To the editor:
Ikaros transcription factors are regulators of lymphocyte development, and changes in their expression result in lymphoma development in mice.1 Although expression of Ikaros family members has been studied in different leukemias,2-4 it has not been previously reported in lymphoma. Thus, we tackle the question whether there is a quantitative difference in distribution of Ikaros, Aiolos, and Helios mRNA between Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL). Formalin-fixed and paraffin-embedded sections of lymph nodes from 41 patients were analyzed.5 NHL is represented by the most frequent indolent follicular center cell lymphoma (FCC), the aggressive diffuse large B-cell lymphoma (DLBCL) and a less frequent anaplastic large cell lymphoma (ALCL; Figure 1). Subclassification of HL (mixed cellularity or nodular) did not reveal any difference in Ikaros family expression, so we rather present them as a single group. The experiments were approved by the Ethics Committee of the University of Zugreb Medical School. Informed consent was obtained in accordance with the Declaration of Helsinki.
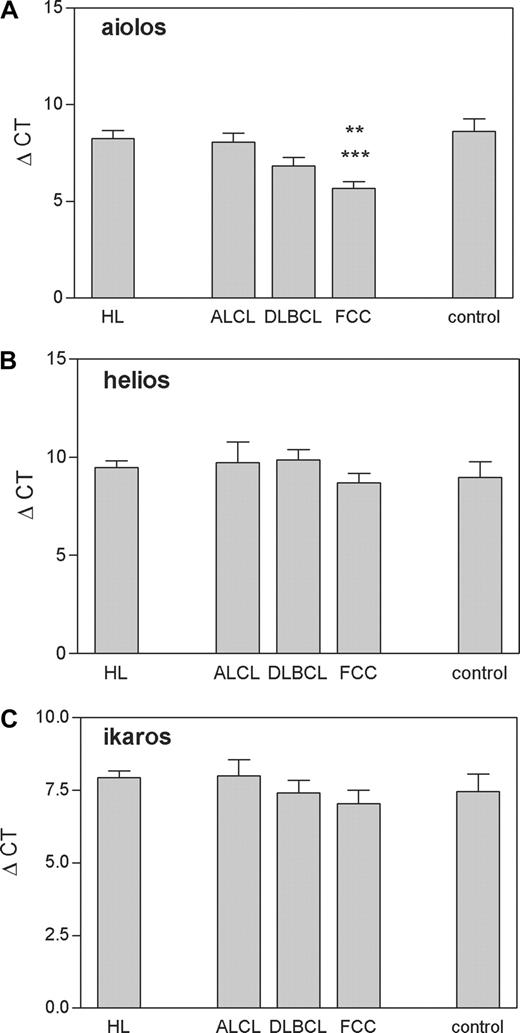
Real-time RT-PCR analysis of Aiolos, Helios, and Ikaros gene expression in HL (N = 10), ALCL (N = 3), DLBCL (N = 15), FCC (N = 13), and control tissues (N = 6) according to the WHO classification. Control includes tonsils and follicular hyperplasia. The results of specific mRNA expression are normalized with the housekeeping gene and illustrated as ΔCt values. Means plus or minus SEM are shown. Aiolos: **P less than .01, FCC versus control; ***P less than .001, FCC versus HL (Tukey test following one-way ANOVA)
Real-time RT-PCR analysis of Aiolos, Helios, and Ikaros gene expression in HL (N = 10), ALCL (N = 3), DLBCL (N = 15), FCC (N = 13), and control tissues (N = 6) according to the WHO classification. Control includes tonsils and follicular hyperplasia. The results of specific mRNA expression are normalized with the housekeeping gene and illustrated as ΔCt values. Means plus or minus SEM are shown. Aiolos: **P less than .01, FCC versus control; ***P less than .001, FCC versus HL (Tukey test following one-way ANOVA)
Figure 1 shows that the relative quantity of Ikaros and Helios mRNA is not statistically different among HL, DLBCL, FCC, ALCL, and normal controls tested (Ikaros: F(4,42) = 0.612, P = .656; Helios: F(4,42) = 0.858, P = .497; one-way ANOVA) On the other hand, analysis of the third Ikaros family member, Aiolos, showed a statistically significant difference among groups (F(4,42) = 7.052, P < .01, one-way ANOVA) due to increase of its expression in FCC-NHL samples (P < .01 vs control; P < .001 vs HL; Tukey test) up to 7-fold, when normalized to the level of gene expression in the control/reference tissue (not shown).
A feature of FCC lymphoma is overexpression of the BCL-2 protein. bcl-2 synergizes potently in tumorigenesis with growth-promoting oncogenes. Probably Bcl-2 overexpression functions in neoplastic transformation by extending the life span of cells, thereby facilitating acquisition of further oncogenic mutations.6 Aiolos expression has been reported to be involved in the control of apoptosis through interaction with Bcl-xL,7 and in DT40 B cells Aiolos promotes cell survival.8 It is our hypothesis that in FCC-NHL overexpression of Aiolos as well as of Bcl-2 contributes to the malignant phenotype.
Ikaros and early B-cell factor (EBF) transcription factors regulate lambda 5 expression of pre-B-cell receptor (BCR) at the pre-BI stage of B-cell development. The transition from pre-BI to pre-BII is marked by pre-BCR down-regulation due to higher Aiolos expression.9 Because one approach to understanding neoplastic transformation is by comparing normal cells to their malignant counterpart, our results would indicate a disregulation of a developmental pathway in FCC-NHL.
Measuring gene expression of many genes simultaneously by microarray profiling can distinguish lymphoid malignancies and also identify previously unrecognized clinically distinct lymphoma subtypes.10 However, in an attempt to crossvalidate the expression of our genes of interest with the publicly available data from several research groups, we couldn't specifically distinguish Ikaros, Aiolos, and Helios transcription factors.11 Therefore, a single gene approach of classical molecular biology is still an important complement to the microarray gene expression profiling for understanding disease processes. Taken together, our results show for the first time Aiolos overexpression in primary lymphoma tissues, which could be exploited in future clinical trials.
Authorship
We thank Dr W. Ellmeier for helpful discussion and critical comments on the work.
This work was supported by the Ministry of Science, Education, and Sport (Project No. 098-0982913-2332) to M.A. and by the Bilateral collaborative project Croatia-Austria to M.A. and W. Ellmeier (OAD No. 911-02/05-06/20).
Contribution: M.A. designed and performed research and wrote the manuscript; L.C.S., S.K., and M.M., performed research and statistical analyses and contributed to the writing of the manuscript; S.D. and M.D. provided primary lymphoma samples fully characterized at the cellular and molecular level and contributed to experimental design and writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mariastefania Antica, Laboratory of Cellular and Molecular Immunology, Division of Molecular Biology, Rudjer Boskovic Institute, Bijenicka 54, 10 000 Zagreb, Croatia; e-mail: antica@irb.hr.