Many biologic markers are associated with poor prognosis in chronic lymphocytic leukemia (CLL), but their mechanistic role remains unclear. Bax is an essential proapoptotic protein and decreased levels in malignant cells lead to resistance to apoptosis. Using a Bax degradation activity (BDA) assay, CLL cells were found to show variable Bax instability. However, BDA did not correlate with Bax protein levels: BDA positive and negative cases had high and low baseline Bax levels. BDA positive cases showed a marked accumulation of poor prognostic markers—unmutated immunoglobulin heavy chain variable genes, ZAP-70/CD38 positivity, 11q22/17p13 deletion, and short lymphocyte doubling time. Patients with BDA positive cells had a shorter median overall survival (OS; 126 months vs not reached, P = .011) and time to first treatment (16 vs 156 months, P = .029) than BDA negative cases. Dual BDA and ZAP-70 positivity had a median OS of 84 months (P = .012). The BDA assay measures the intrinsic ubiquitin/proteasome activity of CLL cells and dynamic changes in Bax protein levels over time. Mechanistically, Bax instability may represent a final common pathway for disparate prognostic markers, as well as being itself an indicator of poor prognosis.
Introduction
The clinical course of B-cell chronic lymphocytic leukemia (CLL) is highly heterogeneous. Disease progression and survival correlate not only with clinical parameters, but also with a plethora of biologic prognostic markers, including immunoglobulin (Ig) heavy chain variable gene (VH) mutation status,1,2 ZAP-70,3,–5 CD38 expression.1 Recurrent cytogenetic abnormalities, with loss of the short arm of chromosome 17 (17p deletion), confer a very poor prognosis6 with the presumed mechanism of action involving (at least) loss of p53. Furthermore, functional loss of p53 activity, even in cases without a detectable p53 mutation, has been suggested as one mechanism underlying the poor prognosis associated with unmutated Ig VH genes.7 The molecular mechanisms underlying the worse prognosis in CLL with unmutated Ig VH, ZAP-70 or CD38 expression also remain elusive, although enhanced cell signaling/survival have been implicated.8,–10 The role of ZAP-70 in enhancing signaling has been questioned in recent work showing that B-cell receptor triggering was not influenced by the ZAP-70 status of CLL cells.11 Clearly, further work is required to elucidate the cellular and molecular mechanisms that underlie the observed clinical value of these prognostic markers
Fundamental to all malignant cells is the property of uncontrolled cell growth, often combined with resistance to apoptosis— a characteristic feature of CLL cells. A key proapoptotic protein is Bax, with low levels of, or absent, Bax expression in malignant cells being associated with resistance to treatment. Several proapoptotic molecules have been shown to be regulated by the ubiquitin/proteasome pathway, including p53,12 Bid,13 Bax,14,15 ARTS16 and NOXA.17 Furthermore, Bax instability was found in malignant cells and cell lines,15,18 as well as primary CLL cells.19 In the accompanying paper (Liu et al20 ), we investigated Bax degradation activity in cell lines, its role in TNF-related apoptosis inducing ligand (TRAIL)-induced apoptosis of CLL cells and its modulation by proteasome inhibition. Bax degradation in cell lines was found to be located in mitochondria, as opposed to the cytosol, and required an active ubiquitin/proteasome machinery. Bax instability was a mechanism of resistance to TRAIL-induced apoptosis, with proteasome inhibitors overcoming this resistance by stabilizing Bax and preventing degradation of ubiquitinated Bax. These findings suggested that testing for Bax instability in CLL may help guide therapeutic choices, with marked Bax degradation activity indicating that combined TRAIL and proteasome inhibition could be useful in some patients. This paper further investigates the utility of measuring Bax instability in CLL. We looked at a cohort of patients with CLL and compared Bax degradation activity with established prognostic markers: Ig VH mutation status, ZAP-70, CD38, cytogenetics, as well as clinical stage and lymphocyte doubling time (LDT).
Methods
Cell culture and clinical samples
The protocol was approved by the East London and The City Research Ethics Committee. The diagnosis of CLL was made by standard criteria: cellular morphology and immunophenotyping (CD5+, CD19+, CD23+ weak surface Ig staining and weak CD79b with FMC7 negativity). All cases had a significant lymphocytosis of more than 40 × 109/L. Peripheral blood was collected after written informed consent from patients with CLL was obtained in accordance with the Declaration of Helsinki. Patients had either never received treatment for their CLL or were at least 6 months after their previous course of treatment. Mononuclear cells were isolated by density centrifugation over Ficoll. Cells were cultured in RPMI-1640 medium (Sigma-Aldrich, St Louis, MO) supplemented with 10% heat-inactivated fetal calf serum (FCS), 25 mM HEPES, 2.0 mM l-glutamine, pH 7.4, at 37°C in a 5% CO2 humidified incubator.
Prognostic markers in CLL: ZAP-70 and CD38 by flow cytometry
ZAP-70 and CD38 by flow cytometry are routinely available as part of the diagnostic work-up for all CLL cases in our immunophenotyping laboratory. Briefly, direct staining of whole blood with conjugated anti–CD38-PE, clone T16, (Beckman Coulter, Villepinte, France) and anti–CD19-FITC, clone J4.119 (Beckman Coulter) was performed, followed by red cell lysis using the Beckman-Coulter ImmunoPreP reagent system and samples were read on a Coulter XL4-MCL flow cytometer (Beckman Coulter). CD38 positivity was set at a threshold of more than 20%. Intracellular staining for ZAP-70 involved: initial surface marking with anti-CD3-PE (UCHT1; Beckman Coulter) and CD56-PE (N901[NKH-1], Beckman Coulter), permeabilization with Dako Intrastain (Dako, Ely, United Kingdom) and labeling with anti-ZAP-70, clone 2F3.2, (Upstate-Millipore, Charlottesville, VA) followed by a goat anti–mouse pan IgG (H + L) FITC (Southern Biotechnology, Birmingham, AL). CLL ZAP-70 expression was assessed by gating out CD3 and CD56 positive cells. Positivity was set at a threshold of more than 20%.
Prognostic markers in CLL: analysis of somatic mutation of the rearranged immunoglobulin heavy chain (IgVH) genes
DNA was extracted from peripheral blood leukemia cells—in some cases from fixed, stained slides—using QIAamp DNA Blood Mini kit (Qiagen, Crawley, United Kingdom) and subjected to polymerase chain reaction (PCR) of the rearranged immunoglobulin heavy chain gene from the framework 1 to the joining region using the BIOMED-2 multiplex PCR protocols (InVivoScribe Technologies, San Diego, CA). PCR products were analyzed by polyacrylamide gel electrophoresis and clonal PCR products were purified using QIAquick PCR purification kits (Qiagen), followed by direct sequencing twice with the consensus primer to the joining region on an ABI 377 DNA sequencer (Applied Biosystems, Foster City, CA). In cases where direct sequencing failed, PCR products were cloned and at least 4 clones were sequenced using vector primers. The consensus sequence in each case was subjected to database search for identification of the variable (V), diversity (D), and joining (J) germ line segments used and somatic mutation in the rearranged V segment using DNAPLOT and VBASE2 (http://www.vbase2.org/).
Prognostic markers in CLL: cytogenetic analyses
Interphase fluorescent in situ hybridization (FISH) analysis was performed using the commercially available Vysis CLL probes (Abbott Diagnostics, Abbott Park, IL) to detect the common CLL-associated abnormalities; deletions of ataxia telangiectasia mutated (ATM) (11q23), D13S319 (13q14) and p53 (17p13), trisomy 12 using a chromosome 12 centromere probe (CEP12). FISH was also set up to exclude the presence of the t(11;14), IGH/CCND1 associated with mantle cell lymphoma. The probes were applied as dual color probe pair sets (ATM/p53, D13S319/CEP12 and IGH/CCND1) and used according to the manufacturers protocol. For each probe set, 100 interphases were scored on a Leica DMXRA microscope (Leica, Wetzlar, Germany) fitted with a COHU CCD camera (Cohu, San Diego, CA) using MacProbe V4.1.1 CGH software (PSI/Applied Imaging, Santa Clara, CA).
In vitro Bax degradation assay
CLL cells were incubated in Buffer A (250 mM sucrose, 10 mM HEPES-KOH, pH 7.4, 10 mM KCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 0.1 mM PMSF, protease inhibitor cocktail 1:100, 50 μg/mL creatine phosphokinase, 10 mM phosphocreatine, 2 mM ATP, and 0.1% Triton X-100) for 20 minutes on ice. Cells were then broken with a glass Dounce homogenizer (Jencons, Leighton Buzzard, United Kingdom) and nuclei removed by spinning at 790g for 10 minutes at 4°C. The postnuclear supernatant was used as the cellular extract for the Bax degradation activity (BDA) assay. After the addition of 2 μg/mL ubiquitin (Sigma, Dorset, United Kingdom), the protein extract in 5 mg/mL concentration was incubated at 37°C for up to 5 hours in Buffer A in the presence of the adenosine triphosphate (ATP) regeneration system and protease inhibitor cocktail. Proteins were taken out hourly and Bax levels determined using Western blotting and subsequent measurement of band density. BDA was calculated as the ratio of the level of Bax at 3 hours in the assay, compared with its level at time 0. Samples with less than 20% Bax degradation (BDA > 0.8) were considered BDA negative.
Western blotting
To assess Bax expression, CLL cells were placed in lysis buffer (1% Triton X-100, 0.5% sodium deoxycholate, 0.1 SDS, 1 mM PMFS, 10 μg/mL aprotinin, 10 μg/mL leupeptin, 1 mM sodium orthovanadate, 1 mM DTT in PBS, pH 7.4). Proteins were subjected to standard SDS-PAGE at 20 to 40 mA/gel and transferred on to PVDF membrane at 100 V for 1 hour. PVDF membranes were blocked with 1% nonfat milk in phosphate-buffered saline containing 0.1% Tween-20 (PBST) for 1 hour and probed for various proteins using the monoclonal anti–Bax 2D2 antibody, clone YTH-2D2 (R&D Systems, Oxford, United Kingdom) or the monoclonal anti–anti-β-actin antibody (Sigma). Bound antibodies were detected using horseradish peroxidase (HRP)-conjugated antimouse antibody (Santa Cruz Biotechnology, Santa Cruz, CA) followed by detection using SuperSignal West Pico Chemiluminescent Substrate (Perbio Science UK, Chester, United Kingdom). The density of each band was analyzed using an AlphaImager 2000 Densitometer (Alpha Innotech, San Jose, CA).
Statistical analysis
Binary comparisons between Bax degradation activity (positive or negative) were made with Binet stage (A or not A), presence of IgVH mutation, ZAP-70 and CD38 positivity, LDT (> 12 months or other), karyotype (11q- or 17p- / other) using chi-square with Yate correction or 2-sided Fisher exact test, as appropriate. Survival time and time to treatment between Bax groups was compared using Kaplan-Meier plots and Cox F test. Multivariate analysis was undertaken using a generalized nonlinear binomial model using logit analysis. Statistical significance was assumed at a P value of less than .05.
Results
Bax instability in CLL cells
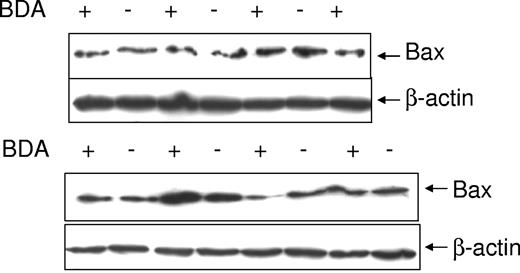
In the accompanying paper, Bax protein levels were found to vary with individual malignant B-cell lines. This variation was not related to Bax mRNA expression, suggesting regulation at the posttranslational level (Liu et al20 ). A specific defect of Bax stability was found in malignant B cells and BDA was higher in these than in control tissues. In this paper, the work has been extended to 40 cases of CLL. In these patients, Bax degradation was positive (active) in 26 of 40 cases, but negative in normal peripheral blood lymphocytes (Figure 1). The level of Bax instability varied and is shown schematically as “+” to “+++” in Figure 1. No degradation in Bak, Bcl-2, Bcl-XL was detected in CLL cells. NOXA and PUMA proteins were not expressed in unstimulated CLL cells (results not shown).
Bax degradation activity in primary CLL cells. The postnuclear supernatants were extracted from peripheral blood lymphocytes (PBL) from a healthy donor and CLL cells and incubated with the ubiquitin and ATP regeneration system for up to 5 hours. Twenty micrograms of protein were loaded in each lane for sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). The Bax (clone 2D2) antibody was used in 1:1000 dilution and the β-actin antibody was used at 1:10 000 dilution. Numbers under each pair of blots are ratios of Bax/β-actin. Top panel shows results for a normal donor; lower 4 panels are cases of CLL.
Bax degradation activity in primary CLL cells. The postnuclear supernatants were extracted from peripheral blood lymphocytes (PBL) from a healthy donor and CLL cells and incubated with the ubiquitin and ATP regeneration system for up to 5 hours. Twenty micrograms of protein were loaded in each lane for sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). The Bax (clone 2D2) antibody was used in 1:1000 dilution and the β-actin antibody was used at 1:10 000 dilution. Numbers under each pair of blots are ratios of Bax/β-actin. Top panel shows results for a normal donor; lower 4 panels are cases of CLL.
Bax degradation activity does not correlate with Bax protein levels
It was important to establish whether the differential responses in the BDA assay were related to the baseline level of Bax protein in CLL cells. Bax protein expression was tested in 15 CLL samples— 8 BDA positive and 7 BDA negative. Figure 2 shows that there was no correlation between BDA and Bax protein levels, with BDA positive and negative cases having both high and low levels of basal Bax.
Bax protein levels in different CLL patients. Proteins were extracted from 15 different CLL samples using the lysis buffer. Twenty micrograms of protein were loaded in each lane for SDS-PAGE. The Bax (clone 2D2) antibody was used in 1:1000 dilution and the β-actin antibody was used at 1:10 000 dilution. BDA positive (+) and negative (−) cases are indicated above each lane.
Bax protein levels in different CLL patients. Proteins were extracted from 15 different CLL samples using the lysis buffer. Twenty micrograms of protein were loaded in each lane for SDS-PAGE. The Bax (clone 2D2) antibody was used in 1:1000 dilution and the β-actin antibody was used at 1:10 000 dilution. BDA positive (+) and negative (−) cases are indicated above each lane.
Bax degradation activity is associated with poor prognostic markers in CLL
The instability of Bax protein in CLL cells, as indicated by the BDA assay, could represent a new prognostic marker with biologic relevance. Therefore, we looked at the correlation of BDA with established prognostic markers. The characteristics of the cohort of 40 cases of CLL are shown in Table 1. Overall, 27% (11/40) of cases were Binet stage B/C at diagnosis, while 48% (19/40) had an LDT of less than 12 months (including cases requiring therapy before the LDT could be established). In terms of biologic parameters, 45% (13/29) of cases had unmutated Ig VH, 38% (15/39) were ZAP-70+, 45% (18/40) were CD38+ and 15% (6/40) had 11q22 or 17p13 deletions. In our diagnostic practice, with a population of nearly 2 million, ZAP-70 and CD38 positivity are typically seen in one third of cases (data not shown). Thus, this cohort of patients with CLL appears to represent a higher risk population than the general CLL population, which is in keeping with the bias of a tertiary referral center, as recognized in the literature.3,5
The identification of 2 populations of CLL cases—those in whom the cells had stable Bax and those that showed Bax instability—led us to investigate the distribution of various prognostic markers in the 2 groups. The 26 cases showing Bax degradation activity (BDA positive) had a marked accumulation of poor prognostic features (Table 2): 10/11 of the Binet stage B/C patients, 9/13 Ig VH unmutated cases, 12/15 of the ZAP-70+, 15/18 of the CD38+, 15/19 of the LDT less than 12 months patients, and all of the 6 11q22 or 17p13 deletion cases. However, probably because of a lack of power, individually, none of these apparent associations with BDA positivity were statistically significant (Table 2).
Bax degradation activity is associated with poor outcomes in CLL
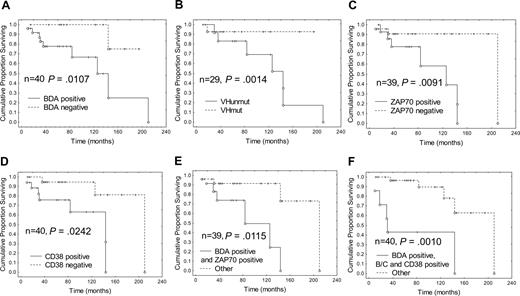
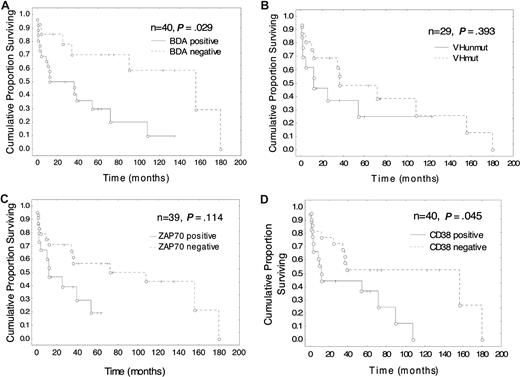
Median overall survival (OS) in the BDA positive group was 126 months, which was significantly worse than in the BDA negative group (P = .011; Figure 3); and the median time to treatment was 16 months compared with 156 months for the BDA negative group (Figure 4; P = .029). The median OS and time to treatment for VH mutation, ZAP-70, and CD38 are also shown in Figures 3 and 4, respectively. The only combination of parameters that improved prognostication were BDA+/ZAP-70+ (84 months, P = .012) and BDA+/Binet stage B/C and CD38+ (31 months, P = .001; Figure 3). Similarly, with respect to time to first treatment, BDA positivity was as useful as the other biologic parameters (Figure 4), and no combination of BDA+ with unmutated VH, ZAP-70+, or CD38+ improved the prognostic value. As Binet stage B/C and LDT less than 12 months are themselves criteria for instituting therapy for CLL, these parameters confound the interpretation of time to treatment analyses. Multivariate analysis looking at survival with BDA, VH status, ZAP-70, CD38, and karyotype only identified unmutated VH as significant (P = .005). With respect to time to first treatment, no parameter was found to be significant in multivariate analysis.
Survival according to Bax degradation activity, VH, ZAP-70, and CD38 status: Bax instability confers a worse prognosis. Kaplan-Meier plots of survival since diagnosis in patients classified according to (A) BDA, (B) VHmut, (C) ZAP-70, and (D) CD38 status, and also in patients with the combined markers (E) BDA+ and ZAP-70+, and (F) BDA+, Binet stage B or C, and CD38+, compared with the remainder. Cox F test was used to compare the 2 groups in each plot. n = number of patients, O indicates patient is dead, and +, patient is alive at time of analysis.
Survival according to Bax degradation activity, VH, ZAP-70, and CD38 status: Bax instability confers a worse prognosis. Kaplan-Meier plots of survival since diagnosis in patients classified according to (A) BDA, (B) VHmut, (C) ZAP-70, and (D) CD38 status, and also in patients with the combined markers (E) BDA+ and ZAP-70+, and (F) BDA+, Binet stage B or C, and CD38+, compared with the remainder. Cox F test was used to compare the 2 groups in each plot. n = number of patients, O indicates patient is dead, and +, patient is alive at time of analysis.
Time to first treatment according to Bax degradation activity, VH, ZAP-70 and CD38 status: Bax instability is associated with the need for early treatment. Kaplan-Meier plots of time to first treatment since diagnosis in patients classified according to (A) BDA, (B) VHmut, (C) ZAP-70, and (D) CD38 status. Cox F test was used to compare the 2 groups in each plot. N indicates number of patients; O, time of first treatment; and +, patient is untreated at time of analysis.
Time to first treatment according to Bax degradation activity, VH, ZAP-70 and CD38 status: Bax instability is associated with the need for early treatment. Kaplan-Meier plots of time to first treatment since diagnosis in patients classified according to (A) BDA, (B) VHmut, (C) ZAP-70, and (D) CD38 status. Cox F test was used to compare the 2 groups in each plot. N indicates number of patients; O, time of first treatment; and +, patient is untreated at time of analysis.
Discussion
B-CLL is characterized by long-lived cells in vivo and high levels of antiapoptotic Bcl-2, and remains an incurable disease requiring repeated treatments. Pro- and antiapoptotic members of the Bcl-2 family of proteins play a key role in the balance between cell death and survival. Low levels, or absence, of the proapoptotic molecule, Bax, have been associated with resistance to apoptosis in a variety of malignant B cells.18,19,21 Bax degradation activity, or loss of Bax protein expression, is associated with poor prognosis in prostate cancer and melanoma.15,22 In CLL, low Bax levels (mRNA and/or protein expression) have been associated with a poor prognosis,23,,–26 but this has not been confirmed in other studies.27,28 The present study has looked at Bax protein instability using the BDA assay. Bax instability was more common in poor prognosis CLL, and patients with BDA-positive cells had a worse prognosis, with a shorter interval from diagnosis to first treatment and a shorter overall survival.
The BDA assay measures the dynamic changes in Bax protein level in CLL cells over time. Interestingly, there was no correlation between BDA and basal cellular Bax protein levels. This highlights the duality of protein regulation, namely, synthesis and degradation. The measurement of a Bax protein level at a single point in time must reflect the balance of these 2 processes, but a high level of Bax could still be associated with high degradation activity if counterbalanced by increased synthesis. Because the BDA assay uses postnuclear extracts, the synthetic arm of Bax regulation has been eliminated and the assay provides an in vitro measure of the ubiquitin/proteasome degradation activity. Thus BDA is a dynamic indicator of intrinsic ubiquitin/proteasome activity in CLL cells and is a one-way “output” (ie, degradation) assay without the synthetic “input” arm. BDA could therefore be a more robust tool for prognostication and may partly explain why the literature contains contradictory reports on the prognostic value of Bax protein measurements in CLL.23,,,,–28
The BDA positive cases showed a marked accumulation of known poor prognostic features, shorter overall survival and time to first treatment compared with the BDA negative group (Table 2 and Figures 3,4). Analysis of the cases with prognostically discordant results further supports the value of the BDA assay. For example, Bax instability (BDA positivity) conferred a poor prognosis in patients without other poor risk markers; stability of the Bax protein (BDA negativity) may have contributed to the longer than expected survival of patients who had other poor prognostic markers, suggesting persistent sensitivity of their cells in vivo to therapeutic agents.
CLL remains an incurable disease requiring repeated courses of treatment, with a decreasing success of therapies due to progressive bone marrow failure, immune suppression, infection, and resistance to therapy. There is a need for therapies with novel mechanisms of action and reduced toxicities. We have shown that Bax instability plays a key role in the resistance of CLL cells and malignant B-cell lines to tumor necrosis factor–related apoptosis-inducing ligand (TRAIL)–induced apoptosis (Liu et al,20 accompanying paper). Furthermore, we showed that it was possible to stabilize Bax with the proteasome inhibitor, bortezomib, thereby sensitizing CLL cells to killing by TRAIL.
Bortezomib has shown antitumor activity in vivo in several cancer types, especially B-cell malignancies.29 In myeloma, both as a single agent and in combination with other treatments, Bortezomib has an established role in the therapeutic armamentarium.30,31 However, the efficacy of bortezomib in patients with CLL has been disappointing.32 The in vitro sensitization of CLL cells to apoptosis by Bortezomib suggests that the combination of proteasome inhibition with other therapies should be investigated in vivo. Recent studies suggest that synergy with bortezomib and TRAIL (Liu et al,20 accompanying manuscript, and Kabore et al33 ), chemotherapeutic agents,34 or bortezomib and monoclonal anti-CD20 and anti-CD52 antibodies35 may allow dose reduction of the individual agents, thereby minimizing their overall toxicities.
The BDA assay described here measures the intrinsic Bax instability of CLL cells and represents a new prognostic marker, with BDA-positivity correlating with shorter survival and earlier treatment. There was a striking accumulation of established markers of poor prognosis in cases with Bax instability, suggesting that Bax instability may be a common “final” pathway found in poor prognosis CLL and possibly other B-cell malignancies (this paper and Liu et al,20 accompanying paper). These findings will be extended to a larger, prospective study of the utility of the ubiquitin-mediated Bax degradation assay at diagnosis in uniformly treated patients, as well as with novel combinations, in CLL.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Research Advisory Board of St Bartholomew's and the Royal London Charitable Foundation, the Leukemia & Lymphoma Society, USA, and Leukaemia Research Fund, United Kingdom.
Authorship
Contribution: S.G.A. designed and performed research, wrote the paper, and raised funds; F.-T.L., C.W., S.S., H.L., and D.L. performed research; D.S. analyzed data; M.Q.D., J.G.G., and A.C.N. contributed design, discussed the project, and raised funds; and L.J. contributed design, performed research, wrote part of the paper, and raised funds.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr S. Agrawal, Stem Cell Laboratory, Haematology, St Bartholomew's Hospital, West Smithfield, London, UK EC1A 7BE; e-mail: s.g.agrawal@qmul.ac.uk.
References
Author notes
S.G.A. and F.-T.L. contributed equally to this work.