Multisystem Langerhans cell histiocytosis (MS-LCH) is associated with high mortality when patients have risk organ involvement (RO+) or are younger than 2 years. In an international randomized trial, LCH-II, we intensified their treatment: arm A consisted of 6 weeks of daily prednisone and weekly vinblastine followed by 18 weeks of daily 6-mercaptopurine with vinblastine/prednisone pulses; etoposide was added in arm B. Considering all 193 randomized risk patients, there were similar outcomes: rapid (6 weeks) response (arm A vs arm B: 63%/71%), 5-year survival probability (74%/79%), disease reactivation frequency (46%/46%), and permanent consequences (43%/37%). However, (1) patients younger than 2 years without RO involvement (RO−) had 100% survival and uniformly high (> 80%) rapid response, (2) RO+ patients not responding within 6 weeks had highest mortality, and (3) importantly, the more intensive arm B reduced mortality in RO+ patients (relative hazard rate, accounting for differences in risk organ involvement, of 0.54; 95% CI = 0.29-1.00). Finally, comparison of RO+ patients in LCH-I and LCH-II confirmed that increasing treatment intensity increased rapid responses (from 43% in arm A LCH-I to 68% in arm B LCH-II; P = .027) and reduced mortality (from 44% in arm A LCH-I to 27% in arm B LCH-II; P = .042). We conclude that intensified treatment significantly increases rapid response and reduces mortality in risk MS-LCH. This trial was registered at http://www.controlled-trials.com as no. ISRCTN57679341.
Introduction
Langerhans cell histiocytosis (LCH) is a reactive clonal proliferation of dendritic cells1,–3 that comprises a wide range of clinical presentations, from localized disease (single-system disease) with excellent outcome to disseminated disease involving 2 and more organs or systems (multisystem disease, MS-LCH).4,–6 Treatment has varied from conservative to intensive combination chemotherapy.7,,–10 When the systems involved are “risk organs” and/or the patient is younger than 2 years at diagnosis, MS-LCH has been considered particularly devastating, and as carrying a potentially fatal prognosis.11,12
In 1991, the Histiocyte Society initiated LCH-I, a randomized international clinical trial for MS-LCH, comparing the efficacy of 6-month single-agent therapy with either vinblastine or etoposide (VP-16).13 The drugs did not differ in disease response, reactivation, permanent consequences, survival, or toxicity. However, the results were inferior in some respects to previous reports based on more aggressive therapy.8,13 That suggested that therapy for MS-LCH patients should be intensified. We therefore designed the next randomized controlled trial, LCH-II, to evaluate combining vinblastine and VP-16, in an intensified 6-month regimen.
Methods
Patient entry and randomization
Eligible patients (confirmed histopathology, multisystem [MS] disease, age younger than 18 years, and no prior specific therapy) were entered, with informed consent obtained in accordance with the Declaration of Helsinki, from 98 pediatric institutions through 7 study subcenters and stratified into 2 groups, risk and low risk.14,15 The study and study protocol have been approved by the Institutional Review Board of each institution participating in the trial. The risk patients, the subject of this report, either had involvement of risk organs (RO+; ie, liver, lung, and hematopoietic system or spleen) or had disease onset at younger than 2 years of age.11,12 Low-risk MS-LCH patients, those 2 years or older and without RO involvement (RO−), will be reported elsewhere.
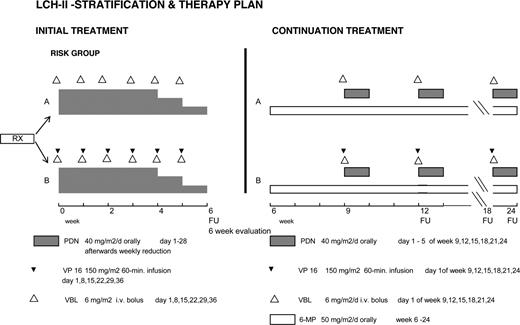
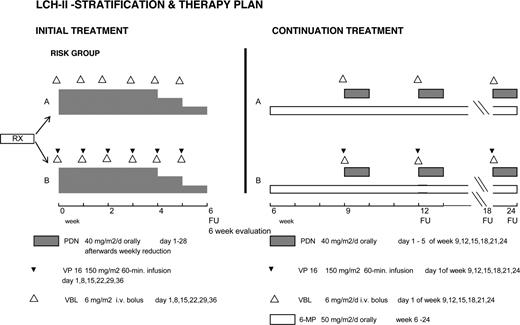
Patients randomized to arm A received an initial treatment of continuous oral prednisone (40 mg/m2 daily in 3 doses for 4 weeks tapering over 2 weeks) and vinblastine (6 mg/m2 intravenous bolus weekly for 6 weeks); those in arm B received etoposide in addition (150 mg/m2 per day, 1-hour infusion weekly for 6 weeks). Continuation therapy was 6-mercaptopurine (50 mg/m2 daily orally) and pulses of oral prednisone (40 mg/m2 daily in 3 doses, days 1-5) and vinblastine (6 mg/m2 per day once every 3 weeks) in arm A, with 150 mg/m2 VP-16 every 3 weeks added in arm B. The total duration of treatment was 24 weeks (Figure 1).
Study objectives
The primary end point was response after 6 weeks of therapy (rapid response), defined as complete resolution (no evidence of active disease, NAD) or continuous regression of all signs and symptoms. Patients who were “worse” (disease progression or death) or “intermediate” (“stable” but active disease or the appearance of new lesions in one site and regression in another)4,5,13 were considered nonresponders. Re-evaluations were performed after 12, 18, and 24 weeks of therapy, then every 3 months for 2 years, every 6 months for 2 years, and then yearly. Secondary end points were comparisons of therapeutic efficacy with respect to overall survival, time to NAD, reactivations after NAD, incidence of permanent consequences (PCs), and treatment-related toxicities. Nonresponse by week 6 was considered treatment failure and the protocol recommended switching from arm A to arm B, or from arm B to a salvage therapy of the LCH-S studies.16,17
Statistical methods
All data, compiled using standardized questionnaires, were reviewed for safety and completeness by an independent review board (Mainz, Germany). Analyses were conducted based on the intention-to-treat principle, at the study reference center (Vienna, Austria).
The primary end points (ie, rapid response rates) of the 2 treatment arms were compared using the χ2 test, and the confidence interval for the difference in response rates was calculated according to Newcombe Method 10.18 Interim analyses were planned using an error-spending procedure with rules for early termination according to O'Brien and Fleming.19 According to our power calculation, the estimated response rate in the control group (prednisone and vinblastine without etoposide) was predicted to be 60% (derived from the LCH-I data13 ). With the addition of etoposide, we sought to detect an improvement of the response rate by 20% (ie, a response rate of 80%). With a sample size of 75 patients in each group and a significance level of 5% (2-sided), the power was considered to be 76%.
The secondary end points were survival, disease resolution, incidence of reactivation, and permanent consequences. Survival probabilities from randomization were estimated to each participant's last follow-up evaluation based on the Kaplan-Meier method and differences in survival were assessed by the log-rank test. The analysis of RO+ patients was stratified according to subcenter. In an exploratory analysis, Cox regression was used to evaluate the impact of each arm of LCH-II accounting for differences in risk organ involvement, as well as increasing treatment intensity in the 4 arms of the 2 sequential randomized studies, LCH-I and LCH-II (LCH-I arm A < LCH-I arm B < LCH-II arm A < LCH-II arm B) on survival. For the evaluation of 6-week response, logistic regression was used.
Cumulative incidence rates were used to estimate the rate of complete disease resolution, disease reactivation after NAD, and PCs, to account for the competing risk of death.20 The rate of development of PCs was calculated in those patients with no evidence of these at diagnosis, and statistical comparisons were performed as described by Gray.21 Toxicity frequencies were compared by the χ2 test and by WHO grade 0-IV using the Cochran-Armitage test for trend.22 Sensitivity analyses were performed to assess the impact of missing data, local pathological diagnosis, or patient recruitment through different subcenters, on the primary end point of the study, the response at 6 weeks. This analysis showed that it is highly unlikely that missing data regarding response at 6 weeks (the nonevaluable patients) could significantly change the conclusions of the findings of the study.
Results
Patient accrual and treatment
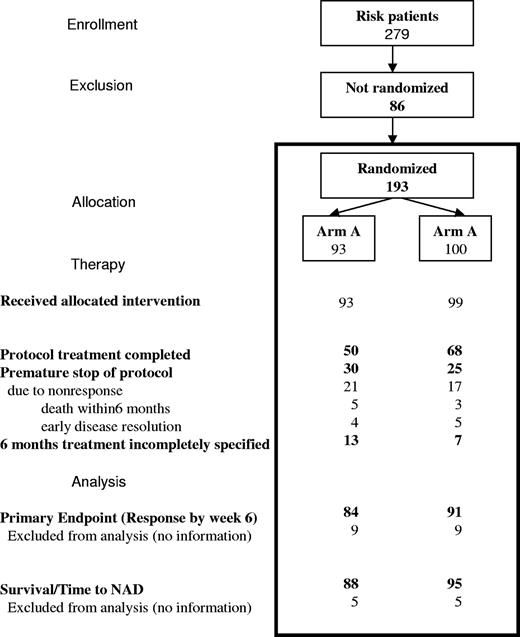
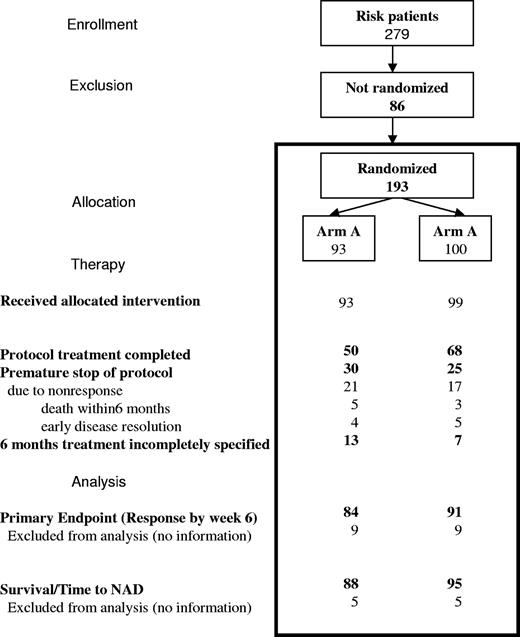
From May 1996 through March 2001, 193 of 279 patients enrolled in the risk group were randomized, 93 to arm A and 100 to arm B. Demographic characteristics between the 2 arms were equivalent (Table 1), except for some unexpected mild differences in the risk profile between the 2 arms. One hundred forty-six of 193 patients were RO+ and 47, who were RO−, were included because of disease onset at younger than 2 years. Of those not randomized, proportionately more were in the RO− group, in which the randomization rate was only 55%, compared with 75% of RO+ patients. Fifteen RO+ patients lacked complete histopathologic confirmation.
Sixty-eight percent of randomized patients (50/80 in arm A, 68/93 in arm B) completed the 24 weeks of treatment per protocol (Figure 2). Nine responders (4 in arm A, 5 in arm B, 5%) with early complete disease resolution stopped therapy prematurely, while 8 patients (all RO+ at diagnosis; 5/85 in arm A, 3/93 in arm B) died during the 6-month treatment period.
Patient flow through the stages of the randomized trial for risk MS-LCH.
Response at week 6
The primary goal of the study was to achieve a higher rate of rapid response. Response data were available on 91% (175/193) of the randomized patients: 63% in arm A and 71% in arm B were rapid responders (Table 2, P = .24; difference = 8%, 95% CI = −6% to 21%). Among the RO+ patients, rapid responses were somewhat less frequent: 56% in arm A and 68% in arm B (P = .18; difference = 12%, 95% CI = −4% to 31%). Importantly, the 42 young (< 2 years old) RO− patients had a much higher rapid response rate (82% arm A, 85% arm B) than did RO+ patients (P = .012; Table 2).
Survival
The 5-year survival probability (pSU) for all risk patients was 0.74 (± 0.05; mean ± SEM) in arm A and 0.79 (± 0.04) in arm B (Table 2, P = .26; difference = 5%, 95% CI = −11% to 21%). Causes of death were disease progression (11 in arm A, 10 in arm B), infectious complications (5 in arm A, 5 in arm B), organ failure (4 in arm A, 2 in arm B), and acute nonlymphoblastic leukemia (1 in arm B); 4 deaths were from unreported causes (3 in arm A, 1 in arm B). Mortality within 6 months (the anticipated treatment duration) was lower in arm B (5% ± 2%) than in arm A (12% ± 4%), and deaths after 2 years from diagnosis were rare (n = 3). Significantly, all patients with disease onset at younger than 2 years but who were RO− survived (Table 2). The unadjusted pSU of the 138 RO+ patients was 0.64 (± 0.06) in arm A and 0.73 (± 0.05) in arm B (P = .14; difference = 9%, 95% CI = −12% to 31%). In an exploratory analysis, RO+ patients were stratified by clinical site, and accounting for differences in risk organ involvement, Cox regression analysis uncovered significantly lower mortality in arm B versus arm A (relative hazard rate: 0.54, 95% CI = 0.29-1.00; P = .049). Finally, responders in each arm had a superior survival rate to nonresponders in that arm (arm A: P < .001, arm B: P = .003; Figure 3).
Rapid response and survival of MS-LCH RO+ patients in LCH-II. Kaplan-Meier analysis of survival of the responders (top line) and the nonresponders (ie, intermediate [IR, middle line] and worse [bottom line]). The bar graphs compare survival in responders (Rs) versus nonresponders (NRs) in each therapy arm. Error bars represent SEM.
Rapid response and survival of MS-LCH RO+ patients in LCH-II. Kaplan-Meier analysis of survival of the responders (top line) and the nonresponders (ie, intermediate [IR, middle line] and worse [bottom line]). The bar graphs compare survival in responders (Rs) versus nonresponders (NRs) in each therapy arm. Error bars represent SEM.
Nonresponse within 6 months triggered a therapy switch, according to the protocol, in 26% (21/80) of patients in arm A and 18% (17/93) in arm B. Of these 38 patients, 11 (52%) in arm A and 7 (41%) in arm B died after switch to a salvage protocol, showing that nonresponse is associated with high mortality that was not averted by a switch to another treatment, and that the rate of mortality after nonresponse to either of the 2 therapy arms was similar.
Disease resolution, reactivation, and permanent consequences
Seventy percent (124/178) of risk patients became disease free. Achievement of NAD was greater in arm B, both within the first year (62%; compared with 49% in arm A) as well as over the entire 5-year study period (P < .036; difference = 7%, 95% CI = −6% to 20%). After 5 years, few patients in each arm still had active disease. Forty-nine percent of RO+ patients and 78% of patients younger than 2 years without RO involvement achieved NAD by 1 year (Table 2).
Reactivations occurred in 45% of the patients who achieved NAD (Table 2), with an equal probability of reactivation within 3 years of NAD in each arm (0.46 ± 0.07) and no substantial difference in the sites of reactivations. Importantly, most reactivations were mild and did not occur in risk organs, but rather in sites such as bone, skin, and pituitary (diabetes insipidus, DI); the 3-year incidence of reactivations in risk organs was only 0.08 (± 0.04) in arm A and 0.13 (± 0.04) in arm B and never occurred in a patient without initial RO involvement.
PCs13,23 resulting from LCH were present at diagnosis in 19% of patients (Table 2) and developed subsequently in an additional 38%. The probability of newly developing PCs within 1 and 5 years after diagnosis, respectively was almost equal in the 2 treatment arms (0.19 ± 0.05 and 0.43 ± 0.07 in arm A, and 0.17 ± 0.04 and 0.37 ± 0.06 in arm B). DI was the most frequent PC, present in 14 patients at diagnosis and developing in 28 patients during follow-up.24 An unexpected common finding of concern was liver fibrosis,25 present at diagnosis in 9 patients and developing subsequently in an additional 9. Seventeen patients had lung fibrosis26,27 (4 patients at diagnosis and 13 subsequently). CNS radiographic abnormalities,28 detected in 15 patients, were associated with clinical symptoms in 4 patients. Other PCs (endocrinopathies, growth failure, orthopedic problems, hearing impairment) were rare. The overall incidence of PCs in LCH-II was similar to that in LCH-I.13
Toxicity
Toxicity was similar in the 2 arms and was comparable with that in LCH-I.13 Seventeen patients, equally distributed between arms A and B, had severe toxicity (WHO score III or IV) including severe thrombocytopenia in 7, leucopenia in 5, and hepatotoxicity in 2. It was not possible to distinguish between disease-induced and therapy-related hematopoietic and hepatic toxicity. One patient developed an atypical acute myeloid leukemia with monosomy 7 at 11 months after diagnosis and a relatively low cumulative dose of 1350 mg/m2 VP-16, raising the question as to whether the leukemia was treatment related29,30 or a separate process.31
Impact of treatment intensity in MS-LCH
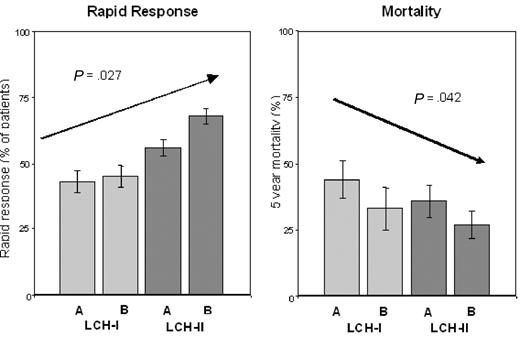
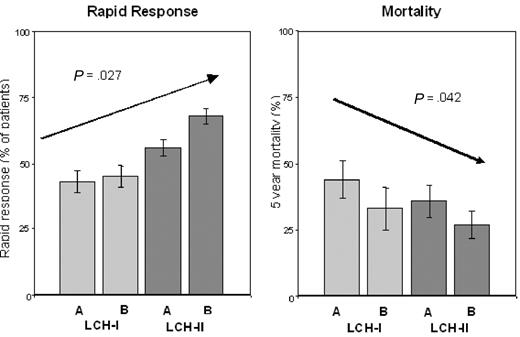
The higher RO+ patient survival in the more intensive arm B in LCH-II led us to perform a combined analysis of rapid response and survival in LCH-II and the less intensive previous LCH-I trial (for which all entry and follow-up criteria were identical), to assess the impact of increasing treatment intensity between the 2 studies. We found a markedly higher rapid response rate (67%) taking all (ie, RO+ and RO−) patients in LCH-II together, versus LCH-I (43%, P < .001). And, the increasing treatment intensity over the 4 arms of these 2 controlled studies was associated with an increase in the rapid response rate, both in all patients (from 47% in arm A LCH-I to 72% in arm B LCH-II; P = .002) and in RO+ patients (from 43% in arm A LCH-I to 68% in arm B LCH-II; P = .027; Figure 4). Very importantly, mortality in RO+ MS-LCH decreased from 44% in arm A LCH-I, to 27% in arm B LCH-II (P = .042; Figure 4).
Rapid response and mortality in the 4 treatment arms of LCH-I and LCH-II. The achievement of a rapid response (left panel) and the 5-year mortality (right panel) of RO+ patients in the individual treatment arms of LCH-I (A and B) and LCH-II (A and B) displayed according to increasing treatment intensity (left to right) are compared. Error bars represent SEM.
Rapid response and mortality in the 4 treatment arms of LCH-I and LCH-II. The achievement of a rapid response (left panel) and the 5-year mortality (right panel) of RO+ patients in the individual treatment arms of LCH-I (A and B) and LCH-II (A and B) displayed according to increasing treatment intensity (left to right) are compared. Error bars represent SEM.
Discussion
LCH-II, an international cooperative prospective randomized clinical trial for the treatment of Langerhans cell histiocytosis, was built upon a strategy developed from findings of our previous study, LCH-I,13 and several earlier single and multicenter nonrandomized trials.6,–8,32,33 These studies showed that systematic approaches are critical to effective treatment and that lack of response after an initial 6-week treatment is a negative prognostic indicator in patients with risk MS-LCH.5,6,8,33,34 Building upon LCH-I, LCH-II intensified treatment during the first 6 weeks of therapy by combining the 2 agents tested individually in LCH-I, vinblastine and etoposide, and adding continuous prednisone, with the ultimate goal of reducing mortality in MS-LCH. Treatment was well tolerated and reported toxicity was mostly mild. The effect of this more intensive therapy in LCH-II was an increased rapid response that was clearly superior to that of LCH-I, showing that the proportion of responders at week 6 is influenced by the intensity of initial treatment.
Despite improvements in the treatment of LCH over the past decades, mortality has remained high.7,8,13 Thus, it was a central goal of LCH-II to improve patient survival by intensifying the initial and continuation phases of the 6-month treatment. At first glance, it appeared that overall survival (ie, of all patients randomized in LCH-II) was only slightly improved over that in LCH-I. However, when we examined survival of RO+ patients, stratified by clinical site and accounting for differences in risk organ involvement, a significantly higher survival rate was associated with the more intensive arm B. We discovered that RO involvement is a discriminating risk factor for mortality. Surprisingly, when RO involvement was used to define mortality risk, young age itself, initially identified by Lahey as a risk factor12 in LCH, no longer appears to be a risk factor for mortality; there was no death of any RO− patient, even those younger than 2 years. Hopefully, this outcome will dispel the widely held opinion that young age, per se, portends a risk of mortality in LCH.4,11,–13,23 Using this more refined definition of the risk category (ie, RO+), we then analyzed the impact of a rapid response on survival, and confirmed that persistence of active disease after an initial 6 weeks of treatment is a second negative prognostic factor. In this high-risk group (RO+ MS-LCH with persistence at week 6), we saw the first signs of an increase in survival using the more intensive arm B (Figure 3).
In an exploratory approach, we performed a comprehensive analysis of the 2 sequential studies, LCH-I and LCH-II, and we observed that rapid response to treatment occurred more likely with the incremental increases in treatment intensity. Rapid response, together with the more intensive continuation therapy of LCH-II, was also reflected in improved survival. Possibly, extending the 6-month total treatment of LCH-II to a full year might further reduce the overall mortality since the Kaplan-Meier analysis showed that almost all mortality occurs within slightly more than a year of the diagnosis of LCH (Figure 3). This is currently being tested in LCH-III. As a corollary, RO+ patients who are nonresponsive should be candidates for early use of experimental therapies, as continuing them on further treatment was shown to be ineffective in inducing disease resolution. This also underscores the importance of early identification of these patients with high risk of mortality (ie, those not responding within 6 weeks). Possible approaches include different drug combinations, such as 2-CdA combined with cytosine-arabinoside. This combination has shown some effectiveness,35 in contrast to 2-CDA used as a single agent in patients who were nonresponders to their initial treatment in LCH-II. We did not observe striking responses to 2-CDA alone in these patients (mortality rate of 46%, compared with the overall mortality rate of 52% among all patients who switched from their initial therapy). Another possible approach that shows promise is allogeneic stem-cell transplantation (SCT) with intensity-reduced conditioning.36
Our findings suggest that vinblastine and etoposide remain mainstays in the treatment of LCH. In fact, until now, these are the only drugs that have been studied and shown to be effective in prospective randomized trials (as single agents in LCH-I and in combination in LCH-II), and it has been shown that these drugs are also efficient in treating recurrent disease. Whether the positive results of our trials can be extended to the use of these drugs in treating adults affected by LCH remains to be determined; a prospective study of this question is currently under way. Similarly, in related variant disorders such as Erdheim-Chester disease, in which anecdotally a few patients have been successfully treated with continuous vinblastine or etoposide, a prospective study may be warranted as well.
Somewhat surprisingly, incidences of reactivation and of PCs were not reduced by the more intensive regimens of LCH-II, compared with LCH-I.13 Intensive therapy appears to have a profound effect on patients with very-high-risk disease but has shown little to no advantage over milder therapy with respect to disease resolution, reactivation, or PCs.13,37,38 These become the problematic aspects of LCH once the risk of mortality has been alleviated.37,–39 Whether a milder but longer continuation treatment could be more effective in preventing reactivations, as suggested by the DAL-HX studies,8,34 remains to be tested and is a central question being asked in the Histiocyte Society LCH-III study.
Two salient points derived from the overall findings are worth reemphasizing. First, in patients with risk organ involvement, the observed increase in the proportion of patients achieving a rapid response to 60% to 70%, the reduction of mortality to 20% to 25%, and the disappearance of risk organ dysfunction in completely responding patients all support the use of relatively intense therapy such as in LCH-II. Second, the results of LCH-II underscore that it is not age itself but the therapeutic response in risk organs that predicts outcome in young (< 2 years old) children with RO+ LCH. In the past, it was quite possibly the relatively high incidence of LCH with risk organ involvement in very young (versus older) children combined with less successful treatment then, than now, that caused the widely held belief that young age is responsible for higher mortality of LCH. With our current knowledge, therapy now can be tailored to the extent of disease rather than the age of the patient.
In conclusion, the results of LCH-II clearly underscore that RO involvement and rapid response to initial therapy are especially important in predicting outcome. When we compare outcomes in the consecutive randomized LCH-I and LCH-II studies, there is a strong indication that increasing treatment intensity was associated with higher rapid response and survival rates. This suggests that therapy intensification, often shunned because of feelings either of hopelessness or of lack of necessity, may be essential for successful treatment of high-risk disease. Based on our experience, we can feel more confident about a positive outcome in the majority of patients and less fearful of “overtreating” certain patient subgroups. We should continue to explore new intensive therapies for the high-risk patients, while low-risk MS-LCH patients, identified by our trials, can be excluded from intensive initial treatment. Of particular significance, patients with high-risk disease who respond rapidly to therapy, and very young patients without RO involvement, can be given a much more optimistic prognosis for survival than has been the case in the past.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the additional members of the LCH-II Study Committee: D. Komp, MD, S. Nicholson, MD, and J. Pritchard, MD, for contributions in planning the study, and the many physicians who participated in the LCH-II Study. We also thank Elfriede Thiem for the excellent data management at the study reference center in Vienna (Austria) and D. J. Michaelis, PhD, head of the independent review board at the Institute of Medical Statistics, Mainz, Germany, for the regular assessment of study progress and stopping rules.
This work was supported by the Histiocytosis Association of America, the Histiocytosis Association of Canada, the Children's Cancer Research Institute (CCRI; Vienna, Austria), Programme Hospitalier de Recherche Clinique 96 (PHRC96) grants, Ministère de la Santé et des Affaires Sociales (France), the Italian Ministry of Health, Ricerca Finalizzata 2004, Associazioni Italiana per la Ricerca sul Cancro e Istiocitosi (AIRI and AIRC; Italy), and the Children's Research Institute (Washington).
Authorship
Contribution: H.G. and S.L. wrote the paper; U.P. and R.M. performed the statistical analyses; N.G. and M.M. contributed to study coordination, data collection, and analysis; M.A., J.B., V.B., J.D., and J.-I. H. made clinical contributions and provided critical paper review; all authors were involved in study design and final approval of the paper.
A list of the Histiocyte Society LCH II study committee and national study coordinators appears in Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Helmut Gadner, Children's Cancer Research Institute, St Anna Kinderspital, Vienna, Austria; e-mail: h.gadner@stanna.at; or Stephan Ladisch, Children's Research Institute, Children's National Medical Center, Washington, DC 20010; e-mail: sladisch@cnmc.org.

![Figure 3. Rapid response and survival of MS-LCH RO+ patients in LCH-II. Kaplan-Meier analysis of survival of the responders (top line) and the nonresponders (ie, intermediate [IR, middle line] and worse [bottom line]). The bar graphs compare survival in responders (Rs) versus nonresponders (NRs) in each therapy arm. Error bars represent SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/5/10.1182_blood-2007-08-106211/2/m_zh80050815710003.jpeg?Expires=1769161754&Signature=IzATVglNQ54HzgkDnPiKbYhjuYEUGICcg1JHxxwhbo23rHU4Jk1LcbMO0KRe3LPn47syHryGFnArGPc~vC8Z6p6DJ3VZre1ls8dvJeuomBem0CHchypQqlwNw33WEiMPz5AkCUPQUieEiSjSDx6HUI8eRj8ZsTk14ZPxIjRMEI8oYdsLHQMdxd75Bamm932oCftno7q07BYZIdJByn0qzxhFalqYE5oxxfUpifD29NtCsyqkohBecGXBLuripnVXQcUghMrg4jwtvgyP77ic8ky0wFKi4Pwyevgb8nYILNHCnxed0HkPSCtvrp2gvRWXQeAVKs7MUvqivYHe6D5pWA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 3. Rapid response and survival of MS-LCH RO+ patients in LCH-II. Kaplan-Meier analysis of survival of the responders (top line) and the nonresponders (ie, intermediate [IR, middle line] and worse [bottom line]). The bar graphs compare survival in responders (Rs) versus nonresponders (NRs) in each therapy arm. Error bars represent SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/5/10.1182_blood-2007-08-106211/2/m_zh80050815710003.jpeg?Expires=1769161755&Signature=UGuhXyjS0RaKQEpPFPRNmQqHA3gS2TdmYQaiJxbFPP9kqswfo5b9bpxXld3S0IH-vSwB1ScfFC9rVpqUPet72zyB9maiQsh0S10eGIc67hV8IxJnoUpIDGNnSGmWRT9zrZCQUmFf63wqW7LA0TGcxG8VOLM18p~yz4lgiNJoHmUgwWbbMkAsLzqmZWyN5LNc1c-H91ggBk-j2~~JE6ocwkou8AQye9uj2RReH9CxF4KT15dvKPoMmOmjt7CNJXhIjgRKzM0gJhxhbqDQwmGy2Cvz2n5ntwWgG-Rohj~9Ev2pztS~SoAFQZpOMsaA~1sxbO4H-avv1szLdLF9fUVSxw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)