Mast cells (MCs) are responsible for host defenses against bacterial, viral, and parasitic infection through the activation of complement-mediated or Toll-like receptor (TLR)–mediated innate immune responses and IgE/antigen-FcϵRI–mediated acquired immune responses.1 However, excessive or inappropriate MC activation also contributes to the development of certain allergic or autoimmune diseases, which are mediated by allergen- or autoantigen-specific effector T cells.1
MCs are capable of presenting antigens to T cells by direct cell-cell interaction through antigen-specific T-cell receptor (TCR) and major histocompatibility complex (MHC) class I– or class II–restricted mechanisms in vitro.1 Exosomes released from MCs, which contain MHC class II and antigen complexes, participate in antigen-specific T-cell expansion/activation independently of direct cell-cell contact between T cells and MCs in vitro.1 However, it has remained unclear whether MCs have such functions in vivo.
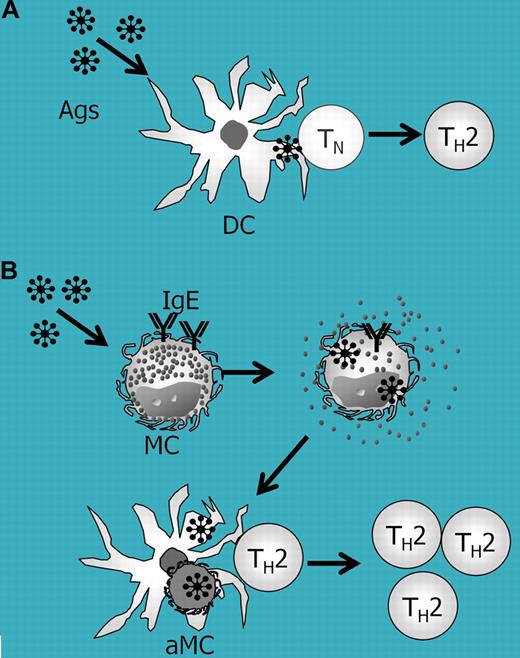
In the February 1 issue of Blood, Kambayashi and coworkers provided a novel molecular mechanism for MHC class II–restricted, antigen-specific T-cell expansion/activation by murine bone marrow–derived cultured MCs (BMCMCs) in a series of well-designed and elegant experiments. Consistent with prior work,2 they demonstrated that naive BMCMCs showed poor antigen-presenting capabilities to CD4+ T cells from MHC class II–restricted and the ovalbumin (OVA)-specific TCR-transgenic (OTII) stain. However, MHC class II–deficient BMCMCs stimulated in advance with anti-OVA IgE and OVA were found to clearly facilitate OTII T-cell activation without further addition of OVA. In addition, IgE/antigen-FcϵRI crosslinking enhanced the internalization of IgE/antigen complexes in MCs, and induced MC apoptosis by down-modulating Bcl-XL expression. The authors also demonstrated that apoptotic MCs carrying intracellularly incorporated antigens were phagocytosed by dendritic cells (DCs). Then, DCs processed the MC-derived intracellular antigens, presented them to CD4+ T cells, and thus induced the expansion/activation of antigen-specific T cells (see figure). These findings suggest that MCs can work as antigen reservoirs and can act as “bridging cells” that transfer the antigen to potent antigen-presenting cells such as DCs.
Role of MCs in IgE- and dendritic-cell–mediated antigen-specific T-cell expansion. (A) In a conventional antigen-presentation model, dendritic cells (DCs) incorporate antigens (Ags) and present antigen to naive T (TN) or memory T (TH2) cells. (B) In the MC-mediated, IgE-mediated, and DC-mediated antigen presentation model, IgE/antigen-FcϵRI crosslinking promotes antigen internalization and apoptosis in MCs. DCs then uptake the apoptotic MCs (aMCs) carrying internalized antigens, and thus can efficiently present the antigen to antigen-specific memory T cells.
Role of MCs in IgE- and dendritic-cell–mediated antigen-specific T-cell expansion. (A) In a conventional antigen-presentation model, dendritic cells (DCs) incorporate antigens (Ags) and present antigen to naive T (TN) or memory T (TH2) cells. (B) In the MC-mediated, IgE-mediated, and DC-mediated antigen presentation model, IgE/antigen-FcϵRI crosslinking promotes antigen internalization and apoptosis in MCs. DCs then uptake the apoptotic MCs (aMCs) carrying internalized antigens, and thus can efficiently present the antigen to antigen-specific memory T cells.
MCs exist in close proximity with T cells in human tonsils3 as well as at the inflammatory sites in mice,2 and in close proximity with DCs in most tissues.4 In addition, MCs can migrate into draining lymph nodes from antigen entry sites.5 Based on the present work by Kambayashi and coworkers, it is possible that IgE/antigen-captured MCs become apoptotic in T-cell zones1 in secondary lymphoid tissues and/or at antigen-challenged inflammatory sites. Thus, DCs can present MC-derived antigens to “antigen-specific,” that is, already antigen-sensitized, memory T helper cells. In contrast to a previous report indicating that MC-mediated T-cell activation is Ig/antigen-FcR independent,2 the findings of Kambayashi and colleagues indicate that MC-mediated T-cell activation is dependent on IgE, and thus it cannot be adopted for less/non-IgE–mediated diseases such as autoimmune disorders. Nevertheless, the present findings provide new insight into our understanding of the molecular mechanisms in-volved in the development of IgE- and MC-mediated allergic diseases.
Conflict-of-interest disclosure: H.S. received a grant from the National Institute of Biomedical Innovation. The other authors declare no competing financial interests. ■