In this issue of Blood, Akagi and colleagues demonstrate the importance of the CCAAT enhancer binding protein β (C/EBPβ) for granulocyte function and survival. Their novel findings further elucidate the variety of C/EBP protein functions in general and the special role of C/EBPβ in particular.
C/EBP proteins are a family of leucine zipper domain–containing transcription factors that form homodimers and heterodimers to regulate expression of their target genes. C/EBP proteins play important roles in differentiation events in diverse tissues, and several of them inhibit proliferation. In the hematopoietic system, C/EBPα is essential for granulocytic lineage commitment. Further downstream, C/EBPϵ directs the differentiation of progenitors into mature neutrophils. These functions are well established by the lack of respective cell populations in genetically modified mice. C/EBPβ is also highly expressed in mature granulocytes, but the lack of C/EBPβ does not alter steady-state bone marrow morphology or peripheral-blood cell counts.1 Thus, the function of C/EBPβ in myeloid development has remained obscure for some time.
Interest in C/EBPβ was fueled by its diverse and seemingly contradictory functions.2 In many instances, C/EBPβ appeared to be redundant with C/EBPα; a C/EBPβ knock-in into the C/EBPα locus rescues most of the C/EBPα deficiency phenotype. However, other reports have suggested that C/EBPβ is required for the oncogenic functions of Ras. The differences between C/EBPα and C/EBPβ are likely to be associated with the cell-specific expression pattern, the close interaction between C/EBPβ and cytokine signaling, and the interaction partners of the 2 proteins. In contrast to C/EBPα, C/EBPβ does not interact with E2F, and this may explain why C/EBPβ does not cause cell-cycle arrest. In addition, expression of the 2 main isoforms of C/EBPα and C/EBPβ, which are controlled translationally, may be regulated differentially depending on cell stimulus.
Analysis of C/EBPβ-deficient mice revealed a high susceptibility to bacterial infections. C/EBPβ-deficient macrophages showed impaired killing of bacteria and tumor cells. Recently, Hiari et al discovered that C/EBPβ plays an important role in emergency granulopoiesis.1 Upon exposure to cytokines or infectious agents in vivo, C/EBPβ expression is induced, which leads to generation of responsive granulocytes even in the absence of C/EBPα. These findings established that C/EBPβ plays an important role in granulopoiesis under critical conditions such as severe infection. The current study by Akagi et al significantly extends these findings: C/EBPβ deficiency decreased cytokine production and colony growth of myeloid, but not erythroid or megakaryocytic progenitors. Importantly, C/EBPβ deficiency also increased apoptotic cell death of granulocytes. This specific feature of C/EBPβ in neutophils also occurs in other cell types. The antiapoptotic functions of C/EBPβ are in direct contrast to other C/EBPs, such as C/EBPα, that induce apoptosis.
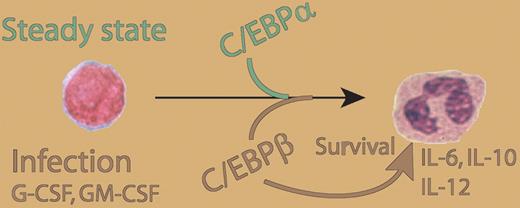
It is intriguing to speculate that granulocytes might be regulated by C/EBPα and C/EBPϵ to maintain a steady state. This would also include regular apoptotic death and a well-maintained balance between proliferation and differentiation. Under critical circumstances, such as severe infection, C/EBPβ not only enhances granulocyte production but also increases granulocyte numbers by inhibiting apoptosis. In addition, upon cytokine stimulation, C/EBPβ boosts granulocyte function and might help to overcome infection. The findings by Akagi et al further integrate growth-factor and cytokine signaling with the transcription-factor networks that govern these processes (see figure). G-CSF is an established treatment for several diseases that cause neutropenia; further studies may show whether C/EBPβ function is crucial for G-CSF treatment successes, such as increased numbers of granulocytes caused by increased production and extended lifespan. Moreover, further studies are needed to elucidate the mechanisms by which C/EBPβ stimulates granulopoiesis and inhibits apoptosis.
Role of C/EBPβ in granulopoiesis and granulocyte function. C/EBPα is required for granulopoiesis in steady-state conditions. As a response to external stimuli, such as sever infection or growth factor exposure, C/EBPβ enhances granulocyte production, inhibits apoptosis, and induces production of IL-6, IL-10, and IL-12 p35.
Role of C/EBPβ in granulopoiesis and granulocyte function. C/EBPα is required for granulopoiesis in steady-state conditions. As a response to external stimuli, such as sever infection or growth factor exposure, C/EBPβ enhances granulocyte production, inhibits apoptosis, and induces production of IL-6, IL-10, and IL-12 p35.
As the authors note, the phenotypic features of C/EBPβ-deficient granulopoiesis resemble those of a myelodysplastic syndrome. Because C/EBPα mutations and/or dysfunction are known to be involved in the pathogenesis of acute myeloid leukemia (AML), it is possible that inactivation of other C/EBP proteins such as C/EBPβ play a role in myeloid disorders (MDSs) as well. For example, C/EBPδ is frequently inactivated by promoter methylation in AML,3 and it is possible that C/EBPβ function might be altered in myeloid malignancies and MDSs. Also, the findings by Akagi et al clearly show that a normal white blood cell count in a genetic mouse model can, under steady-state conditions, conceal an important function of the gene in hematopoiesis.
The critical importance of granulopoiesis and granulocyte function in hematology and clinical medicine is obvious, and we have made a lot of progress in deciphering its mechanisms. Nonetheless, tools to more effectively and specifically manipulate granulocyte functions and productions are necessary. The findings presented by Akagi and colleagues might help to design new strategies for overcoming neutropenia and granulocyte dysfunction.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■