-globin and F4/80 antibodies to identify primitive erythroid cells and mature macrophage cells, respectively.21,22 Very few F4/80-positive macrophages were identified in the spleen until E15.5, at which time their frequency was still very low compared with the liver (Figure 1Bi). Furthermore, the mature macrophage cells in the spleen did not appear to be associated with the small number of primitive erythroid cells detected within the spleen (Figure 1Bii). In contrast, large numbers of primitive erythroid cells were identified within large fetal vessels (Figure 1Biii). These findings suggest that the developing splenic rudiment does not play a significant role in the enucleation of primitive erythroid cells.
Previous investigators have suggested that primitive erythroid cells enucleate by karyolysis.23,24 If karyolysis occurs as primitive erythroblasts circulate, then we would expect to see morphologic evidence of nuclear fragmentation within primitive orthochromatic erythroblasts. Careful examination of Wright-Giemsa–stained fetal blood smears from E12.5 to E17.5 fetuses failed to reveal any evidence of nuclear fragmentation (data not shown). To more positively identify all primitive erythroid cells, fetal blood was stained with anti–ϵγ-globin antibodies and HO. No evidence of karyolysis in circulating primitive erythroid cells was detected using this staining approach.
To examine whether terminal primitive erythroid maturation is associated with active DNA cleavage as seen in apoptosis, DNA was isolated from E15.5 peripheral blood cells, where greater than 98% of the nucleated cells are primitive erythroblasts.12 As shown in Figure 1C, there was no evidence of DNA laddering in primary primitive erythroblasts (lane 1). Even after 24 hours of treatment with the apoptosis inducer staurosporine,25 there was no evidence of DNA laddering (Figure 1C lane 2). In contrast, DNA isolated from control adult marrow cells treated for 6 or 24 hours with staurosporine showed marked evidence of DNA fragmentation (Figure 1C lanes 3 and 4, respectively).
Appearance of extruded nuclei (“pyrenocytes”) in the bloodstream
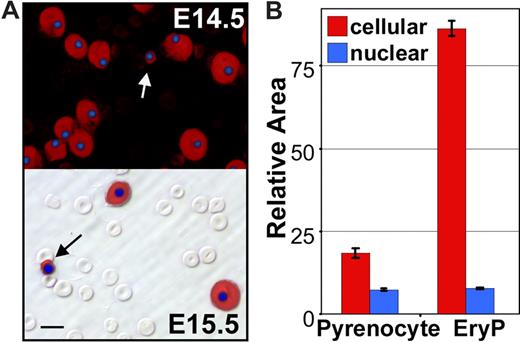
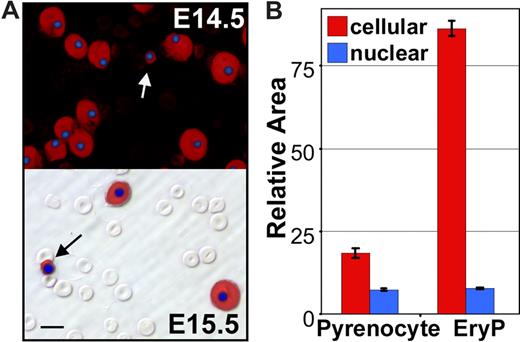
Careful examination of the dual anti–ϵγ-globin– and HO-stained E14.5 and E15.5 fetal blood smears revealed the presence of rare cells containing condensed nuclei surrounded by small rims of ϵγ-globin–positive cytoplasm (Figure 2A arrows). Their small size, high nuclear to cytoplasmic ratio, and embryonic hemoglobin content suggested that they were the products of nuclear extrusion from primitive erythroid cells. We reasoned that if this were the case they should be distinctly smaller in overall cell size when compared with late-stage primitive erythroid precursors and yet have a similar nuclear size. To test this prediction, a morphometric analysis of the nuclear and total cell areas of these small ϵγ-globin–positive cells and neighboring ϵγ-globin–positive primitive erythroblasts was conducted. As shown in Figure 2B, there was a significant difference in total cell area, but no difference in nuclear area, suggesting that these small cells constitute a distinct primitive erythroid cell population. Because of their highly condensed nucleus and high nuclear to cytoplasmic ratio, we termed these cells “pyrenocytes.”
Appearance of primitive “pyrenocytes” in the fetal bloodstream. (A) Immunohistochemistry fetal blood, stained with anti–ϵγ-globin antibodies (red) and DAPI (blue) to label primitive erythroid cells and nuclei, respectively. The upper panel is a darkfield combined fluorescent image of E14.5 blood. The numerous smaller definitive erythrocytes are not evident because they are both ϵγ-globin– and DAPI-negative. The lower panel is E15.5 blood with fluorescent images layered over a Hoffman contrast image to show the abundant unstained definitive erythrocytes compared with the larger ϵγ-globin–positive primitive erythroblasts. Primitive pyrenocytes with small rim of ϵγ -globin–positive cytoplasm are indicated by arrows. Scale bar = 10 μm. (B) Morphometric analysis of E14.5 blood images indicates that pyrenocytes are significantly (P < .001) smaller than surrounding primitive orthochromatic erythroblasts (EryP). However, there is no significant difference in the size of their nuclei. Error bars are SEM.
Appearance of primitive “pyrenocytes” in the fetal bloodstream. (A) Immunohistochemistry fetal blood, stained with anti–ϵγ-globin antibodies (red) and DAPI (blue) to label primitive erythroid cells and nuclei, respectively. The upper panel is a darkfield combined fluorescent image of E14.5 blood. The numerous smaller definitive erythrocytes are not evident because they are both ϵγ-globin– and DAPI-negative. The lower panel is E15.5 blood with fluorescent images layered over a Hoffman contrast image to show the abundant unstained definitive erythrocytes compared with the larger ϵγ-globin–positive primitive erythroblasts. Primitive pyrenocytes with small rim of ϵγ -globin–positive cytoplasm are indicated by arrows. Scale bar = 10 μm. (B) Morphometric analysis of E14.5 blood images indicates that pyrenocytes are significantly (P < .001) smaller than surrounding primitive orthochromatic erythroblasts (EryP). However, there is no significant difference in the size of their nuclei. Error bars are SEM.
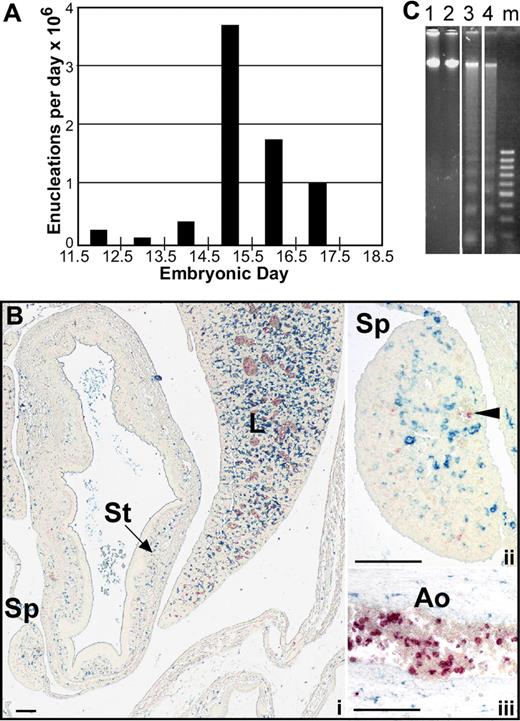
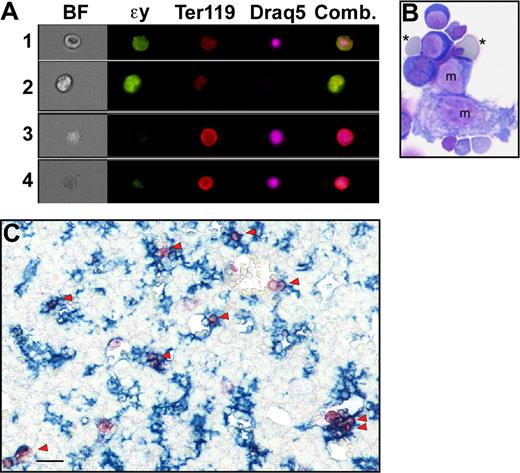
To further explore whether pyrenocytes constitute a distinct cell population, while avoiding the bias of their manual identification on blood smears, we took advantage of the multispectral imaging capabilities of the ImageStream flow cytometer (Amnis). This technology captures concurrent brightfield and 4 fluorescent images of single cells that can be analyzed for a combination of morphologic and fluorescent intensity perimeters. Fetal mouse blood was fixed, permeabilized, and stained with anti–ϵγ-globin and Ter119 antibodies and Draq5, to identify nucleated primitive erythroid cells. As shown in Figure 3A (red line), 2 distinct populations of nucleated primitive erythroid cells were identified at E15.5 of gestation. The larger population consisted of primitive orthochromatic erythroblasts (Figure 3B rows 1 and 2). The smaller population, constituting approximately 2% of the total nucleated primitive erythroid cells, consisted of pyrenocytes, characterized on the ImageStream by their small size and extremely high nuclear to cytoplasmic ratio (Figure 3B rows 3 and 4). The ImageStream software was used to compare the nuclear size of these 2 cell populations. As shown in Figure 3C, both late-stage primitive erythroid precursors (EryP) and primitive pyrenocytes have a similar nuclear size. These findings confirm the morphometric analysis of the blood smears and indicate that primitive pyrenocytes constitute a distinct and previously unrecognized population of cells in the fetal bloodstream. Analysis of E12.5 fetal blood failed to reveal evidence of circulating pyrenocytes (Figure 3A green line). However, a minor population, constituting less than 1% of nucleated primitive erythroid cells, was clearly evident at E14.5 (Figure 3A blue line). These results further support the concept that primitive pyrenocytes are the product of enucleation because their temporal appearance coincides with that of primitive erythroid enucleation events (Figure 1A).
Primitive pyrenocytes constitute a distinct cellular population in the fetal bloodstream. (A) Relative size of nucleated primitive erythroid cells as determined by ImageStream analysis reveals that pyrenocytes account for approximately 2% of nucleated blood cells in E15.5 blood (red line), less than 1% at E14.5 (blue line), and are not detected at E12.5 (green line). (B) Example of images of fetal blood cells visualized using the ImagesStream. Concurrent brightfield (BF) images and fluorescent images (anti–ϵγ-globin antibodies [ϵγ], Ter119, and the DNA stain [Draq5]) of larger primitive orthochromatic erythroblasts (rows 1 and 2) and of smaller primitive pyrenocytes (rows 3 and 4). (C) Primitive pyrenocytes and primitive orthochromatic erythroblasts (EryP) in the E15.5 circulation have similar nuclear sizes. Error bars represent SD. (D) E15.5 blood stained with annexin V (AnV), Ter119, and Draq5 (shown as merged images), and 7AAD analyzed by the ImageStream. Row 1 is a primitive pyrenocyte that is annexin V–positive and 7AAD-negative. Row 2 is a primitive orthochromatic erythroblasts that is negative for annexin V and 7AAD.
Primitive pyrenocytes constitute a distinct cellular population in the fetal bloodstream. (A) Relative size of nucleated primitive erythroid cells as determined by ImageStream analysis reveals that pyrenocytes account for approximately 2% of nucleated blood cells in E15.5 blood (red line), less than 1% at E14.5 (blue line), and are not detected at E12.5 (green line). (B) Example of images of fetal blood cells visualized using the ImagesStream. Concurrent brightfield (BF) images and fluorescent images (anti–ϵγ-globin antibodies [ϵγ], Ter119, and the DNA stain [Draq5]) of larger primitive orthochromatic erythroblasts (rows 1 and 2) and of smaller primitive pyrenocytes (rows 3 and 4). (C) Primitive pyrenocytes and primitive orthochromatic erythroblasts (EryP) in the E15.5 circulation have similar nuclear sizes. Error bars represent SD. (D) E15.5 blood stained with annexin V (AnV), Ter119, and Draq5 (shown as merged images), and 7AAD analyzed by the ImageStream. Row 1 is a primitive pyrenocyte that is annexin V–positive and 7AAD-negative. Row 2 is a primitive orthochromatic erythroblasts that is negative for annexin V and 7AAD.
Definitive pyrenocytes, when generated in vitro, rapidly lose phosphatidylserine asymmetry.10 We therefore asked whether circulating primitive pyrenocytes maintain their phosphatidylserine asymmetry. E15.5 blood was stained with annexin V, 7AAD, Ter119, and Draq5 and analyzed by ImageStream to distinguish primitive pyrenocytes (Figure 3D row 1) and orthochromatic erythroblasts (Figure 3D row 2). A total of 35% of the pyrenocyte population, but only 6% of the larger primitive erythroblasts, were annexin V–positive and 7AAD-negative (Table 1). The lack of 7AAD staining on this cell population indicates that the annexin V staining was not the result of increased membrane permeability. Bone marrow treated with staurosporine, which served as a positive control, revealed similar high levels of annexin V staining compared with untreated primitive pyrenocytes (Table 1).
Primitive erythroblasts interact with fetal liver macrophage cells in vivo
The discovery of primitive pyrenocytes in the bloodstream suggested that enucleation of primitive erythroblasts may also be occurring within the circulation. However, careful examination of peripheral blood smears failed to reveal evidence of enucleating primitive erythroid forms (data not shown). We therefore investigated whether late-stage primitive erythroblasts, like their definitive counterparts, might be enucleating within the fetal liver. Late-stage primitive erythroblasts (Figure 4A row 1) and primitive erythrocytes (Figure 4A row 2) could be distinguished from definitive erythroid precursors (Figure 4A rows 3 and 4) using ImageStream analysis with a combination of ϵγ-globin and Ter119 expression as well as quantifiable morphologic criteria, including cell size, nuclear size, and the brightfield characteristics of cell contrast and gradient RMS (a measure of surface irregularity). To examine whether primitive orthochromatic erythroblasts are enriched in the liver, we determined the relative number of nucleated and enucleated primitive erythroid cells in paired samples of blood and liver derived from 4 independent E15.5 litters. The percent enucleation of circulating primitive erythroid cell in these 4 litters ranged from 15% to 76% (Table 2). We reasoned that the ratio of nucleated to enucleated primitive erythroid cells in the bloodstream and the liver should be identical if primitive erythroblasts are freely flowing through the liver. As shown in Table 2, this comparison revealed a consistent and significant enrichment (1.2- to 1.7-fold, P ≤ .02) of nucleated cells in the liver compared with the bloodstream, indicating that late-stage primitive erythroblasts are preferentially retained in the liver at E15.5.
Primitive erythroblasts may associate with fetal liver macrophage cells in vivo. (A) ImageStream images of liver cells viewed in brightfield (BF) or stained with anti–ϵγ-globin (ϵγ) and Ter119 antibodies and Draq5. The right panel is a combination of images of the 3 fluorescent stains. ImageStream analysis software facilitates the identification of different erythroid populations in the liver by quantifying brightfield characteristics (contrast and gradient RMS, ie, irregular surface) as well as fluorescent stain intensities. Primitive orthochromatic erythroblasts (row 1) were contrasthi, gradient RMShi, ϵγ-globinhi, Ter119lo, large, Draq5+, small. Primitive enucleated erythrocytes (row 2) were contrasthi, gradient RMShi, ϵγ-globinhi, Ter119lo, large, Draq5−. Immature and mature definitive erythroblasts (rows 3 and 4, respectively) were distinguished from primitive erythroid cells by their nuclear size as well as by being contrastlo, gradient RMSlo, ϵγ-globinlo, and Ter119hi. (B) Cytospin preparations of E14.5 liver erythroblast islands stained with Wright-Giemsa reveal the presence of small numbers of primitive erythroid cells (*) attached to macrophage cells (m). (C) Immunohistochemistry of E15.5 liver reveals the very common spatial association (arrowheads) of ϵγ-globin–positive primitive erythroid cells (red) with F4/80-positive macrophage cells (blue). Scale bar = 20 μm.
Primitive erythroblasts may associate with fetal liver macrophage cells in vivo. (A) ImageStream images of liver cells viewed in brightfield (BF) or stained with anti–ϵγ-globin (ϵγ) and Ter119 antibodies and Draq5. The right panel is a combination of images of the 3 fluorescent stains. ImageStream analysis software facilitates the identification of different erythroid populations in the liver by quantifying brightfield characteristics (contrast and gradient RMS, ie, irregular surface) as well as fluorescent stain intensities. Primitive orthochromatic erythroblasts (row 1) were contrasthi, gradient RMShi, ϵγ-globinhi, Ter119lo, large, Draq5+, small. Primitive enucleated erythrocytes (row 2) were contrasthi, gradient RMShi, ϵγ-globinhi, Ter119lo, large, Draq5−. Immature and mature definitive erythroblasts (rows 3 and 4, respectively) were distinguished from primitive erythroid cells by their nuclear size as well as by being contrastlo, gradient RMSlo, ϵγ-globinlo, and Ter119hi. (B) Cytospin preparations of E14.5 liver erythroblast islands stained with Wright-Giemsa reveal the presence of small numbers of primitive erythroid cells (*) attached to macrophage cells (m). (C) Immunohistochemistry of E15.5 liver reveals the very common spatial association (arrowheads) of ϵγ-globin–positive primitive erythroid cells (red) with F4/80-positive macrophage cells (blue). Scale bar = 20 μm.
We next asked whether late-stage primitive erythroblasts are associated, like their definitive counterparts, with fetal liver macrophage cells. E14.5 fetal livers were gently but rapidly partially dissociated and cytospin preparations were stained with Wright-Giemsa or anti–ϵγ-globin antibodies and DAPI. Rare erythroblast islands containing nucleated and enucleated primitive erythroblasts intermixed with maturing definitive erythroblasts were evident (Figure 4B). These results suggest that primitive erythroblasts associate with erythroblast islands in the fetal liver. However, the short preparation time of this experimental approach minimizes, but does not exclude, the possibility that these primitive erythroid cells became associated with macrophage cells during the dissociation step.
To further determine whether primitive erythroblasts associate with macrophage cells in vivo, we examined the spatial distribution of primitive erythroblasts and macrophage cells. Initial experiments were performed to identify the spatial and temporal distribution of mature macrophage cells in the mid-gestation mouse embryo using the F4/80 antibody. At E14.5 to E15.5, when the bulk of primitive erythroblasts enucleate (Figure 1A), macrophage cells were at highest density in the fetal liver and only scattered F4/80-positive macrophage cells were present in other organs, the body wall, and the placenta (Figure 1C and data not shown). We then examined the distribution of ϵγ-globin–positive primitive erythroid cells in relation to F4/80-positive macrophage cells. As shown in Figure 4C (arrowheads), many primitive erythroid cells were found adjacent to fetal liver macrophage cells at E15.5 of gestation.
Primitive erythroblasts physically interact with fetal liver macrophage cells in vitro
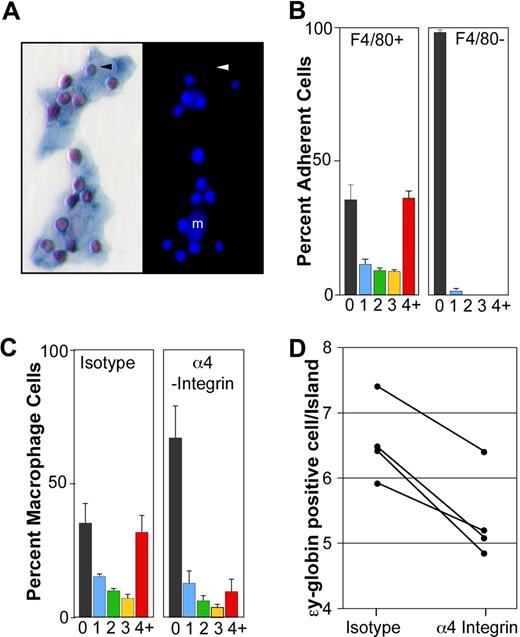
The colocalization of many primitive erythroid cells with macrophage cells in the E14.5 to E15.5 liver raised the possibility that these cell types are physically interacting. To test this hypothesis, we developed an in vitro erythroblast island reconstitution assay. Erythroblast islands from E14.5 livers were attached to microscope slides, endogenous erythroblasts stripped away from the adherent macrophage cells, and E14.5 peripheral blood incubated with the attached liver cells. Immunohistochemical analysis with anti–ϵγ-globin and F4/80 antibodies revealed the surprisingly frequent and specific association of primitive erythroblasts with macrophage cells (Figure 5A). While primitive erythroid cells represented only 31% of the circulating erythroid cells at E14.5, they constituted 99% of the cells attached to macrophage cells in vitro.
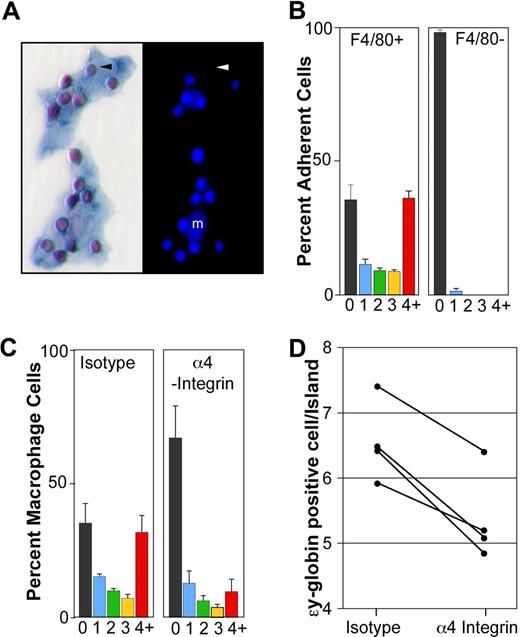
Primitive erythroblasts interact with fetal liver macrophage cells in vitro. (A) Immunohistochemical analysis of 2 erythroblast islands reconstituted with E14.5 blood and E14.5 liver macrophages. (Left panel) ϵγ-globin–positive primitive erythroid cells (red) attached to F4/80-positive macrophage cells (blue). (Right panel) The same erythroblast islands with nuclei stained by HO. Small bright nuclei of the primitive erythroblasts are seen as well as larger fainter macrophage nuclei (m). An enucleated primitive erythrocyte is indicated by an arrowhead. (B) Primitive blood cells preferentially bind F4/80-positive macrophages compared with F4/80-negative adherent cells. The percentage of F4/80-positive macrophages (left panel) and F4/80-negative adherent cells (right panel) with 0, 1, 2, 3, or 4 or more ϵγ-globin–positive cells attached is plotted. These data represent the mean plus or minus SEM of 3 independent experiments. (C) The presence of α4-integrin blocking antibody during the reconstitution of erythroblast islands resulted in both a significant decrease in the percentage of macrophages in erythroblast islands, defined as containing 4 or more erythroblasts (4+, red bars) and a significant increase in the percentage of macrophages lacking any attached primitive blood cells (0, black bars) compared with isotype controls (P < .02). These data represent the mean plus or minus SEM of 3 independent experiments. (D) The addition of α4-integrin blocking antibody also caused a decrease in the average number of primitive erythroid cells per reconstituted erythroblast island (defined as 4 or more primitive erythroid cells per macrophage) compared with isotype controls (P ≤ .005). The results of 4 independent experiments are shown.
Primitive erythroblasts interact with fetal liver macrophage cells in vitro. (A) Immunohistochemical analysis of 2 erythroblast islands reconstituted with E14.5 blood and E14.5 liver macrophages. (Left panel) ϵγ-globin–positive primitive erythroid cells (red) attached to F4/80-positive macrophage cells (blue). (Right panel) The same erythroblast islands with nuclei stained by HO. Small bright nuclei of the primitive erythroblasts are seen as well as larger fainter macrophage nuclei (m). An enucleated primitive erythrocyte is indicated by an arrowhead. (B) Primitive blood cells preferentially bind F4/80-positive macrophages compared with F4/80-negative adherent cells. The percentage of F4/80-positive macrophages (left panel) and F4/80-negative adherent cells (right panel) with 0, 1, 2, 3, or 4 or more ϵγ-globin–positive cells attached is plotted. These data represent the mean plus or minus SEM of 3 independent experiments. (C) The presence of α4-integrin blocking antibody during the reconstitution of erythroblast islands resulted in both a significant decrease in the percentage of macrophages in erythroblast islands, defined as containing 4 or more erythroblasts (4+, red bars) and a significant increase in the percentage of macrophages lacking any attached primitive blood cells (0, black bars) compared with isotype controls (P < .02). These data represent the mean plus or minus SEM of 3 independent experiments. (D) The addition of α4-integrin blocking antibody also caused a decrease in the average number of primitive erythroid cells per reconstituted erythroblast island (defined as 4 or more primitive erythroid cells per macrophage) compared with isotype controls (P ≤ .005). The results of 4 independent experiments are shown.
The binding of primary, late-stage primitive erythroblasts to macrophage cells resulted in the reproducible in vitro reconstitution of erythroblast islands. The frequency of macrophage cells with 0, 1, 2, 3, or 4 or more primitive erythroid cells is shown in Figure 5B (left panel). Reconstituted erythroblast islands, defined as 4 or more erythroblasts per macrophage, contained 6.6 plus or minus 0.2 (mean ± SEM, n = 5) primitive erythroid cells per in vitro reconstituted island. Because E14.5 primitive erythroblasts can bind to homochronic fetal liver macrophages, we asked whether they could also bind to heterochronic and heterotopic macrophage cells. Specifically, we found that late-stage primitive erythroblasts efficiently bound macrophage cells isolated from E11.5 and E17.5 livers and adult marrow (6.1, 6.3, and 5.1 ϵγ-globin–positive cells/reconstituted island, respectively). In addition, we found that nucleated primitive erythroid cells bound specifically to F4/80-positive macrophages, but not to adherent F4/80-negative cells in the same culture (Figure 5B right panel). These results, taken together, indicate that late-stage primitive erythroid precursors are capable of specifically binding macrophage cells.
α4 integrin is expressed by definitive erythroid precursors and mediates some of their physical interactions with macrophage cells through VCAM-1, the α4 integrin counter-receptor expressed by macrophage cells.19 α4 integrin is expressed on the surface of a subpopulation of primitive erythroid cells.14 We found by flow cytometry that the majority of F4/80-positive liver cells at E15.5 express VCAM-1 on their cell surface (data not shown), confirming recently published immunohistochemical data that fetal liver macrophage cells express VCAM-1.26 Therefore, we tested whether α4 integrin mediates adhesion of primitive erythroblasts to macrophage cells. The addition of α4 integrin blocking antibody, compared with isotype control antibody, resulted in a marked reduction in the number of macrophages that form erythroblast islands (Figure 5C). In contrast, cultures treated with the isotype control antibody had a similar proportion of macrophages in erythroblast islands compared with untreated cultures (Figure 5B,C left panels). Furthermore, the addition of α4 integrin blocking antibody caused a significant reduction in the average number of primitive erythroid cells per erythroblast island (Figure 5D), while isotype control-treated cultures had no significant effect compared with untreated cultures (6.5 ± 0.3 vs 6.6 ± 0.2, respectively). These results are consistent with a role for α4 integrin in the adhesive interactions that occur between primitive erythroblasts and macrophage cells.
Primitive erythroblasts do not autonomously enucleate in vitro but may enucleate when associated with macrophages
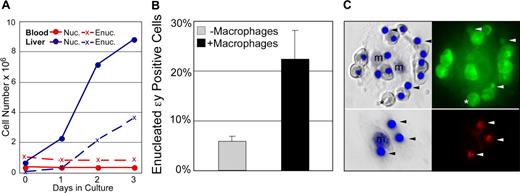
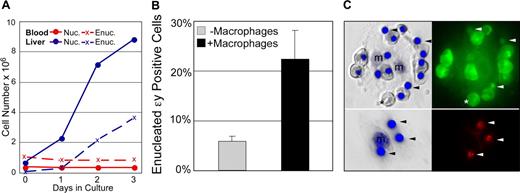
The findings that primitive erythroblasts preferentially localize to the fetal liver in vivo and can physically interact with macrophage cells in vitro raises the question whether primitive erythroid cells, like their definitive counterparts, normally enucleate while associated with macrophages. In control experiments, we first tested whether primitive erythroblasts, like their definitive counterparts, can enucleate without macrophage cells.10,27,–29 E14.5 blood and fetal liver cell suspensions, with macrophage cells removed by differential adhesion, were cultured in cytokine conditions optimized to limit the differentiation of contaminating myeloid progenitors. The culture of E14.5 fetal liver cells in vitro led to the progressive increase in both nucleated and enucleated definitive erythroid cells (Figure 6A blue lines). In contrast, culture of fetal blood in the same conditions revealed no change in the number of nucleated primitive erythroid cells or accumulation of more enucleated cells (Figure 6A red lines).
Primitive erythroid cells enucleate in association with macrophages. (A) Late-stage primitive erythroblasts derived from fetal blood do not autonomously enucleate when cultured in vitro. In control cultures, immature definitive erythroblasts derived from the fetal liver (E14.5 liver, solid blue line) continue to divide as they mature. This maturation includes autonomous enucleation as evidenced by the increasing accumulation of erythrocytes (dashed blue line). The nucleated cells in E14.5 blood are late-stage primitive erythroblasts that have ceased dividing. Unlike definitive cells, late-stage primitive erythroblasts did not complete their maturation by enucleating in vitro as evidenced by the persistence of nucleated cells (solid red line) and the lack of increasing numbers of enucleated erythrocytes (dashed red line). (B) E14.5 primitive erythroblasts enucleate in vitro when cocultured with macrophage cells. The enucleation of primitive blood cells attached to macrophages was significantly higher than that of the same blood cells cultured in parallel without macrophages. The mean plus or minus SEM of 7 independent experiments is plotted. (C) Nuclear engulfment by macrophages is evident after reconstitution of erythroid islands with E14.5 primitive erythroid cells. E14.5 blood was prelabeled with cytotracker dyes to determine the source of nuclei. The left panels are Hoffman contrast images overlayed with HO staining of nuclei. The right panels are fluorescent images revealing the prestained cytotracker green (top) or cytotracker red (bottom) cells. After 48 hours of reconstitution, most of the attached cells from E14.5 blood (top panels) are primitive orthochromatic erythroblasts with occasional enucleated primitive erythrocytes (*). Additional small nuclei, independent of orthochromatic erythroblasts, are also evident (arrowheads). Stripping of erythroblast islands (lower panels) reveals the persistence of labeled nuclei engulfed by macrophage cells.
Primitive erythroid cells enucleate in association with macrophages. (A) Late-stage primitive erythroblasts derived from fetal blood do not autonomously enucleate when cultured in vitro. In control cultures, immature definitive erythroblasts derived from the fetal liver (E14.5 liver, solid blue line) continue to divide as they mature. This maturation includes autonomous enucleation as evidenced by the increasing accumulation of erythrocytes (dashed blue line). The nucleated cells in E14.5 blood are late-stage primitive erythroblasts that have ceased dividing. Unlike definitive cells, late-stage primitive erythroblasts did not complete their maturation by enucleating in vitro as evidenced by the persistence of nucleated cells (solid red line) and the lack of increasing numbers of enucleated erythrocytes (dashed red line). (B) E14.5 primitive erythroblasts enucleate in vitro when cocultured with macrophage cells. The enucleation of primitive blood cells attached to macrophages was significantly higher than that of the same blood cells cultured in parallel without macrophages. The mean plus or minus SEM of 7 independent experiments is plotted. (C) Nuclear engulfment by macrophages is evident after reconstitution of erythroid islands with E14.5 primitive erythroid cells. E14.5 blood was prelabeled with cytotracker dyes to determine the source of nuclei. The left panels are Hoffman contrast images overlayed with HO staining of nuclei. The right panels are fluorescent images revealing the prestained cytotracker green (top) or cytotracker red (bottom) cells. After 48 hours of reconstitution, most of the attached cells from E14.5 blood (top panels) are primitive orthochromatic erythroblasts with occasional enucleated primitive erythrocytes (*). Additional small nuclei, independent of orthochromatic erythroblasts, are also evident (arrowheads). Stripping of erythroblast islands (lower panels) reveals the persistence of labeled nuclei engulfed by macrophage cells.
Because late-stage primitive erythroblasts did not appear capable of autonomous enucleation, we asked whether their coculture with macrophage cells might enhance their enucleation. The in vitro reconstitution assay was used to examine the primitive erythroid cell enucleation in the context of macrophage islands. In control experiments, where fetal blood was cultured without adherent cells, no evidence of autonomous enucleation was found, consistent with the results above (Figure S2). However, after 24 hours of coculture with adherent fetal liver cells, there was a significant increase in enucleation of primitive erythroid cells attached to F4/80-positive macrophages compared with blood cells cultured in parallel without adherent cells (Figure 6B). Furthermore, examination of the macrophage cells after coculture revealed internalized nuclei. The primitive erythroid source of these nuclei was confirmed by prelabeling the nucleated blood cells with cytotracker dyes (Figure 6C). These results, taken together, suggest that primitive erythroblasts enucleate while associated with macrophage cells.
Discussion
Enucleation is the hallmark of erythropoiesis in mammals.1 Definitive erythroid precursors in the fetal liver and postnatal bone marrow enucleate by nuclear extrusion. This process results in the production of reticulocytes and small, nucleated cells with a thin rim of cytoplasm and a cytoplasmic membrane that have been referred to as “extruded erythroblast nuclei.”4,,–7 These “extruded nuclei” undergo rapid loss of cell membrane phosphatidylserine asymmetry and are engulfed by macrophage cells.10,30 Because the cell membrane plays an important role in the biology of these cells, “extruded nucleus” is an inadequate, if not misleading, term. Because they contain an extremely condensed nucleus and have a very high nuclear to cytoplasmic ratio, we propose that these cells be termed “pyrenocytes,” derived from the Greek words “pyren” (πυρην, the pit of a stone fruit) and “cyte” (κυτος, cell).
In contrast to this process of nuclear extrusion seen in definitive erythropoiesis, it has been suggested that primitive erythroid cells in rodents enucleate by karyolysis.23,24 However, we did not find evidence of internal lysis of the nucleus in circulating late-stage primitive erythroblasts or evidence of DNA laddering in these cells even when exposed to staurosporine. In contrast, we identified a transient population of primitive pyrenocytes in the bloodstream and fetal liver of mouse embryos that appeared concurrent with the onset of primitive erythroblast enucleation. A similar, transient population of primitive pyrenocytes, described as “nuclei with a small amount of cytoplasm,” has been described in the bloodstream of hamster fetuses.11 Our studies support the concept that primitive erythroblasts undergo a semisynchronous wave of enucleation by nuclear extrusion to form anucleate primitive erythrocytes and pyrenocytes.
It was observed 50 years ago that definitive erythroblasts mature while attached to a central macrophage cell within erythroblast islands.31 Subsequently, it was found that erythroblast islands could be isolated in vitro and that erythroblast cells could be stripped from, and subsequently added back to, the macrophage cells to reconstitute islands.22,26,32,–34 Using a similar experimental approach, we found that circulating late-stage primitive erythroid precursors can reconstitute erythroblast islands by attaching to fetal liver– or adult marrow–derived macrophage cells. This surprising finding indicates that circulating primitive erythroblasts have the potential to physically interact with macrophage cells. Studies of erythroblast islands in the bone marrow indicate that several adhesion molecules mediate the physical interactions between definitive erythroblasts and macrophage cells, including α4 integrin, emp, and ICAM-4.19,34,35 α4 integrin has been found on the surface of a subset of primitive erythroblasts and its counter-receptor VCAM-1 is expressed by fetal liver macrophage cells.14,26 Here we have demonstrated a functional role for α4 integrin in primitive erythroblast-macrophage interactions. Preliminary studies indicate that primitive erythroblasts also express emp and ICAM4 (data not shown). It will be of interest to determine which other adhesion molecules are expressed by primitive erythroid cells and whether these molecules contribute to the physical interactions that occur with macrophage cells.
Several findings support the concept that primitive erythroblast-macrophage interactions may occur in vivo. First, the ratio of nucleated to enucleated primitive erythroblasts is higher in the fetal liver than in the bloodstream, indicating that nucleated primitive erythroblasts are enriched in the liver. Second, immunohistochemical studies indicate that primitive erythroid cells and macrophage cells are in close physical proximity in the fetal liver but not the fetal spleen or placenta. Finally, primitive erythroid cells were occasionally found to constitute components of erythroblast islands isolated from the fetal liver. Macrophage cells in the fetal liver and bone marrow engulf definitive pyrenocytes that have lost phosphatidylserine asymmetry.10,30 We found that a significant proportion of primitive pyrenocytes in the fetal bloodstream are annexin V–positive and that nuclei derived from primitive erythroblasts are present within macrophage cells after in vitro coculture. It is likely that the enucleation of primitive erythroblasts in close physical proximity to macrophage cells facilitates the engulfment of newly formed pyrenocytes and that some primitive pyrenocytes may temporarily escape this fate in vivo leading to their transient appearance in the bloodstream.
A fundamental difference between primitive and definitive erythropoiesis is the maturation of primitive erythroid cells in the circulation while definitive erythroid cells mature extravascularly in erythroblast islands. It has been proposed that macrophage cells serve several roles to facilitate the maturation of definitive erythroid precursors including the provision of cytokines and iron, the enhancement of erythroblast proliferation, and the bringing of erythroid cells in close proximity for regulatory purposes as, for example, fas–FasL interactions.36,,,–40 In contrast, primitive erythroid cells mature in the bloodstream and thus appear to have circumvented macrophage interactions during early steps of erythroblast maturation. A specific role for macrophages in the process of definitive erythroid cell enucleation remains unclear,33,35,41 particularly because large percentages of definitive erythroblasts can enucleate in vitro in the absence of macrophage or stromal cells10,27,29,42,–44 and in vitro culture of maturing definitive erythroblasts does not enhance their enucleation.39 In striking contrast, primitive erythroid cells are unable to enucleate autonomously in vitro; however, coculture of late-stage primitive erythroblasts with macrophages leads to a small, but significant, increase in enucleation compared with blood cells cultured in parallel without macrophages. It is currently not known whether circulating primitive erythroblasts contain inhibitory mechanisms preventing enucleation or whether they lack intrinsic machinery necessary for autonomous nuclear extrusion. The continued study of the similarities and differences between primitive and definitive erythropoiesis will lead to an improved understanding of erythroblast maturation and the terminal steps of erythroid differentiation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Rachael Emerson and Sarah Mack for assistance with immunohistochemistry.
This work was supported by National Institutes of Health and Dean's Research Award (University of Rochester).
National Institutes of Health
Authorship
Contribution: K.E.M. designed and performed experiments, analyzed data, and wrote the manuscript; P.D.K. performed experiments and generated antibody, fetal dissections, and morphometry; A.D.K. performed experiments and animal husbandry; R.L.P. performed experiments; T.P.B. assisted with flow cytometry; J.P. designed experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James Palis, University of Rochester Medical Center, Center for Pediatric Biomedical Research, Box 703, 601 Elmwood Ave, Rochester, NY 14642; e-mail: james_palis@urmc.rochester.edu.

![Figure 3. Primitive pyrenocytes constitute a distinct cellular population in the fetal bloodstream. (A) Relative size of nucleated primitive erythroid cells as determined by ImageStream analysis reveals that pyrenocytes account for approximately 2% of nucleated blood cells in E15.5 blood (red line), less than 1% at E14.5 (blue line), and are not detected at E12.5 (green line). (B) Example of images of fetal blood cells visualized using the ImagesStream. Concurrent brightfield (BF) images and fluorescent images (anti–ϵγ-globin antibodies [ϵγ], Ter119, and the DNA stain [Draq5]) of larger primitive orthochromatic erythroblasts (rows 1 and 2) and of smaller primitive pyrenocytes (rows 3 and 4). (C) Primitive pyrenocytes and primitive orthochromatic erythroblasts (EryP) in the E15.5 circulation have similar nuclear sizes. Error bars represent SD. (D) E15.5 blood stained with annexin V (AnV), Ter119, and Draq5 (shown as merged images), and 7AAD analyzed by the ImageStream. Row 1 is a primitive pyrenocyte that is annexin V–positive and 7AAD-negative. Row 2 is a primitive orthochromatic erythroblasts that is negative for annexin V and 7AAD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/4/10.1182_blood-2007-08-107581/3/m_zh80030814500003.jpeg?Expires=1766249020&Signature=BKRDAXgrXglYe2F3n3viu1G2B63CDs2Uh60CbNOU605mgWPxi5LSUPJvceREowPof2d50OxGrSvPs8R4OCl720Rnz2XkUIzXokCBJcuLAtsd2Ihzy7WcQZ7QSffw2ge6agrFPQrEJRimfjJU7WMOiF52j90HIWjWTu-Xi4oh0JPXTHMQWvSfUW09xm-XKRtTtceSje3T~0-LgllfNhSCDyEQxDkD1xO7bmDi5UkhblPYxLue4VhkfutPPMc3JVwCHbDQnLCsw5703cZ~dXCE6SXTuwmoTamY02U8Fbfa04PYxKUndTMrkaKUipCZCql0GMKoYgUNtcTXUbhdTlsw~A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 3. Primitive pyrenocytes constitute a distinct cellular population in the fetal bloodstream. (A) Relative size of nucleated primitive erythroid cells as determined by ImageStream analysis reveals that pyrenocytes account for approximately 2% of nucleated blood cells in E15.5 blood (red line), less than 1% at E14.5 (blue line), and are not detected at E12.5 (green line). (B) Example of images of fetal blood cells visualized using the ImagesStream. Concurrent brightfield (BF) images and fluorescent images (anti–ϵγ-globin antibodies [ϵγ], Ter119, and the DNA stain [Draq5]) of larger primitive orthochromatic erythroblasts (rows 1 and 2) and of smaller primitive pyrenocytes (rows 3 and 4). (C) Primitive pyrenocytes and primitive orthochromatic erythroblasts (EryP) in the E15.5 circulation have similar nuclear sizes. Error bars represent SD. (D) E15.5 blood stained with annexin V (AnV), Ter119, and Draq5 (shown as merged images), and 7AAD analyzed by the ImageStream. Row 1 is a primitive pyrenocyte that is annexin V–positive and 7AAD-negative. Row 2 is a primitive orthochromatic erythroblasts that is negative for annexin V and 7AAD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/4/10.1182_blood-2007-08-107581/3/m_zh80030814500003.jpeg?Expires=1767406709&Signature=w7~YX8SETJbRc8Ug8RrELqAP3u1oRy9i-Mup9VWpguq6pKzllWf~89~NCfGzZ11q734hCq6vH~EtvhGtZcBelDW2DZd~zLNstJwmXZI5xGQeeK9xdjE3b0lKxl-QWw066jasHyRf0tp5V9NqLucVxfX3IJKwRXZyfkMd1rrgWhm0HinXpyu2ijXFrj8JPxu3FgB8qI61mRFjFQQFJ8vsxUUxRPPDKL5TcxoUKDMSCxVaeFguNaZuRqu5obM6dRAoeNIgNnwDv1bldTwQ78DEJzY021GSmTNuQWMZEZlClLyGVavpULTAwXyc3MTTwVdby7Re9CeYLFXqipTBfg8PzQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)