Abstract
Monocyte recruitment and differentiation into dendritic cells or macrophages play a critical role in defense mechanisms against pathogens and in inflammatory and autoimmune diseases. Important contributions have been made on the molecular events controlling neutrophil and lymphocyte extravasation under steady state or inflammation. However, the molecules involved in monocyte rolling during their migration to antigen capture areas and lymphoid organs during infection remain undefined. Here we have analyzed the homing molecules controlling mouse monocyte rolling in an experimental model of Leishmania major infection. Monocyte migration through inflamed dermal venules was dependent on interactions of PSGL-1 with P- and E-selectins, and of L-selectin with PNAd, whereas migration through lymph node high endothelial venules relied essentially on L-selectin–PNAd interactions. These results might have important implications regarding the induction of immune responses against pathogens and future immunotherapeutic protocols of inflammatory and autoimmune diseases, based on selective inhibition of monocyte migration to specific inflammatory foci.
Introduction
Monocyte recruitment to the skin and lymph nodes (LNs) was demonstrated to be essential to the immune response against Leishmania major in a recent report showing that dendritic cells (DCs) differentiated from monocytes at the infection site were responsible for the induction of protective T-cell responses.1 The relevance of monocyte recruitment and differentiation into DCs was also proposed for immune responses against viruses or bacteria.2 Moreover, macrophage differentiation from monocytes recruited to inflammatory foci plays a critical role in protection against pathogens and autoimmune diseases.3-5 Analyzing the mechanisms controlling monocyte migration might contribute to understanding immunity against infection and development of autoimmunity. Several investigators have analyzed the molecules directing lymphocyte and neutrophil extravasation,6 but although various reports have addressed the involvement of chemokines and integrins in monocyte recruitment during inflammation,7-13 the mechanisms controlling monocyte rolling during migration to antigen-capture areas and lymphoid organs during infection, remain undefined. Here we have analyzed the molecules involved in monocyte rolling during Leishmania major infection by performing transendothelial migration blockade experiments with monoclonal antibodies (mAbs) against leukocyte rolling molecules. Monocyte migration through inflamed dermal venules (DVs) depended on interactions of PSGL-1 with P/E-selectin, and of L-selectin with PNAd, whereas migration through high endothelial venules (HEVs) relied essentially on L-selectin–PNAd interactions
Methods
Procedures were approved by the Centro Nacional de Biotecnologia Animal Care and Use Committee and complied with national and European legislation.
Endogenous monocyte migration blockade
C57BL/6 Ly-5.2+ mice received 2 × 106Leishmania major promastigotes in the footpad. Three weeks after infection, C57BL/6 Ly-5.2+ mice received an intravenous injection for 3 days of anti–PSGL-1, P-selectin, E-selectin, PNAd, or L-selectin mAbs kindly provided by Dr Vestweber (anti–PSGL-1, anti–P-selectin), Dr Hallman (anti–E-selectin), and Dr Engelhardt (anti-PNAd).
Transferred monocyte migration blockade
These experiments were performed as described for endogenous monocytes, except that C57BL/6 Ly-5.2+ mice received an intravenous injection of 3 to 5 × 106 monocytes purified from C57BL/6 Ly-5.1+ mice,1 together with the first mAb injection.
Flow cytometry
The dermis and popliteal LNs (PO-LNs) were stained with fluorescein-conjugated anti–Ly-6C, phycoerythrin-conjugated anti-CD11b, allophycocyanin-conjugated anti-CD11c, and biotin-conjugated anti-F4/80, followed by streptavidin-PerCP. Transferred monocytes were stained with fluorescein-conjugated anti–Ly-6C, phycoerythrin-conjugated anti–Ly-5.1, allophycocyanin-conjugated anti-CD11c, and biotin-conjugated anti-CD11b, followed by streptavidin-PerCP.
PNAd expression
PNAd was analyzed on dermis and PO-LN cryostat sections, 10 minutes after intravenous injection of Alexa-594–conjugated anti-PNAd at 4 weeks after infection.
Results and discussion
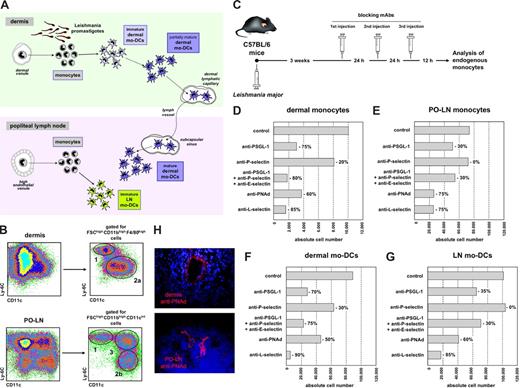
Monocyte migration was analyzed based on a previous study on monocyte differentiation during Leishmania major infection,1 demonstrating that monocytes were recruited to the skin and PO-LNs, through DVs and HEVs, respectively. Newly recruited monocytes differentiated into monocyte-derived DCs (mo-DCs), generating “dermal mo-DCs,” that migrated to the PO-LNs, and “LN mo-DCs,” after monocyte recruitment to the PO-LNs (Figure 1A). At 3 to 4 weeks after infection, corresponding to the peak of the immune response,1 monocytes, dermal mo-DCs, and LN mo-DCs were identified by CD11c, Ly-6C, CD11b, and F4/80 expression (Figure 1B). Monocytes were defined as Ly-6Chigh CD11c−/low cells. Dermal mo-DCs were Ly-6Cintemediate CD11clow/intermediate cells in the dermis, and Ly-6Clow CD11cintermediate in the PO-LNs. Differences in Ly-6C/CD11c expression between dermal mo-DCs in the dermis and PO-LNs reflect their maturation after migration to the PO-LNs. LN mo-DCs were Ly-6Chigh CD11cintermediate cells. Monocyte transendothelial migration was analyzed after treating C57BL/6 mice at week 3 after infection with mAbs against molecules involved in lymphocyte and/or neutrophil rolling (Figure 1C). The effect of mAb-mediated blockade was analyzed on monocytes and mo-DCs in the dermis and PO-LNs (Figure 1D-G).
Analysis of endogenous monocyte migration to the dermis and PO-LNs during Leishmania major infection. (A) Diagram of monocyte recruitment and differentiation during Leishmania major infection. (B) Identification of dermal and PO-LN monocytes and mo-DCs at week 3 after infection. 1 indicates monocytes; 2, dermal mo-DCs in the dermis (2a) and after migration to the PO-LNs (2b); 3, LN mo-DCs. (C) Experimental model for the analysis of endogenous monocyte migration. (D-G) Effect of treatment (200 μg/injection) with mAbs anti–PSGL-1 (clone 4RA10), P-selectin (clone RB40.34), E-selectin (clone UZ4), PNAd (clone MECA 79), and/or L-selectin (clone MEL-14) on the absolute number of endogenous monocytes in the dermis (D), monocytes in the PO-LNs (E), dermal mo-DCs after migration to the PO-LNs (F), and LN mo-DCs (G). Data are representative of 4 independent experiments with similar results. (H) Cryostat sections of the dermis and PO-LNs of Leishmania major–infected mice at 4 weeks after infection, after intravenous injection of Alexa-594–conjugated anti-PNAd mAbs (clone MECA-79).
Analysis of endogenous monocyte migration to the dermis and PO-LNs during Leishmania major infection. (A) Diagram of monocyte recruitment and differentiation during Leishmania major infection. (B) Identification of dermal and PO-LN monocytes and mo-DCs at week 3 after infection. 1 indicates monocytes; 2, dermal mo-DCs in the dermis (2a) and after migration to the PO-LNs (2b); 3, LN mo-DCs. (C) Experimental model for the analysis of endogenous monocyte migration. (D-G) Effect of treatment (200 μg/injection) with mAbs anti–PSGL-1 (clone 4RA10), P-selectin (clone RB40.34), E-selectin (clone UZ4), PNAd (clone MECA 79), and/or L-selectin (clone MEL-14) on the absolute number of endogenous monocytes in the dermis (D), monocytes in the PO-LNs (E), dermal mo-DCs after migration to the PO-LNs (F), and LN mo-DCs (G). Data are representative of 4 independent experiments with similar results. (H) Cryostat sections of the dermis and PO-LNs of Leishmania major–infected mice at 4 weeks after infection, after intravenous injection of Alexa-594–conjugated anti-PNAd mAbs (clone MECA-79).
Compared with untreated mice, the number of dermal monocytes was reduced approximately 75% after anti-PSGL-1 treatment, 20% after treatment with anti–P-selectin (a PSGL-1 ligand), and 80% after treatment with mAbs against PSGL-1 and its ligands, P- and E-selectin. These data confirm previous reports on PSGL-1 involvement in leukocyte or neutrophil rolling on inflamed DVs.14,15 Dermal monocyte number was reduced approximately 60% after anti-PNAd treatment and 85% after anti–L-selectin treatment (Figure 1D). Thus, monocyte migration through DVs depended not only on PSGL-1 interactions with P- and E-selectin but also on L-selectin/PNAd interactions (Figure 2G). Although PNAd was described to be upregulated on venous endothelia during inflammatory and autoimmune diseases,16-19 and to participate in lymphocyte rolling on inflamed venules, neither PNAd upregulation on DVs during infectious processes nor its involvement in monocyte migration during inflammation was previously reported. PNAd expression by inflamed DVs was confirmed on dermal cryostat sections after intravenous injection of Alexa 594–conjugated anti-PNAd mAbs (Figure 1H). The stronger monocyte migration blockade obtained with anti–L-selectin than with anti-PNAd suggests that L-selectin could bind to a second ligand. Indeed, L-selectin interaction with N-glycan ligands during homeostasis and inflammation was recently described.20 Alternatively, L-selectin/PSGL-1 interactions could mediate monocyte secondary rolling, a process allowing leukocytes to roll on other leukocytes attached to inflamed endothelia.21,22
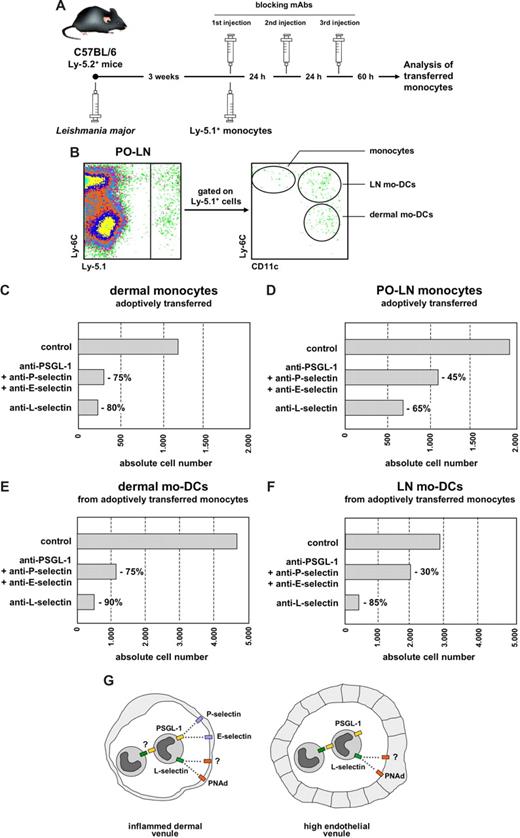
Analysis of adoptively transferred monocyte migration to the dermis and PO-LNs during Leishmania major infection. (A) Experimental model for the analysis of adoptively transferred monocyte migration. (B) Identification of adoptively transferred Ly-5.1+ monocytes and mo-DCs at 3 weeks after infection. (C-F) Effect of treatment with mAbs against rolling molecules on the absolute number of monocytes in the dermis (C), monocytes in the PO-LNs (D), dermal mo-DCs after migration to the PO-LNs (E), and LN mo-DCs (F). (G) Proposed model for the differential participation of PSGL-1 and L-selectin on monocyte transendothelial migration through inflamed DVs and HEVs. Data are representative of 3 independent experiments with similar results.
Analysis of adoptively transferred monocyte migration to the dermis and PO-LNs during Leishmania major infection. (A) Experimental model for the analysis of adoptively transferred monocyte migration. (B) Identification of adoptively transferred Ly-5.1+ monocytes and mo-DCs at 3 weeks after infection. (C-F) Effect of treatment with mAbs against rolling molecules on the absolute number of monocytes in the dermis (C), monocytes in the PO-LNs (D), dermal mo-DCs after migration to the PO-LNs (E), and LN mo-DCs (F). (G) Proposed model for the differential participation of PSGL-1 and L-selectin on monocyte transendothelial migration through inflamed DVs and HEVs. Data are representative of 3 independent experiments with similar results.
The number of PO-LN monocytes was severely reduced after treatment with anti-L-selectin or anti-PNAd (Figure 1E), suggesting that L-selectin was essential for monocyte migration through HEVs during infection (Figure 2G). Previous reports had demonstrated a role for L-selectin in lymphocyte rolling on HEVs and the participation of the chemokines CCL2 and CXCL910,11 in monocyte migration to the LNs. L-selectin involvement in monocyte migration through HEVs had not been demonstrated before, although it was proposed in a report analyzing the in vitro binding of a monocytic cell line to HEVs on frozen sections.23 Our data suggest that monocyte binding to HEVs is preferentially mediated by L-selectin, although a role for N-glycan ligands cannot be excluded. A moderate effect on monocyte migration through HEVs was observed after treatment with anti–PSGL-1 alone or in combination with anti–P- and E-selectin; no effect was observed after anti–P-selectin treatment. These findings suggest that, during monocyte migration through inflamed HEVs, PSGL-1 would participate in secondary rolling (Figure 2G) but not mediate direct binding to HEVs. However, P- and E-selectins participated in leukocyte and plasmacytoid DC migration through inflamed HEVs.24,25 Similar variations to those reported for dermal and PO-LN monocytes were noticed when the number of dermal mo-DCs and LN mo-DCs present in the PO-LNs was analyzed (Figure 1F,G).
To confirm these data on the contribution of L-selectin and PSGL-1 to monocyte migration, we next analyzed the effect of mAbs against these molecules on the migration of adaptively transferred monocytes (Figure 2A). The rationale for these experiments was to avoid a nonaccurate interpretation because of the possibility that a fraction of monocytes, newly formed during the last hours before the analysis, was not blocked by the mAbs. These experiments confirmed the differential contribution of L-selectin and PSGL-1 in monocyte migration through DVs (Figure 2C,E) and the essential role of L-selectin in monocyte migration through HEVs (Figure 2D,F).
This report analyzes for the first time the molecules involved in mouse monocyte rolling during an infectious process. Moreover, to our knowledge, even in steady state, no previous reports had investigated the rolling molecules controlling mouse monocyte transendothelial migration. Our data demonstrate the involvement of PSGL-1 and L-selectin in this process and of their differential contribution to monocyte migration through DVs and HEVs. Our results suggest that PNAd is induced on DVs during infection, confirming previous data on PNAd upregulation during lymphocyte homing in autoimmune and inflammatory reactions.16-19 These data have important implications regarding the analysis of the immune responses in experimental models involving lymphocyte homing blocking and immunotherapeutic protocols of inflammatory and autoimmune diseases based on the selective inhibition of monocyte migration to specific inflammatory foci.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Jens Stein for scientific advice and for the Alexa-594–conjugated anti-PNAd mAb and Drs Dietmar Vestweber, Rupert Hallman, and Britta Engelhardt for reagents.
This work was supported by the Spanish Ministry of Research (SAF-2006-07 609) and the Comunidad de Madrid (S-SAL-0304-2006).
Authorship
Contribution: B.L. designed and performed research, and analyzed data; and C.A. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Carlos Ardavín, Departamento de Inmuno-logía y Oncología, Centro Nacional de Biotecnología/CSIC, Universidad Autónoma, Madrid 28049, Spain; e-mail: ardavin@cnb.uam.es.